Seven New Drimane-Type Sesquiterpenoids from a Marine-Derived Fungus Paraconiothyrium sporulosum YK-03
Abstract
1. Introduction
2. Results and Discussion
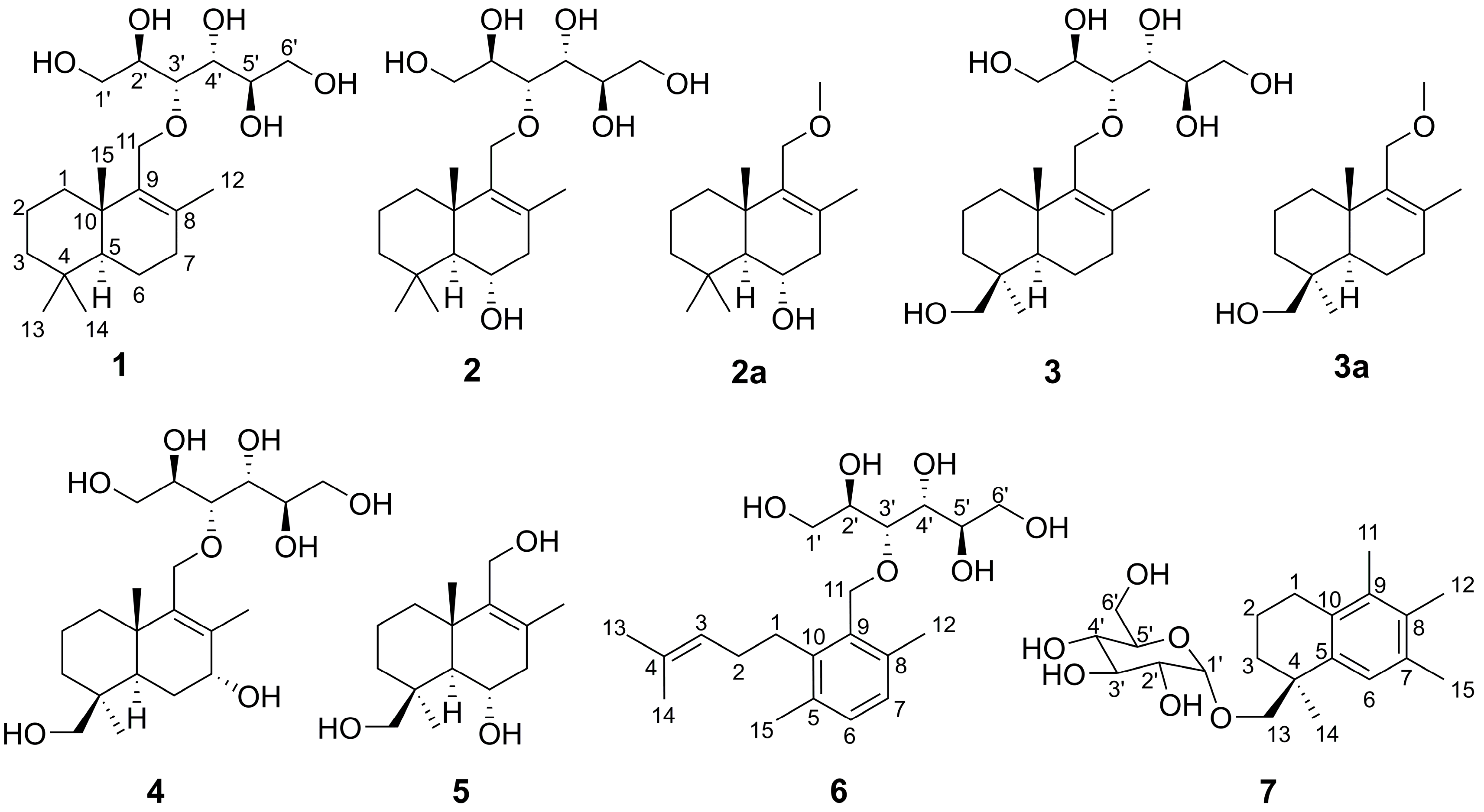
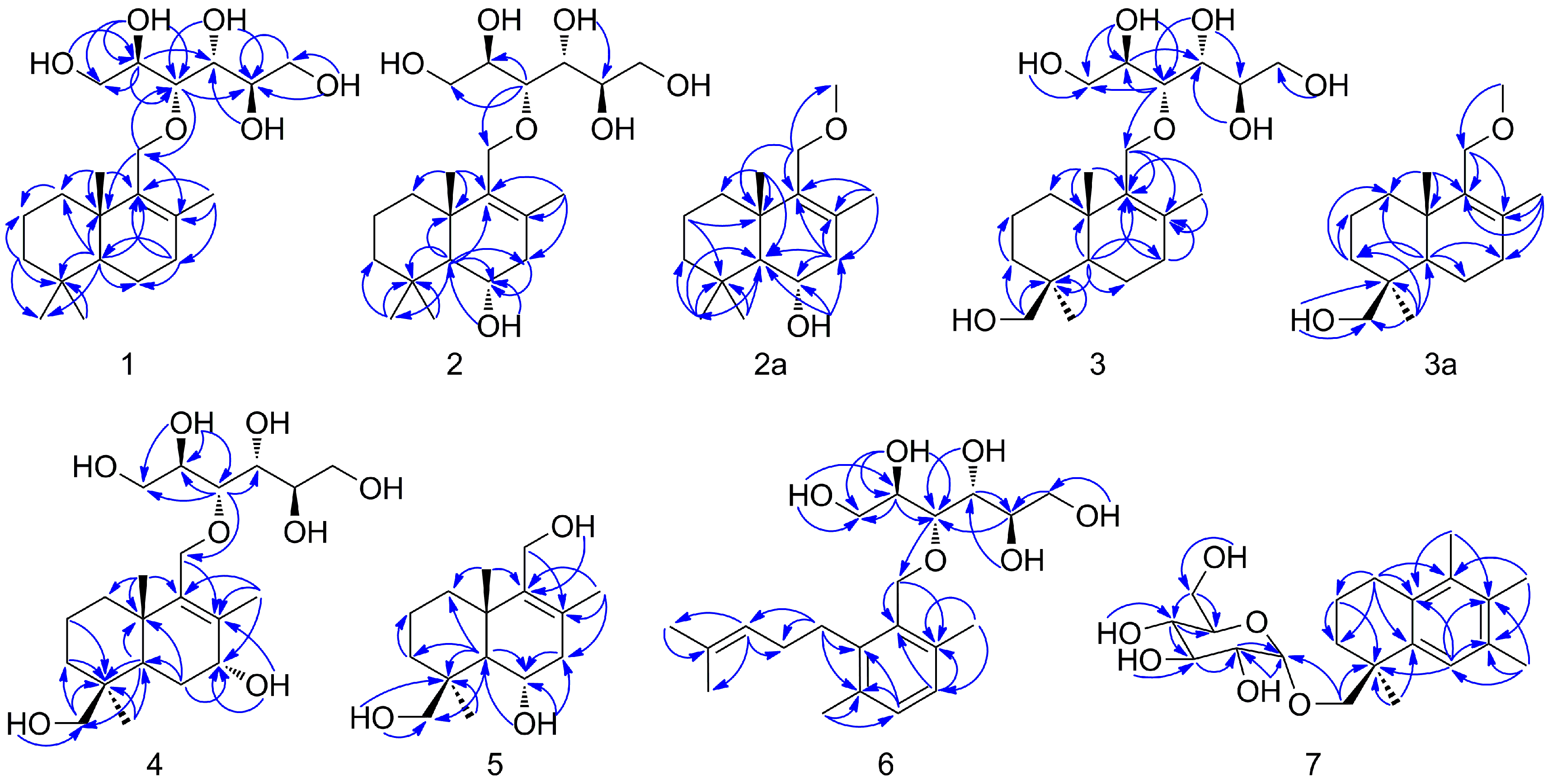
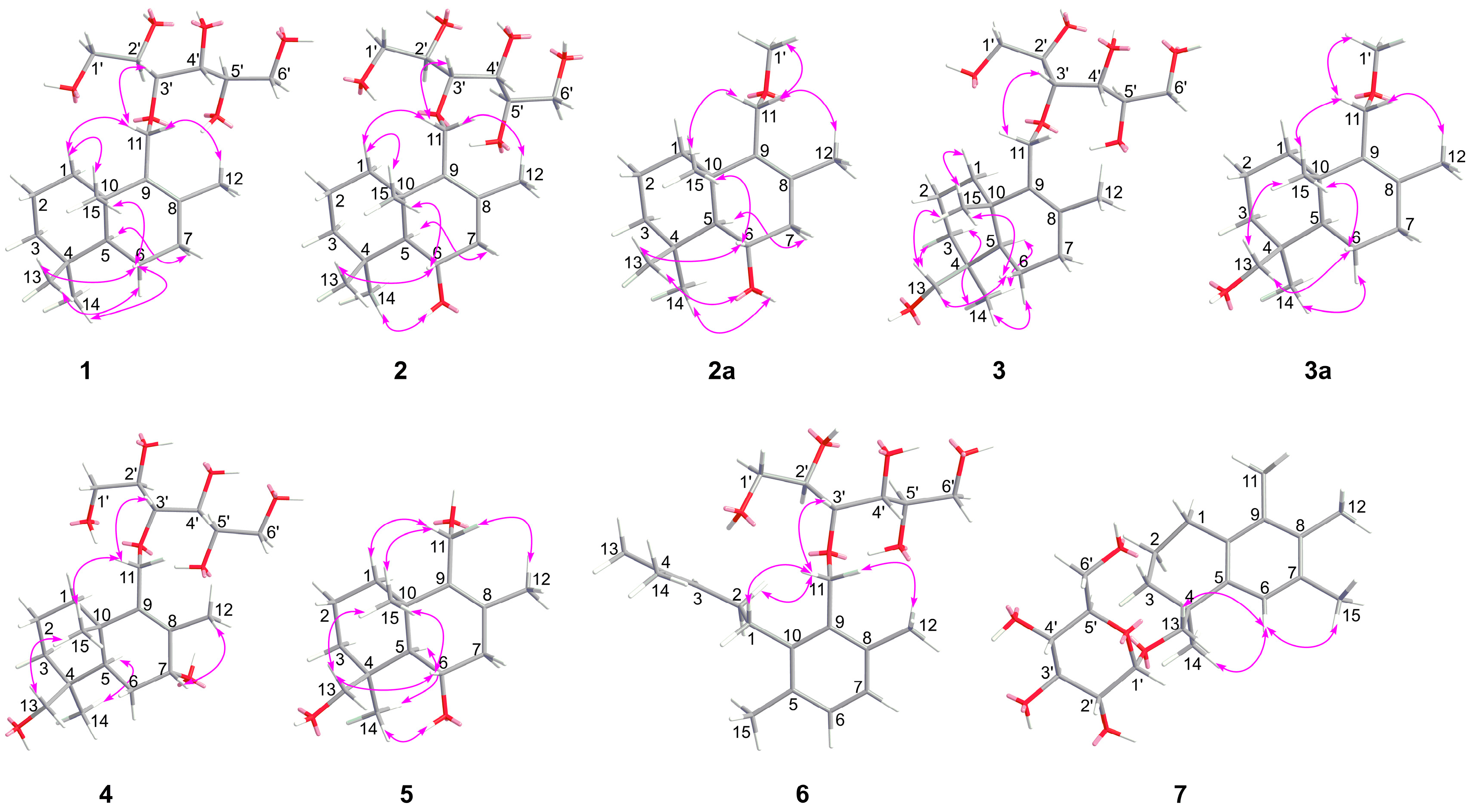
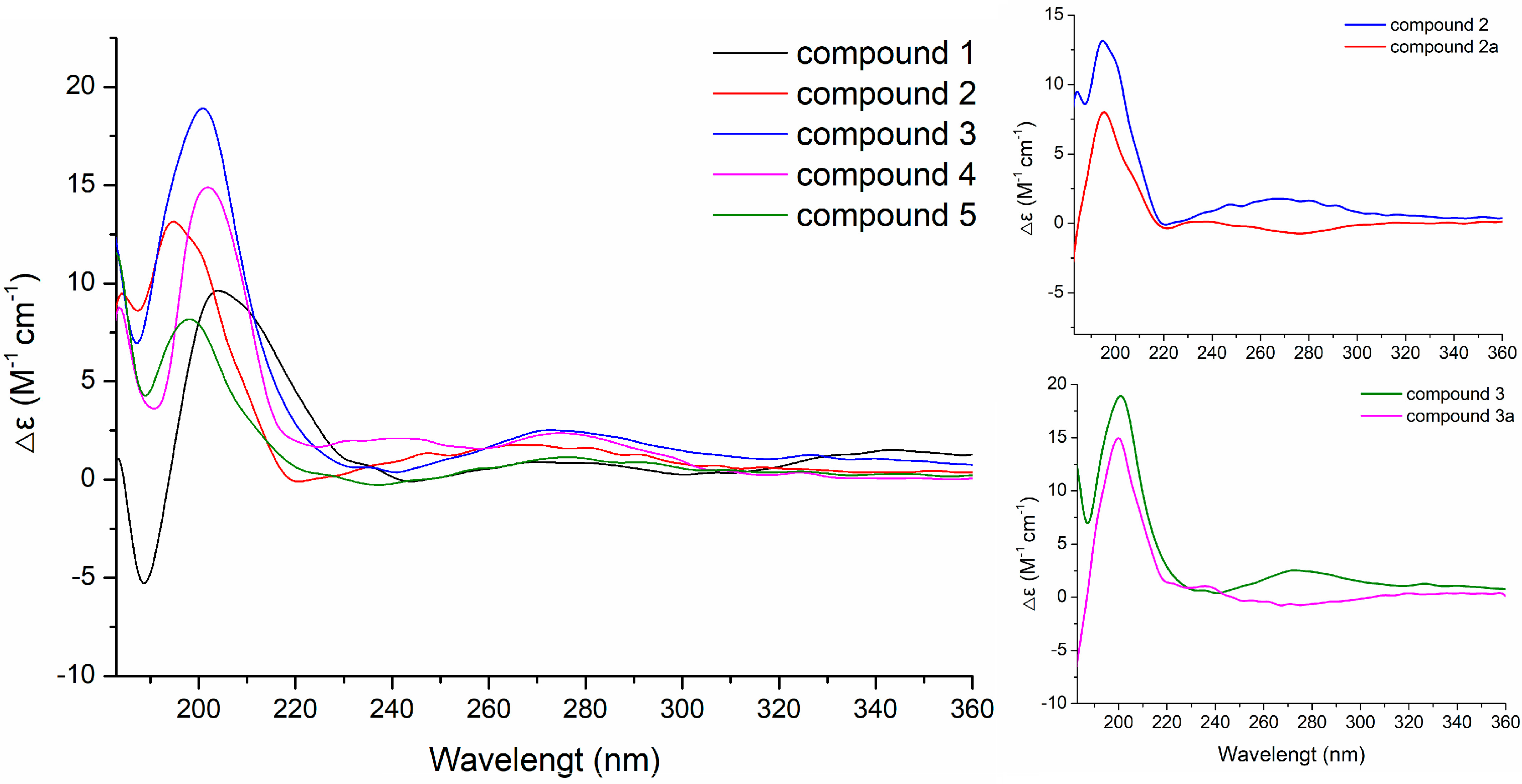
2.1. Structural Elucidation
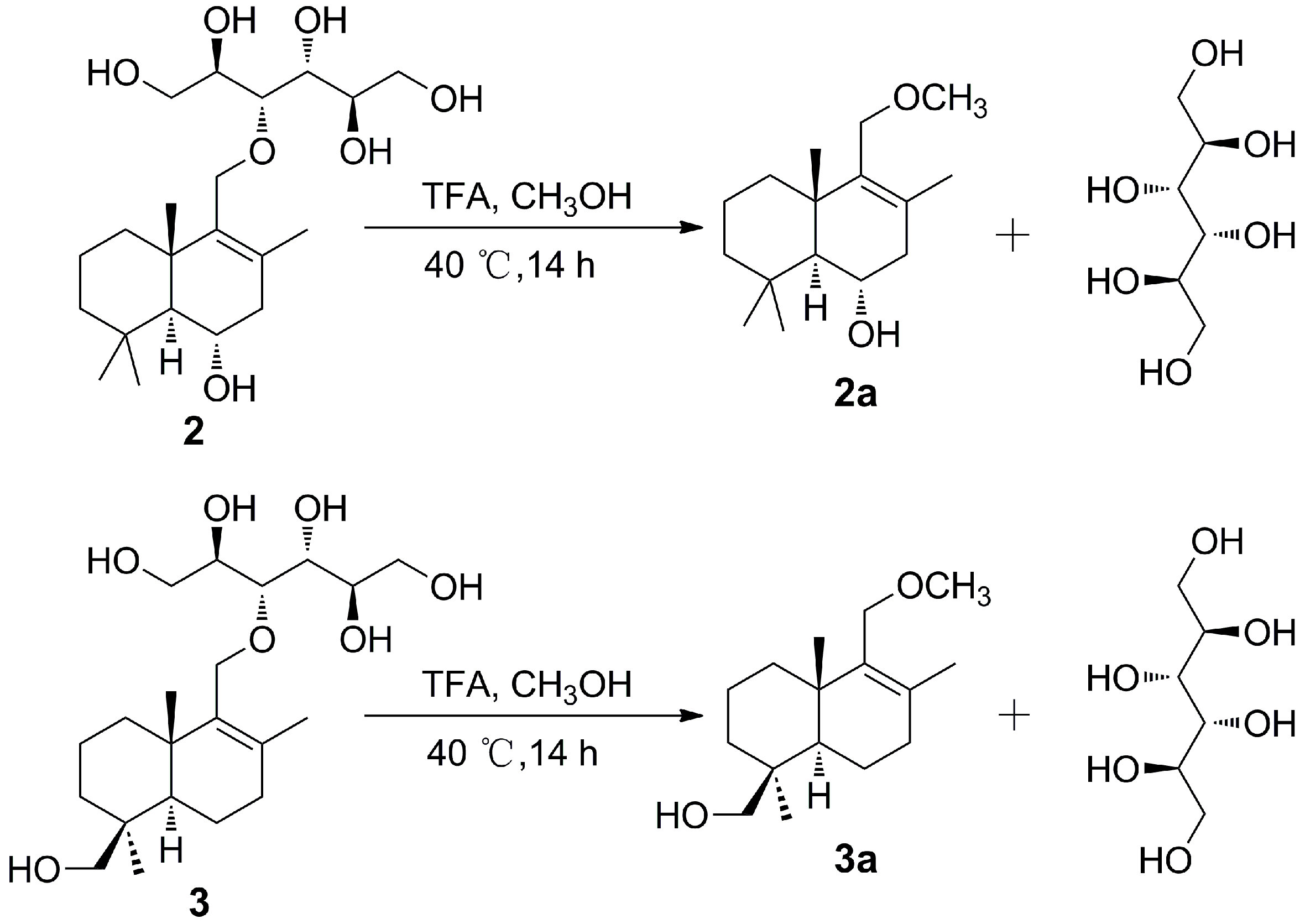
2.2. Investigation on the Origin of Mannitol Moiety
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation
3.4. Extraction and Isolation
3.5. X-ray Crystallographic Analysis of Compounds 2a and 7
3.6. Acid Hydrolysis of Compounds 2 and 3
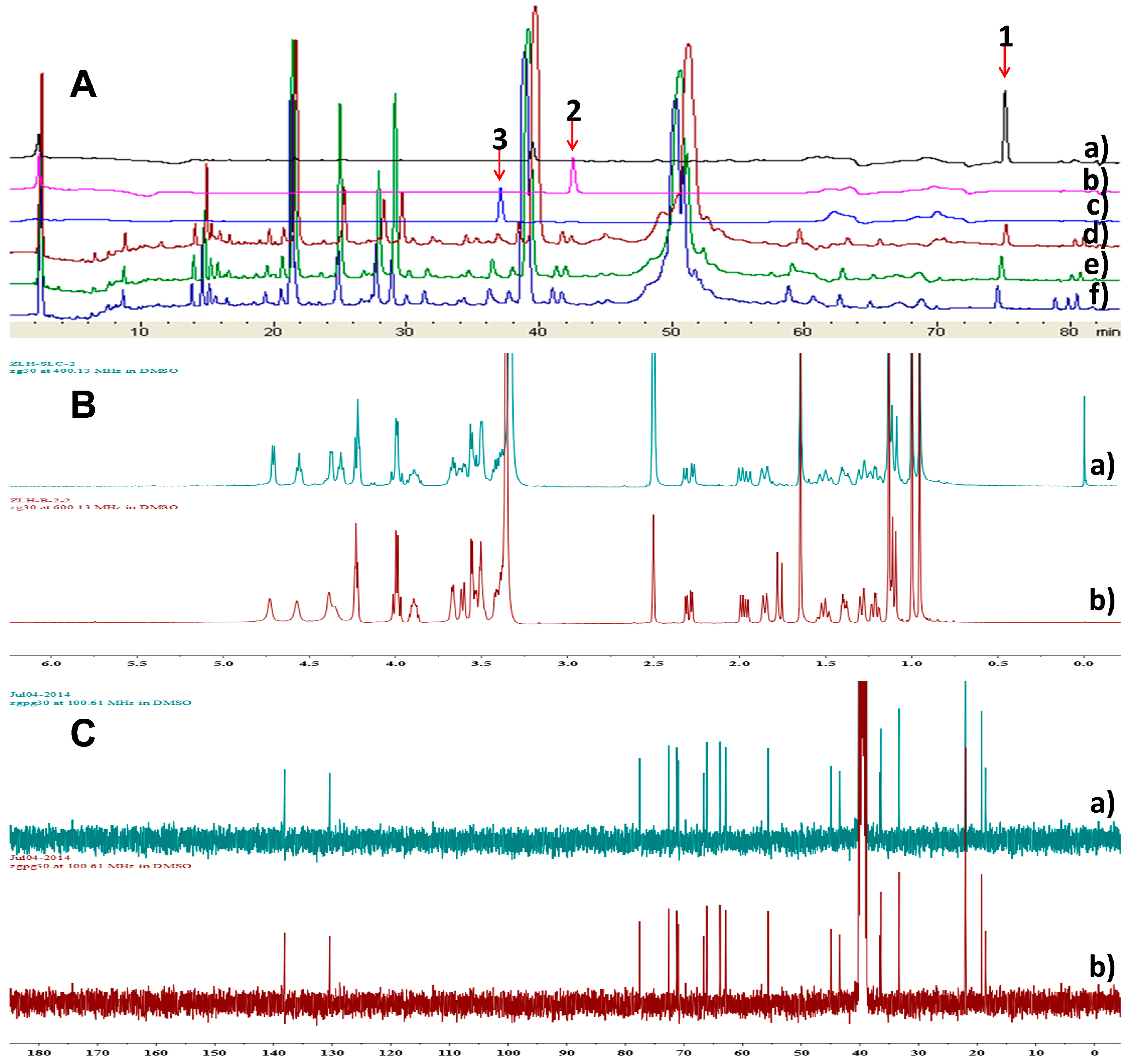
3.7. Analysis of the Refrence Compounds 1–3 and Metabolites of P. Sporulosum YK-03 in Different Mediums Using HPLC and NMR Methods
3.8. Cytotoxic Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef]
- Barbero, H.; Diez-Poza, C.; Barbero, A. The Oxepane Motif in Marine Drugs. Mar. Drugs 2017, 15, 361. [Google Scholar] [CrossRef]
- Overy, D.P.; Bayman, P.; Kerr, R.G.; Bills, G.F. An assessment of natural product discovery from marine (sensu strictu) and marine-derived fungi. Mycology 2014, 5, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Verkley, G.J.M.; Silva, M. d.; Wicklow, D.T.; Crou, P.W. Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Stud. Mycol. 2004, 50, 323–335. [Google Scholar]
- Takano, K.; Itoh, Y.; Ogino, T.; Kurosawa, K.; Sasaki, K. Phylogenetic analysis of manganese-oxidizing fungi isolated from manganese-rich aquatic environments in Hokkaido, Japan. Limnology 2006, 7, 219–223. [Google Scholar] [CrossRef]
- Liu, L.; Gao, H.; Chen, X.; Cai, X.; Yang, L.; Guo, L.; Yao, X.; Che, Y. Brasilamides A-D: Sesquiterpenoids from the Plant Endophytic Fungus Paraconiothyrium brasiliense. Eur. J. Org. Chem. 2010, 3302–3306. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Li, D.; Zhang, Y.; Li, L.; Guo, L.; Cao, Y.; Che, Y. Bisabolane Sesquiterpenoids from the Plant Endophytic Fungus Paraconiothyrium brasiliense. J. Nat. Prod. 2015, 78, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ren, F.; Che, Y.; Liu, G.; Liu, L. New Bergamotane Sesquiterpenoids from the Plant Endophytic Fungus Paraconiothyrium brasiliense. Molecules 2015, 20, 14611–14620. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Y.; Zhao, C.; Ren, F.; Liu, X.; Che, Y. Hawaiinolides E-G, cytotoxic cassane and cleistanthane diterpenoids from the entomogenous fungus Paraconiothyrium hawaiiense. Fitoterapia 2014, 99, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Y.; Niu, S.; Liu, X.; Che, Y. Cytotoxic cleistanthane and cassane diterpenoids from the entomogenous fungus Paraconiothyrium hawaiiense. J. Nat. Prod. 2014, 77, 1513–1518. [Google Scholar] [CrossRef]
- Mohamed, I.E.; Kehraus, S.; Krick, A.; Konig, G.M.; Kelter, G.; Maier, A.; Fiebig, H.-H.; Kalesse, M.; Malek, N.P.; Gross, H. Mode of Action of Epoxyphomalins A and B and Characterization of Related Metabolites from the Marine-Derived Fungus Paraconiothyrium sp. J. Nat. Prod. 2010, 73, 2053–2056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-H.; Feng, B.-M.; Chen, G.; Li, S.-G.; Sun, Y.; Wu, H.-H.; Bai, J.; Hua, H.-M.; Wang, H.-F.; Pei, Y.-H. Sporulaminals A and B: A pair of unusual epimeric spiroaminal derivatives from a marine-derived fungus Paraconiothyrium sporulosum YK-03. RSC Adv. 2016, 6, 42361–42366. [Google Scholar] [CrossRef]
- Shiono, Y.; Kikuchi, M.; Koseki, T.; Murayama, T.; Kwon, E.; Aburai, N.; Kimura, K.-i. Isopimarane diterpene glycosides, isolated from endophytic fungus Paraconiothyrium sp. MY-42. Phytochemistry 2011, 72, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, Z.; Li, H.; Wang, Y.; Fu, P.; Zhu, W. Pafuranones A and B, two dimeric polyketides from a rare marine algae-derived fungus Paraconiothyrium sp. Chin. Chem. Lett. 2019. [Google Scholar] [CrossRef]
- Suzuki, T.; Ariefta, N.R.; Koseki, T.; Furuno, H.; Kwon, E.; Momma, H.; Harneti, D.; Maharani, R.; Supratman, U.; Kimura, K.I.; et al. New polyketides, paralactonic acids A-E produced by Paraconiothyrium sp. SW-B-1, an endophytic fungus associated with a seaweed, Chondrus ocellatus Holmes. Fitoterapia 2019, 132, 75–81. [Google Scholar] [PubMed]
- Zhao, C.; Fu, P.; Zhang, Y.; Liu, X.; Ren, F.; Che, Y. Sporulosol, a New Ketal from the Fungus Paraconiothyrium sporulosum. Molecules 2018, 23, 1263. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Nhiem, N.X.; Tai, B.H.; Yen, P.H.; Dung, D.T.; Ngan, N.T.T.; Le Tuan Anh, H.; Van Minh, C.; Van Kiem, P. Secondary metabolites from the marine-derived fungus Paraconiothyrium sp. VK-13. Vietnam J. Chem. 2018, 56, 434–439. [Google Scholar]
- Ren, F.; Chen, S.; Zhang, Y.; Zhu, S.; Xiao, J.; Liu, X.; Su, R.; Che, Y. Hawaiienols A-D, Highly Oxygenated p-Terphenyls from an Insect-Associated Fungus, Paraconiothyrium hawaiiense. J. Nat. Prod. 2018, 81, 1752–1759. [Google Scholar] [CrossRef]
- Almeida, C.; El Aouad, N.; Martin, J.; Perez-Victoria, I.; Gonzalez-Menendez, V.; Platas, G.; de la Cruz, M.; Monteiro, M.C.; de Pedro, N.; Bills, G.F.; et al. Graminin B, a furanone from the fungus Paraconiothyrium sp. J. Antibiot. 2014, 67, 421–423. [Google Scholar] [CrossRef]
- Xu, G.-B.; Mi, J.; Yang, T.; Wu, L.-W.; Yuan, X.-H.; Li, G.-Y. Two new polyketide metabolites isolated from Paraconiothyrium brasiliense. Chem. Nat. Compd. 2017, 53, 870–873. [Google Scholar] [CrossRef]
- Cho, N.; Ransom, T.T.; Sigmund, J.; Tran, T.; Cichewicz, R.H.; Goetz, M.; Beutler, J.A. Growth Inhibition of Colon Cancer and Melanoma Cells by Versiol Derivatives from a Paraconiothyrium Species. J. Nat. Prod. 2017, 80, 2037–2044. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Li, S.-G.; Wu, H.-H.; Chen, G.; Li, L.; Bai, J.; Hua, H.-M.; Wang, H.-F.; Pei, Y.-H. 3,4-Dihydroisocoumarin derivatives from the marine-derived fungus Paraconiothyrium sporulosum YK-03. Phytochem. Lett. 2017, 20, 200–203. [Google Scholar] [CrossRef]
- De Gusmño, N.B.; Kaouadji, M.; Steiman, R.; Seigle-murandi, F.; Ulrichc, J. Coniothyriol, an uncommon polyketide from Coniothyrium sporulosum. Nat. Prod. Lett. 1993, 2, 287–292. [Google Scholar] [CrossRef]
- Guiraud, P.; Steiman, R.; Seigle-Murandi, F.; de Gusmao, N.B. Antimicrobial and Antitumor Activities of Mycosporulone. J. Nat. Prod. 1999, 62, 1222–1224. [Google Scholar] [CrossRef]
- Quang, T.H.; Kim, D.C.; Van Kiem, P.; Van Minh, C.; Nhiem, N.X.; Tai, B.H.; Yen, P.H.; Thi ThanH-Ngan, N.; Kim, H.J.; Oh, H. Macrolide and phenolic metabolites from the marine-derived fungus Paraconiothyrium sp. VK-13 with anti-inflammatory activity. J. Antibiot. 2018, 71, 826–830. [Google Scholar] [CrossRef]
- Jansen, B.J.M.; De Groot, A. The occurrence and biological activity of drimane sesquiterpenoids. Nat. Prod. Rep. 1991, 8, 309–318. [Google Scholar] [CrossRef]
- Jansen, B.J.M.; de Groot, A. Occurrence, biological activity and synthesis of drimane sesquiterpenoids. Nat. Prod. Rep. 2004, 21, 449–477. [Google Scholar] [CrossRef] [PubMed]
- Erkel, G.; Lorenzen, K.; Anke, T.; Velten, R.; Gimenez, A.; Steglich, W. Kuehneromycins A and B, two new biological active compounds from a Tasmanian Kuehneromyces sp. (Strophariaceae, Basidiomycetes). Naturforsch. C Biosci. 1995, 50, 1–10. [Google Scholar] [CrossRef]
- Fleck, W.F.; Schlegel, B.; Hoffmann, P.; Ritzau, M.; Heinze, S.; Graefe, U. Isolation of isodrimenediol, a possible intermediate of drimane biosynthesis from Polyporus arcularius. J. Nat. Prod. 1996, 59, 780–781. [Google Scholar] [CrossRef]
- Derita, M.; Montenegro, I.; Garibotto, F.; Enriz, R.D.; Fritis, M.C.; Zacchino, S.A. Structural requirements for the antifungal activities of natural drimane sesquiterpenes and analogues, supported by conformational and electronic studies. Molecules 2013, 18, 2029–2051. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Alves, T.M.; Ribeiro, F.L.; Kloos, H.; Zani, C.L. Polygodial, the fungitoxic component from the Brazilian medicinal plant Polygonum punctatum. Mem. Inst. Oswaldo Cruz 2001, 96, 831–833. [Google Scholar] [CrossRef]
- Grabley, S.; Thiericke, R.; Zerlin, M.; Goert, A.; Phillips, S.; Zeeck, A. Secondary metabolites by chemical screening. 33. New albrassitriols from Aspergillus sp. (FH-A 6357). J. Antibiot. 1996, 49, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Anke, H.; Sterner, O. Comparison of the antimicrobial and cytotoxic activities of twenty unsaturated sesquiterpene dialdehydes from plants and mushrooms. Planta Med. 1991, 57, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Liu, X.-X.; Zhang, W.-J.; Zang, L.-Y.; Wang, G.; Ng, S.W.; Tan, R.-X.; Ge, H.-M. Sesquiterpenoids isolated from an endophyte fungus Diaporthe sp. RSC Adv. 2015, 5, 17559–17565. [Google Scholar] [CrossRef]
- Abad, A.; Agulló, C.; Cuñat, A.C.; González-Coloma, A.; Pardo, D. Preparation of 9α-Fluorinated Sesquiterpenic Drimanes and Evaluation of Their Antifeedant Activities. Eur. J. Org. Chem. 2010, 2182–2198. [Google Scholar] [CrossRef]
- Montenegro, I.; Pino, L.; Werner, E.; Madrid, A.; Espinoza, L.; Moreno, L.; Villena, J.; Cuellar, M. Comparative study on the larvicidal activity of drimane sesquiterpenes and nordrimane compounds against Drosophila melanogaster til-til. Molecules 2013, 18, 4192–4208. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.; Hardie, J.; Pickett, J.A. Laboratory evaluation of antifeedant compounds for inhibiting settling by cereal aphids. Entomol. Exp. Appl. 1997, 84, 189–193. [Google Scholar] [CrossRef]
- Ying, B.-P.; Peiser, G.; Ji, Y.-Y.; Mathias, K.; Tutko, D.; Hwang, Y.-S. Phytotoxic sesquiterpenoids from Canella winterana. Phytochem. Lett. 1995, 38, 909–915. [Google Scholar] [CrossRef]
- Minale, L.; Riccio, R.; Sodano, G. Avarol, a novel sesquiterpenoid hydroquinone with a rearranged drimane skeleton from the sponge Disidea avara. Tetrahedron Lett. 1974, 3401–3404. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Edu, C.; Biriiac, A.; Lungu, L.; Aricu, A.; D’Ambrosio, M.; Shova, S.; Nicolescu, A.; Deleanu, C.; Vornicu, N. Synthesis of Polyfunctional Drimanes from Drim-7,9(11)-diene and Drim-8-en-7-one. Synth. Commun. 2013, 43, 3020–3033. [Google Scholar] [CrossRef]
- Domingo, V.; Prieto, C.; Siva, L.; Rodilla, J.M.; Quilez del Moral, J.F.; Barrero, A.F. Iodine, a Mild Reagent for the Aromatization of Terpenoids. J. Nat. Prod. 2016, 79, 831–837. [Google Scholar] [CrossRef]
- Henquet, M.G.L.; Prota, N.; van der Hooft, J.J.J.; Varbanova-Herde, M.; Hulzink, R.J.M.; de Vos, M.; Prins, M.; de Both, M.T.J.; Franssen, M.C.R.; Bouwmeester, H.; et al. Identification of a drimenol synthase and drimenol oxidase from Persicaria hydropiper, involved in the biosynthesis of insect deterrent drimanes. Plant J. 1052, 90, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Schnarr, G.W.; Vyas, D.M.; Szarek, W.A. Carbon-13 nuclear magnetic resonance spectra of acyclic carbohydrate derivatives: Alditols, 1,-bis(phenylhydrazones), and dithioacetals. J. Chem. Soc. Perkin Trans. 1979, 2, 496–503. [Google Scholar] [CrossRef]
- Ueoka, R.; Nakao, Y.; Kawatsu, S.; Yaegashi, J.; Matsumoto, Y.; Matsunaga, S.; Furihata, K.; van Soest, R.W.M.; Fusetani, N. Gracilioethers A-C, Antimalarial Metabolites from the Marine Sponge Agelas gracilis. J. Org. Chem. 2009, 74, 4203–4207. [Google Scholar] [PubMed]
Sample Availability: Not Available. |

| No. | 1 | 2 | 2a b | 3 | 3a b | 4 | 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δC | δH, mult (J, Hz) | δC | δH, mult (J, Hz) | δC | δH, mult (J, Hz) | δC | δH, mult (J, Hz) | δC | δH, mult (J, Hz) | δC | δH, mult (J, Hz) | δC | δH, mult (J, Hz) | |
| 1 | 36.1 | 1.89, d (13.2); 1.18, td (13.2, 2.4) | 36.7 | 1.84, d (13.2); 1.20, td (13.2, 3.0) | 36.9 | 1.71, d (12.6); 1.18, td (13.2, 3.6) | 36.2 | 1.89, d (13.2); 1.19, dd (10.8, 3.6) | 36.4 | 1.74, d (13.2); 1.16, d (13.2) | 35.8 | 1.88, d (13.2); 1.17, td (13.2, 3.6) | 37.0 | 1.78, m; 1.29, m |
| 2 | 18.7 | 1.55, m; 1.43, m | 18.6 | 1.51, qt (13.6, 3.8); 1.37, dt (13.6, 3.5) | 18.9 | 1.50, qt (13.2, 3.6); 1.38, dt (13.2, 3.6) | 18.5 | 1.49, m; 1.38, m | 18.6 | 1.50, m; 1.37, m | 18.5 | 1.55, m; 1.39, m | 18.6 | 1.45, m; 1.33, m |
| 3 | 41.4 | 1.36, m; 1.11, td (12.6, 3.6) | 43.5 | 1.28, d (13.2); 1.10, d (10.8) | 43.9 | 1.29, d (13.2); 1.11, m | 35.1 | 1.77, d (13.2); 0.78, td (13.2, 3.6) | 35.4 | 1.77, d (13.2); 0.78, td (13.2, 3.6) | 35.2 | 1.78, d (13.8); 0.82, td (13.2, 3.6) | 38.1 | 1.58, d (13.2); 0.90, td (13.2, 4.0) |
| 4 | 33.2 | 33.4 | 33.8 | 38.4 | 38.7 | 38.0 | 38.7 | |||||||
| 5 | 51.1 | 1.05, d (12.6) | 55.7 | 1.09, d (11.1) | 56.1 | 1.08, d (10.8) | 51.9 | 1.14, d (12.6) | 52.2 | 1.13, d (12.6) | 45.4 | 1.49, d (13.2) | 56.5 | 1.21, d (11.2) |
| 6 | 18.6 | 1.61, m; 1.37, m | 66.1 | 3.88, m | 66.5 | 3.89, m | 18.7 | 1.67, m; 1.36, m | 19.0 | 1.68, m; 1.37, m | 28.8 | 1.68, d (12.6); 1.57, dd (12.6, 4.8) | 66.9 | 3.96, m |
| 7 | 33.3 | 2.00, m; 2.01, m | 45.0 | 2.29, dd (17.8, 6.3); 1.98, dd (17.8, 8.9) | 45.2 | 2.30, dd (18.0, 6.0); 1.96, dd (18.0, 9.0) | 33.8 | 1.94, m; 1.95, m | 33.9 | 1.95, m | 68.2 | 3.69, m | 44.5 | 2.20, dd (17.2, 6.0); 1.94, dd (17.2, 9.6) |
| 8 | 131.7 | 130.4 | 131.0 | 131.6 | 131.9 | 133.5 | 128.5 | |||||||
| 9 | 138.4 | 138.2 | 137.6 | 138.4 | 137.8 | 140.6 | 140.8 | |||||||
| 10 | 37.5 | 40.0 | 40.5 | 37.5 | 37.7 | 38.2 | 40.4 | |||||||
| 11 | 66.8 | 4.02, d (15.0); 4.00, d (15.0) | 66.7 | 3.98, d (16.7); 3.97, d (16.7) | 67.8 | 3.80, d (10.5); 3.69, d (10.5) | 66.8 | 4.00, d (12.6); 3.99, d (12.6) | 67.9 | 3.80, d (10.2); 3.68, d (10.2) | 66.8 | 4.02, d (10.2); 3.99, d (10.2) | 56.5 | 3.91, dd (11.6, 4.4); 3.81, dd (11.6, 4.8) |
| 12 | 19.8 | 1.65, s | 19.3 | 1.64, s | 19.4 | 1.59, s | 19.7 | 1.63, s | 19.6 | 1.57, s | 17.6 | 1.74, s | 19.2 | 1.61, s |
| 13 | 21.5 | 0.81, s | 22.0 | 0.99, s | 22.4 | 0.94, s | 62.6 | 3.52, m; 3.14, m | 62.9 | 3.52, m; 3.13, m | 62.8 | 3.54, m; 3.13, dd (10.8, 5.4) | 65.1 | 3.78, dd (10.4, 4.8); 3.37, dd (10.4, 4.4) |
| 14 | 33.1 | 0.86, s | 36.5 | 1.12, s | 36.8 | 1.13, s | 27.3 | 0.87, s | 27.6 | 0.87, s | 27.0 | 0.86, s | 31.4 | 1.13, s |
| 15 | 20.7 | 0.92, s | 22.0 | 0.94, s | 22.3 | 0.94, s | 21.3 | 0.89, s | 21.4 | 0.88, s | 19.5 | 0.85, s | 22.9 | 0.98, s |
| 6-OH | 4.22, d (6.6) | 4.43, d (4.8) | ||||||||||||
| 7-OH | 4.56, d (5.4) | 4.11, t (4.8) | ||||||||||||
| 13-OH | 4.14, t (4.8) | 4.16, t (5.4) | ||||||||||||
| 1′ | 63.0 | 3.54, dd (10.2, 4.8); 3.41, m | 63.0 | 3.52, m; 3.40, m | 63.0 | 3.53, m; 3.40, m | 62.9 | 3.51, m; 3.42, m | ||||||
| 2′ | 72.8 | 3.68, m | 72.6 | 3.65, m | 72.7 | 3.66, m | 72.8 | 3.67, m | ||||||
| 3′ | 77.6 | 3.57, d (4.8) | 77.7 | 3.54, d (4.8) | 77.6 | 3.55, d (4.8) | 77.6 | 3.57, d (4.8) | ||||||
| 4′ | 71.1 | 3.51, m | 71.0 | 3.51, m | 71.0 | 3.50, m | 71.1 | 3.51, m | ||||||
| 5′ | 71.4 | 3.51, m | 71.3 | 3.49, m | 71.3 | 3.50, m | 71.3 | 3.51, m | ||||||
| 6′ | 64.0 | 3.61, dd (10.2, 4.2); 3.35, m | 63.9 | 3.60, d (10.8); 3.36, m | 63.9 | 3.60, m; 3.36, m | 63.9 | 3.61, dd (9.6, 6.0); 3.36, m | ||||||
| 1’-OH | 4.57, t (5.4) | 4.57, br s | 4.54, t (4.8) | 4.61, t (5.4) | ||||||||||
| 2’-OH | 4.72, d (4.8) | 4.72, br s | 4.70, d (4.8) | 4.77, d (5.4) | ||||||||||
| 4’-OH | 4.20, br s | 4.23, d (5.4) | 4.20, d (4.8) | 4.25, br d (4.8) | ||||||||||
| 5’-OH | 4.37, br s | 4.38, br s | 4.36, br s | 4.42, br d (4.8) | ||||||||||
| 6’-OH | 4.32, t (5.4) | 4.35, br s | 4.30, t (4.8) | 4.34, t (5.4) | ||||||||||
| No. | 6 a | 7 b | ||
|---|---|---|---|---|
| δC | δH, mult (J, Hz) | δC | δH, mult (J, Hz) | |
| 1 | 29.2 | 2.71, t (8.4) | 28.2 | 2.53, t (8.4) |
| 2 | 29.5 | 2.05, m | 19.2 | 1.72, m |
| 3 | 124.3 | 5.25, t (6.0) | 33.0 | 1.84, m 1.44, m |
| 4 | 131.0 | 37.8 | ||
| 5 | 134.9 | 139.2 | ||
| 6 | 127.6 | 6.90, d (7.8) | 126.1 | 7.01, s |
| 7 | 129.7 | 6.98, d (7.8) | 133.0 | |
| 8 | 140.2 | 132.1 | ||
| 9 | 135.6 | 134.1 | ||
| 10 | 133.2 | 132.7 | ||
| 11 | 67.4 | 4.67, d (10.2) 4.60, d (10.2) | 15.8 | 2.07, s |
| 12 | 19.5 | 2.25, s | 15.9 | 2.09, s |
| 13 | 25.6 | 1.66, s | 75.6 | 5.59, d (9.2) 3.24, d (9.2) |
| 14 | 17.5 | 1.55, s | 27.1 | 1.25, s |
| 15 | 19.7 | 2.34, s | 21.0 | 2.18, s |
| 1′ | 63.0 | 3.56, m 3.43, m | 99.3 | 4.64, d (3.6) |
| 2′ | 72.3 | 3.72, m | 72.6 | 3.20, m |
| 3′ | 78.1 | 3.76, d (5.1) | 73.7 | 3.40, m |
| 4′ | 70.9 | 3.58, m | 70.7 | 3.05, td (9.1, 5.2) |
| 5′ | 71.3 | 3.54, m | 73.3 | 3.28, m |
| 6′ | 63.8 | 3.63, m 3.41, m | 61.4 | 3.56, m 3.40, m |
| 1’-OH | 4.59, t (5.4) | |||
| 2’-OH | 4.75, d (5.4) | 4.60, d (6.4) | ||
| 3’-OH | 4.74, d (4.8) | |||
| 4’-OH | 4.30, d (5.4) | 4.85, d (5.2) | ||
| 5’-OH | 4.40, d (5.4) | |||
| 6’-OH | 4.35, t (5.4) | 4.38, t (6.0) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-H.; Chen, G.; Sun, Y.; Wang, H.-F.; Bai, J.; Hua, H.-M.; Pei, Y.-H. Seven New Drimane-Type Sesquiterpenoids from a Marine-Derived Fungus Paraconiothyrium sporulosum YK-03. Molecules 2019, 24, 1817. https://doi.org/10.3390/molecules24091817
Zhang L-H, Chen G, Sun Y, Wang H-F, Bai J, Hua H-M, Pei Y-H. Seven New Drimane-Type Sesquiterpenoids from a Marine-Derived Fungus Paraconiothyrium sporulosum YK-03. Molecules. 2019; 24(9):1817. https://doi.org/10.3390/molecules24091817
Chicago/Turabian StyleZhang, Li-Hua, Gang Chen, Yi Sun, Hai-Feng Wang, Jiao Bai, Hui-Ming Hua, and Yue-Hu Pei. 2019. "Seven New Drimane-Type Sesquiterpenoids from a Marine-Derived Fungus Paraconiothyrium sporulosum YK-03" Molecules 24, no. 9: 1817. https://doi.org/10.3390/molecules24091817
APA StyleZhang, L.-H., Chen, G., Sun, Y., Wang, H.-F., Bai, J., Hua, H.-M., & Pei, Y.-H. (2019). Seven New Drimane-Type Sesquiterpenoids from a Marine-Derived Fungus Paraconiothyrium sporulosum YK-03. Molecules, 24(9), 1817. https://doi.org/10.3390/molecules24091817





