Glycerol and Q-Tubes: Green Catalyst and Technique for Synthesis of Polyfunctionally Substituted Heteroaromatics and Anilines
Abstract
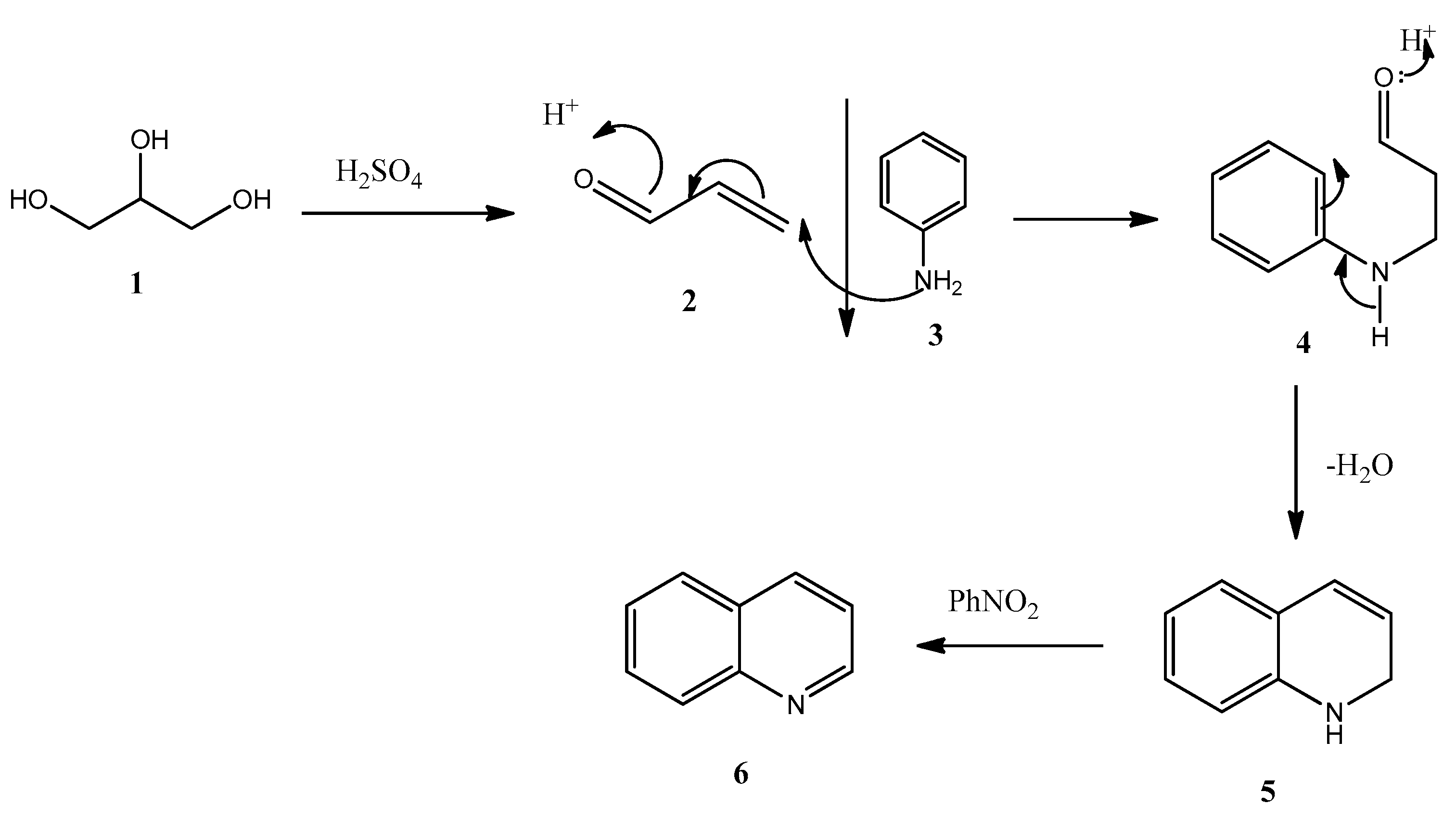
1. Introduction
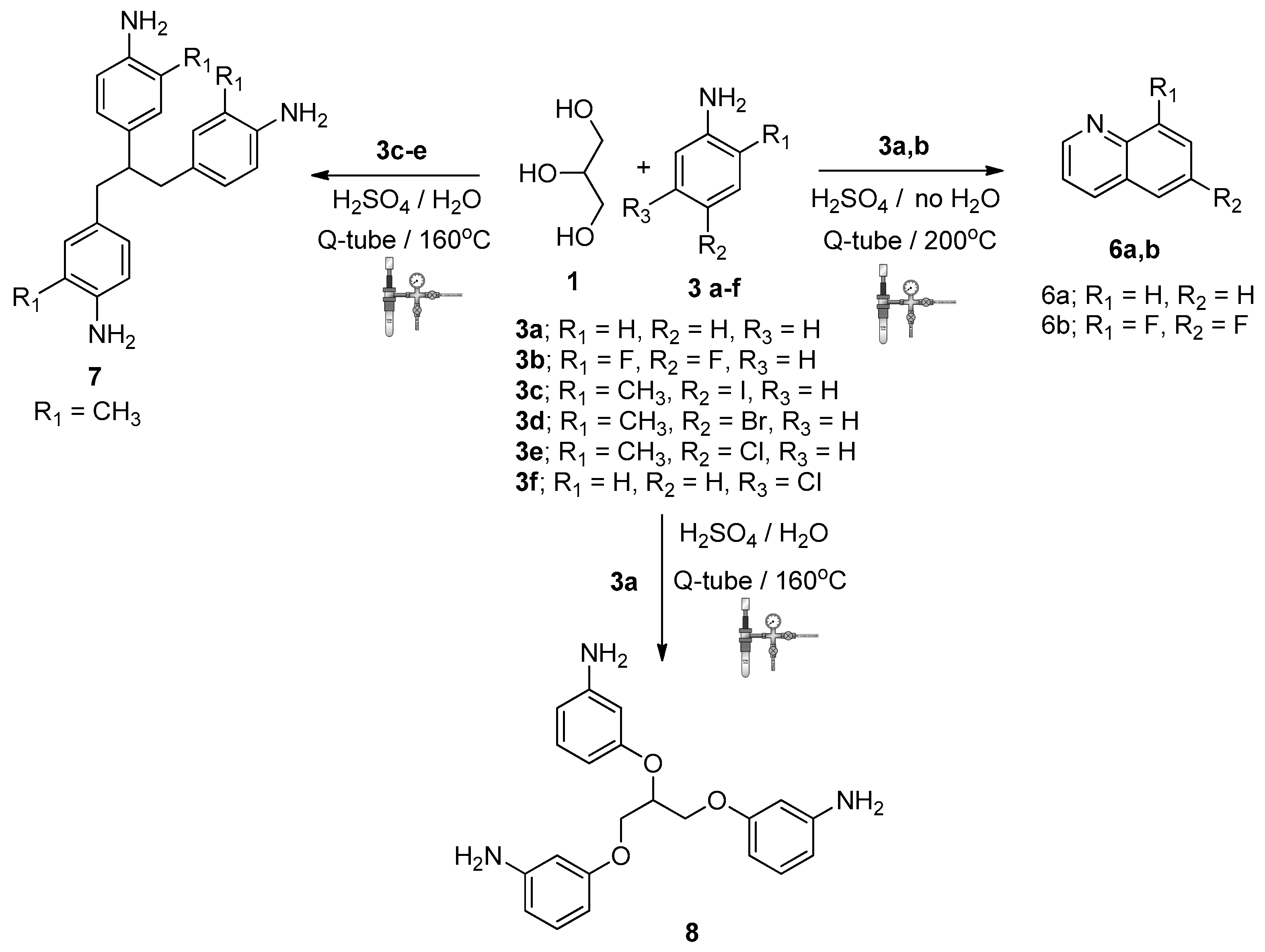
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Procedures for Q-Tube-Assisted Synthesis of Quinolines 6a,b
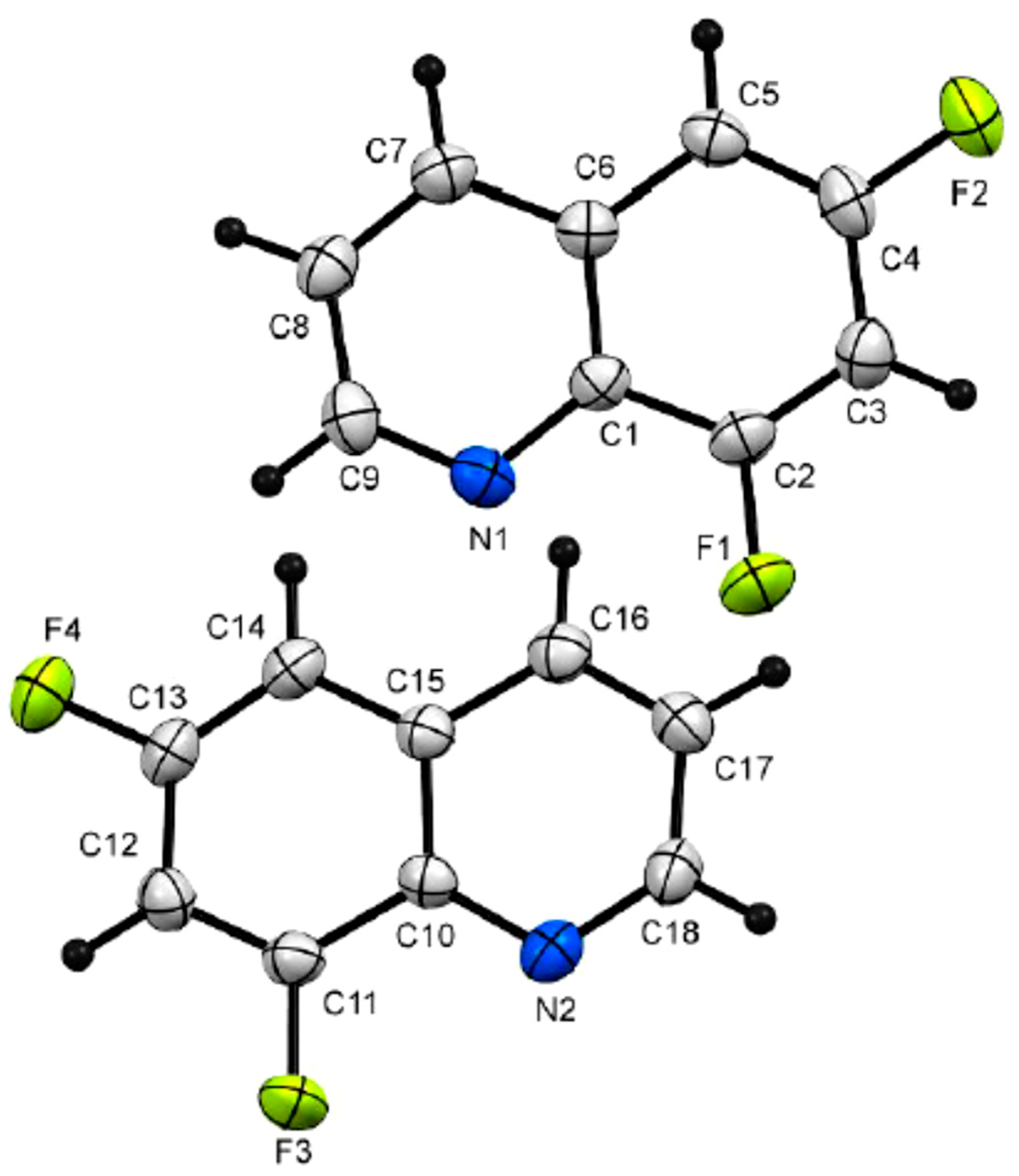
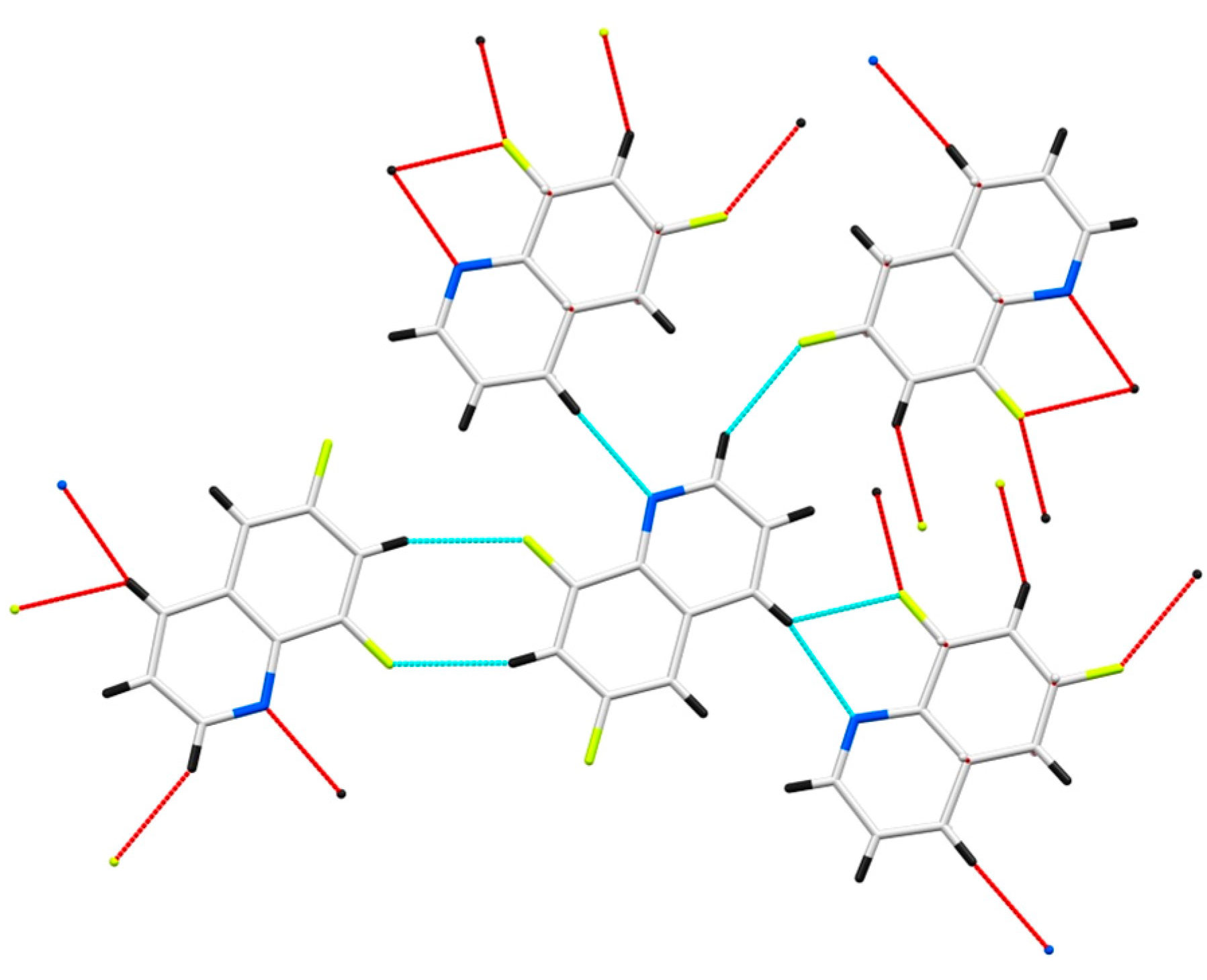
3.3. 6,8-Difloroquinoline 6b
3.4. General Procedure to Aniline Trimers 7 and 8
3.4.1. 4,4′,4″-(propane-1,2,3-triyl)tris(2-methylaniline) 7
3.4.2. 4-(2-(4-amionphenoxy)-3-(4-amoinophenoxy))aniline 8
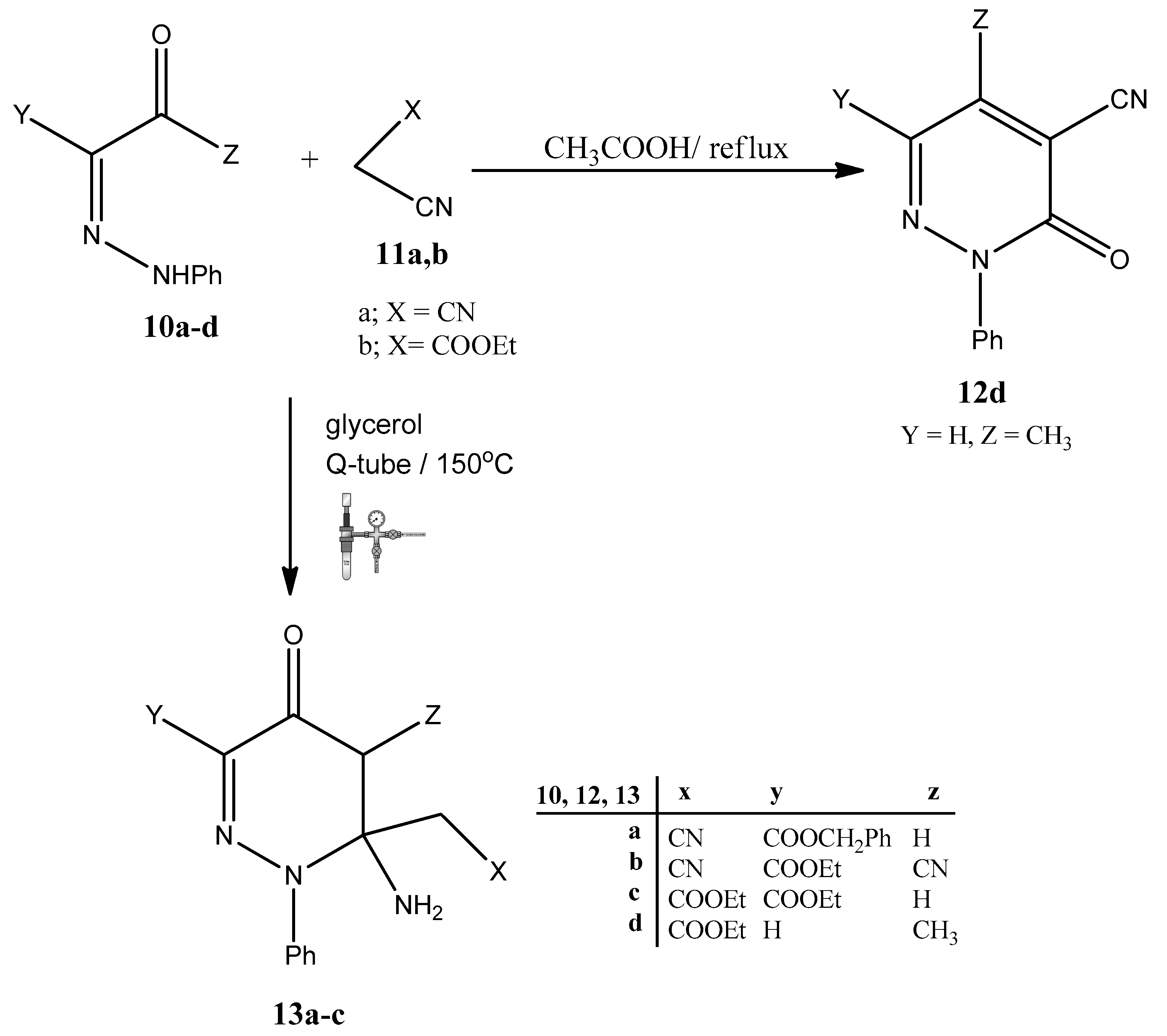
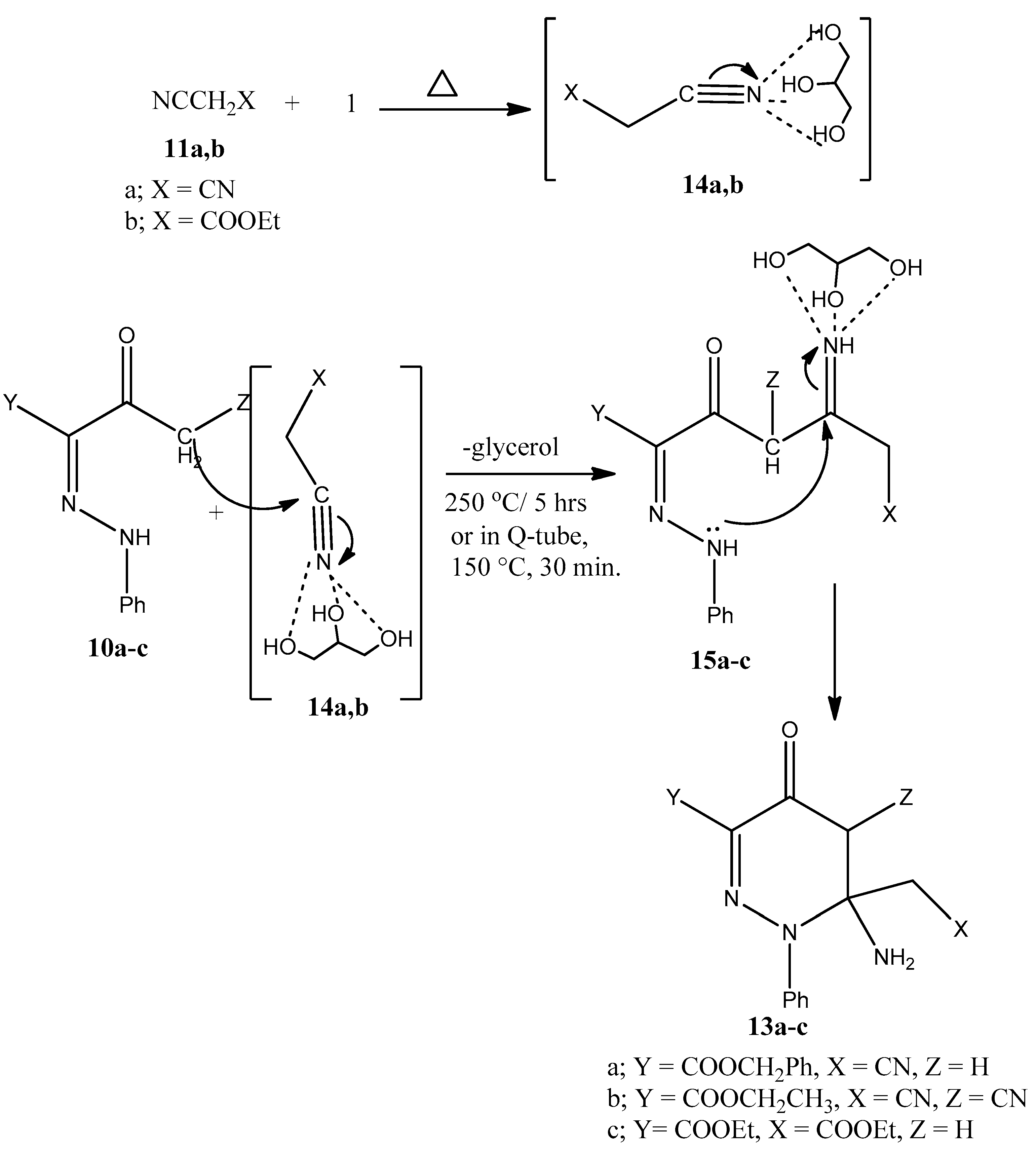
3.5. General Procedure for Syntheses of 13a–c
3.5.1. Method A: Conventional Heating
3.5.2. Method B: Q-Tube Assisted Reactions
3.6. Benzyl 6-amino-6-(cyanomethyl)-4-oxo-1-phenyl-1,4,5,6-tetrahydropyridazine-3-carboxylate 13a
3.7. Ethyl-6-amino-5-cyano-6-(cyanomethyl)-4-oxo-1-phenyl-1,4,5,6-tetrahydropyridazine-3-carboxylate 13b
3.8. Ethyl-6-amino-6-(2-ethoxy-2-oxoethyl)-4-oxo-1-phenyl-1,4,5,6-tetrahydropyridazine-3-carboxylate 13c
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smeaton, W.A. Carl Wilhelm Scheele (1742–1786): Provincial Swedish pharmacist and world-famous chemist. Endeavour 1992, 16, 128–131. [Google Scholar] [CrossRef]
- Urdang, G. Pictorial life history of the apothecary chemist Carl Wilhelm Scheele. Am. Inst. Hist. Pharm. 1942, 17–19. [Google Scholar]
- Pagliaro, M.; Rossi, M. Glycerol: Properties and production. In The Future of Glycerol, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 1–2. [Google Scholar]
- García, J.; García-Marín, H.; Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar]
- Pinto, A.C.; Guarieiro, L.L.; Rezende, M.J.; Ribeiro, N.M.; Torres, E.A.; Lopes, W.A.; Pereira, P.A.; Andrade, J.B. Biodiesel: An overview. J. Braz. Chem. Soc. 2005, 16, 1313–1330. [Google Scholar] [CrossRef]
- Díaz-Álvarez, A.E.; Francos, J.; Croche, P.; Cadierno, V. Recent advances in the use of glycerol as green solvent for synthetic organic chemistry. Curr. Green Chem. 2014, 1, 51–65. [Google Scholar] [CrossRef]
- Vo, Y.H.; Le, T.V.; Nguyen, H.D.; To, T.A.; Ha, H.Q.; Nguyen, A.T.; Phan, A.N.; Phan, N.T. Synthesis of quinazolinones and benzazoles utilizing recyclable sulfated metal-organic framework-808 catalyst in glycerol as green solvent. J. Ind. Eng. Chem. 2018, 64, 107–115. [Google Scholar] [CrossRef]
- Li, S.; Deng, W.; Li, Y.; Zhang, Q.; Wang, Y. Catalytic conversion of cellulose-based biomass and glycerol to lactic acid. J. Energy Chem. 2019, 32, 138–151. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Xiao, G. Sustainable Value-added C3 Chemicals from Glycerol Transformations: A Mini Review for Heterogeneous Catalytic Processes. Chin. J. Chem. Eng. 2019, in press. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.; Rajaei, K.; Tarighi, S. Oxidation of bio-renewable glycerol to value-added chemicals through catalytic and electro-chemical processes. Appl. Energy 2018, 230, 1347–1379. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol. 2019, in press. [Google Scholar]
- Zhao, H.; Zheng, L.; Li, X.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol to 1, 2-propanediol over Cu-based catalysts: A short review. Catal. Today 2019, in press. [Google Scholar] [CrossRef]
- Cespi, D.; Passarini, F.; Mastragostino, G.; Vassura, I.; Larocca, S.; Iaconi, A.; Chieregato, A.; Dubois, J.L.; Cavani, F. Glycerol as feedstock in the synthesis of chemicals: A life cycle analysis for acrolein production. Green Chem. 2015, 17, 343–355. [Google Scholar] [CrossRef]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Prati, L.; Hutchings, G.J. Glycerol oxidation using gold-containing catalysts. Acc. Chem. Res. 2015, 17, 1403–1412. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. APPL Catal. B-Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. Sci. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M.; Yehye, W.A. Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew. Sustain. Energy Rev. 2015, 1, 113–127. [Google Scholar] [CrossRef]
- Garlapati, V.K.; Shankar, U.; Budhiraja, A. Bioconversion technologies of crude glycerol to value added industrial products. Biotechnol. Rep. (Amst.) 2016, 1, 9–14. [Google Scholar] [CrossRef]
- Tan, H.W.; Aziz, A.A.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 1, 118–127. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 11, 4434–4440. [Google Scholar] [CrossRef]
- Díaz-Álvarez, A.; Cadierno, V. Glycerol: A promising green solvent and reducing agent for metal-catalyzed transfer hydrogenation reactions and nanoparticles formation. Appl. Sci. Basel 2013, 3, 55–69. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Dumeignil, F. Recent developments in the field of catalytic dehydration of glycerol to acrolein. ACS Catal. 2013, 3, 1819–1834. [Google Scholar] [CrossRef]
- Varma, R.S.; Len, C. Glycerol valorization under continuous flow conditions-recent advances. Curr. Opin. Green Sustain. Chem. 2019, 15, 83–90. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a green solvent for high product yields and selectivities. Environ. Chem. Lett. 2007, 5, 67–71. [Google Scholar] [CrossRef]
- Gu, Y.; Jérôme, F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010, 12, 1127–1138. [Google Scholar] [CrossRef]
- Sotto, N.; Cazorla, C.; Villette, C.; Billamboz, M.; Len, C. Toward the sustainable synthesis of biosourced divinylglycol from glycerol. ACS Sustain. Chem. Eng. 2016, 4, 6996–7003. [Google Scholar] [CrossRef]
- Arrhenius, S. About the dissociation heat and the influence of temperature on the degree of dissociation of the electrolytes. J. Phys. Chem. 1889, 4, 96–116. [Google Scholar]
- Hamid, A.A.; Abd-Elmonem, M.; Hayallah, A.M.; Elsoud, F.A.; Sadek, K.U. Glycerol: A Promising Benign Solvent for Catalyst Free One-Pot Multi-Component Synthesis of Pyrano [2, 3-c] pyrazoles and Tetrahydro-benzo [b] pyrans at Ambient Temperature. Chem. Select. 2017, 2, 10689–10693. [Google Scholar]
- Safaei, H.R.; Shekouhy, M.; Rahmanpur, S.; Shirinfeshan, A. Glycerol as a biodegradable and reusable promoting medium for the catalyst-free one-pot three component synthesis of 4H-pyrans. Green Chem. 2012, 14, 1696–1704. [Google Scholar] [CrossRef]
- He, F.; Li, P.; Gu, Y.; Li, G. Glycerol as a promoting medium for electrophilic activation of aldehydes: Catalyst-free synthesis of di (indolyl) methanes, xanthene-1, 8 (2 H)-diones and 1-oxo-hexahydroxanthenes. Green Chem. 2009, 11, 1767–1773. [Google Scholar] [CrossRef]
- Radatz, C.S.; Silva, R.B.; Perin, G.; Lenardão, E.J.; Jacob, R.G.; Alves, D. Catalyst-free synthesis of benzodiazepines and benzimidazoles using glycerol as recyclable solvent. Tetrahedron. Lett. 2011, 52, 4132–4136. [Google Scholar] [CrossRef]
- Skraup, Z.H. Synthetic experiments in the quinoline series. Monatsh. Chem. 1881, 2, 139–170. [Google Scholar]
- Wang, H. Comprehensive Organic Name Reactions. Skraup Reaction; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Soap and Detergent Association. Glycerine: An overview. Terms, Technical Data, Properties, Performance; Soap and Detergent Association: Washington, DC, USA, 1990. [Google Scholar]
- Nainwal, L.M.; Tasneem, S.; Akhtar, W.; Verma, G.; Khan, M.F.; Parvez, S.; Shaquiquzzaman, M.; Akhter, M.; Alam, M.M. Green recipes to quinoline: A review. Eur. J. Med. Chem. 2019, 164, 121–170. [Google Scholar] [CrossRef]
- Găină, L.; Cristea, C.; Moldovan, C.; Porumb, D.; Surducan, E.; Deleanu, C.; Mahamoud, A.; Barbe, J.; Silberg, I. Microwave-Assisted Synthesis of Phenothiazine and Qinoline Derivatives. Int. J. Mol. Sci. 2007, 8, 70–80. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Hasan, M.A. 1-(1-Alkylsulfonic)-3-methylimidazolium chloride Brönsted acidic ionic liquid catalyzed Skraup synthesis of quinolines under microwave heating. Tetrahedron. Lett. 2014, 55, 3319–3321. [Google Scholar] [CrossRef]
- Selivanova, G.A.; Reshetov, A.V.; Bagryanskaya, I.Y.; Shteingarts, V.D. Skraup-like cyclization of polyfluoro-2-naphthylamines: Vicarious electrophilic substitution of fluorine. J. Fluorine Chem. 2012, 137, 113–116. [Google Scholar] [CrossRef]
- Ramann, G.; Cowen, B. Recent advances in metal-free quinoline synthesis. Molecules 2016, 21, 986. [Google Scholar] [CrossRef]
- Li, A.; Yang, Z.; Yang, T.; Luo, C.W.; Chao, Z.S.; Zhou, C.S. High efficiency microwave-assisted synthesis of quinoline from acrolein diethyl acetal and aniline utilizing Ni/Beta catalyst. Catal. Commun. 2018, 115, 21–25. [Google Scholar] [CrossRef]
- Rao, M.S.; Sarkar, S.; Hussain, S. Microwave-assisted synthesis of 3-aminoarylquinolines from 2-nitrobenzaldehyde and indole via SnCl2-mediated reduction and facile indole ring opening. Tetrahedron Lett. 2019, 60, 1221–1225. [Google Scholar] [CrossRef]
- Hu, W.; Yang, W.; Yan, T.; Cai, M. An efficient heterogeneous gold (I)-catalyzed intermolecular cycloaddition of 2-aminoaryl carbonyls and internal alkynes leading to polyfunctionalized quinolines. Synth. Commun. 2019, 49, 799–813. [Google Scholar] [CrossRef]
- Karamshahi, Z.; Ghorbani-Vaghei, R.; Sarmast, N. Efficient synthesis of multiply substituted 7H-indeno [2, 1-c] quinoline using 7-aminonaphthalene-1, 3-disulfonic acid supported on LDHs as catalyst. Mater. Sci. Eng. C 2019, 1, 45–54. [Google Scholar] [CrossRef]
- Singhal, A.; Kumari, P.; Nisa, K. Facile One-Pot Friedlander Synthesis of Functionalized Quinolines using Graphene Oxide Carbocatalyst. Curr. Org. Chem. 2019, 16, 154–159. [Google Scholar] [CrossRef]
- Snieckus, V.; Curto, J.M. Cobalt-Catalyzed Synthesis of Quinolines and Acridines from Anthranils. Synfacts 2019, 15, 354. [Google Scholar]
- Dadhania, H.; Raval, D.; Dadhania, A. A Highly Efficient and Solvent-Free Approach for the Synthesis of Quinolines and Fused Polycyclic Quinolines Catalyzed by Magnetite Nanoparticle-Supported Acidic Ionic Liquid. Polycycl. Aromat. Comp. 2019, 1–4. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Meng, X.; Sun, Y.; Man, X.; Li, J.; Sun, F. Manganese (II)-catalysed dehydrogenative annulation involving C–C bond formation: Highly regioselective synthesis of quinolines. Dalton Trans. 2019, 48, 4474–4478. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, X.; Zhou, Y.; Geng, X.; Wang, C.; Wu, Y.D.; Wu, A.X. Direct Synthesis of 2, 3-Diaroyl Quinolines and Pyridazino [4, 5-b] quinolines via an I2-Promoted One-Pot Multicomponent Reaction. Org. Lett. 2019, 21, 2708–2711. [Google Scholar] [CrossRef]
- Mao, H.K.; Chen, B.; Chen, J.; Li, K.; Lin, J.F.; Yang, W.; Zheng, H. Recent advances in high-pressure science and technology. MRE 2018, 1, 59. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Tang, X.; Zheng, H.; Li, K.; Lin, X.; Fang, L.; Sun, G.A.; Chen, X.; Xie, L.; et al. Pressure-Induced Diels–Alder Reactions in C6H6-C6F6 Cocrystal towards Graphane Structure. Angew. Chem. Int. Ed. 2019, 58, 1468–1473. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, L. Recent advance in high-pressure solid-state metathesis reactions. MRE 2018, 3, 95. [Google Scholar] [CrossRef]
- Sadek, K.U.; Selim, M.A.; Alnajjar, A.A.; Atallah, M.; Elnagdi, M.H. Multicomponent reactions under increased pressure: On the reaction of arylhydrazonals, aromatic aldehydes and malononitrile in Q-Tube. Eur. J. Chem. 2016, 7, 468–472. [Google Scholar] [CrossRef]
- AL-Johani, M.; Al-Zaydi, K.; Mousally, S.; Alqahtani, N.; Elnagdi, N.; Elnagdi, M. Multi Component Reactions under Increased Pressure: On the Mechanism of Formation of Pyridazino [5, 4, 3-de] [1,6] naphthyridine Derivatives by the Reaction of Malononitrile, Aldehydes and 2-Oxoglyoxalarylhydrazones in Q-Tubes. Molecules 2017, 22, 2114. [Google Scholar] [CrossRef]
- Alzaydi, K.M.; Abojabal, N.S.; Elnagdi, M.H. Multicomponent reactions in Q-Tubes™: One-pot synthesis of benzo [c] chromen-6-one and phenanthridin-6 (5H)-one derivatives in a four-component reaction. Tetrahedron Lett. 2016, 57, 3596–3599. [Google Scholar] [CrossRef]
- Nacca, F.G.; Merlino, O.; Mangiavacchi, F.; Krasowska, D.; Santi, C.; Sancineto, L. The Q-tube system, a nonconventional technology for Green Chemistry Practitioners. Curr. Green Chem. 2017, 4, 58–66. [Google Scholar] [CrossRef]
- Chen, R.; Hoffmann, R.; Cammi, R. The Effect of Pressure on Organic Reactions in Fluids—A New Theoretical Perspective. Angew. Chem. Int. Ed. 2017, 56, 11126–11142. [Google Scholar] [CrossRef]
- Galy, N.; Nguyen, R.; Yalgin, H.; Thiebault, N.; Luart, D.; Len, C. Glycerol in subcritical and supercritical solvents. J. Chem. Technol. Biotechnol. 2017, 92, 14–26. [Google Scholar] [CrossRef]
- Nqoro, X.; Tobeka, N.; Aderibigbe, B. Quinoline-based hybrid compounds with antimalarial activity. Molecules 2017, 22, 2268. [Google Scholar] [CrossRef]
- Foley, M.; Tilley, L. Quinoline antimalarials: Mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther. 1998, 79, 55–87. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Ennis, K.; Vercaigne, L.; Walkty, A.; Gin, A.S.; Embil, J.; Smith, H.; Hoban, D.J. A critical review of the fluoroquinolones. Drugs 2002, 62, 13–59. [Google Scholar] [CrossRef]
- Scholar, M. Fluoroquinolines: Past, present and future of a novel group of antibacterial agents. Am. J. Pharm. Educ. 2002, 66, 164–171. [Google Scholar]
- Saggadi, H.; Luart, D.; Thiebault, N.; Polaert, I.; Estel, L.; Len, C. Quinoline and phenanthroline preparation starting from glycerol via improved microwave-assisted modified Skraup reaction. RSC Adv. 2014, 4, 21456–21464. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Elnagdi, M.H. Personal communication. Arkivoc in preparation.
- Nagawade, R.R.; Khanna, V.V.; Bhagwat, S.S.; Shinde, D.B. Synthesis of new series of 1-Aryl-1,4-dihydro-4-oxo-6-methyl pyridazine-3-carboxylic acid as potential antibacterial agents. Eur. J. Med. Chem. 2005, 40, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Kandile, N.G.; Mohamed, M.I.; Zaky, H.; Mohamed, H.M. Novel pyridazine derivatives: Synthesis and antimicrobial activity evaluation. Eur. J. Med. Chem. 2009, 44, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Ahmad, A.; Bhandari, A.; Ram, V. Synthesis and antitubercular activity of pyridazinone derivatives. J. Chil. Chem. Soc. 2011, 56, 778–780. [Google Scholar] [CrossRef]
- Guan, L.P.; Sui, X.; Deng, X.Q.; Quan, Y.C.; Quan, Z.S. Synthesis and anticonvulsant activity of a new 6-alkoxy- [1,2,4]triazolo[4,3-b]pyridazine. Eur. J. Med. Chem. 2010, 45, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.S.; Sharma, P.K.; Nema, R.K. Synthesis and anticonvulsant activity of pyridazinone derivatives. Int. J. ChemTech. Res. 2009, 1, 522–525. [Google Scholar]
- Mishra, R.; Siddiqui, A.A.; Husain, A.; Rashid, M.; Goda, C. Design, synthesis and antihypertensive screening of novel pyridazine substituted s-triazin-2-imine/one/thione derivatives. J. Enzym. Inhib. Med. Chem. 2013, 28, 552–559. [Google Scholar] [CrossRef]
- Asif, M. General study of pyridazine compounds against cyclooxygenase enzyme and their relation with analgesic, antiinflammatory and anti-arthritic activities. Chron. Young Sci. 2010, 1, 3–9. [Google Scholar]
- Asif, M. Analgesic activity of some 6-phenyl-4-substituted benzylidene tetrahydro pyridazin-3(2H)-ones. Glob. J. Pharmacol. 2011, 5, 18–22. [Google Scholar]
- Ghozlan, S.; Abdelhamid, I.; Elnagdi, M.H. Functionally substituted arylhydrazones as building blocks in heterocyclic synthesis: Routes to pyridazines and pyridazinoquinazolines. Arkivoc 2006, 1, 147–157. [Google Scholar]
- Fahmy, S.; Abed, N.; Mohareb, R.; Elnagdi, M. Activated nitriles in heterocyclic synthesis: Novel synthesis of pyridazines, pyridines, and isoxazoles. Synthesis 1982, 11, 490–493. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 22a–c are available from the authors. |



| Entry | R1 | R2 | R3 | Reaction Conditions | Product | Yield% | MP. (°C) | m/z | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Medium | Time (min.) | Temp (°C) | ||||||||
| 3a | H | H | H | glycerol | 60 | 200 | 6a | 58 | BP = 238 | |
| 3a | H | H | H | glycerol + H2O | 15 | 160 | 8 | 73 | 240 | 365.17 |
| 3b | F | F | H | glycerol | 60 | 200 | 6b | 60 | 243 | 165.03 |
| 3c | CH3 | I | H | glycerol + H2O | 15 | 160 | 7 | 75 | 180 | 359.23 |
| 3d | CH3 | Br | H | glycerol + H2O | 20 | 160 | 7 | 70 | 180 | 359.23 |
| 3e | CH3 | Cl | H | glycerol + H2O | 20 | 160 | 7 | 70 | 180 | 359.23 |
| 3f | H | H | Cl | glycerol + H2O | 30 | 160 | not concluded | - | 410 | |
| Product | x | y | z | Yield Percentage | |
|---|---|---|---|---|---|
| Conventional Heating | Under Pressure | ||||
| 13a | CN | COOCH2Ph | H | 52 | 76 |
| 13b | CN | COOEt | CN | 64 | 88 |
| 13c | COOEt | COOEt | H | 70 | 92 |
| 12d | CN | H | CH3 | 86.16 [74] | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlMarzouq, D.S.; Elnagdi, N.M.H. Glycerol and Q-Tubes: Green Catalyst and Technique for Synthesis of Polyfunctionally Substituted Heteroaromatics and Anilines. Molecules 2019, 24, 1806. https://doi.org/10.3390/molecules24091806
AlMarzouq DS, Elnagdi NMH. Glycerol and Q-Tubes: Green Catalyst and Technique for Synthesis of Polyfunctionally Substituted Heteroaromatics and Anilines. Molecules. 2019; 24(9):1806. https://doi.org/10.3390/molecules24091806
Chicago/Turabian StyleAlMarzouq, Douaa Salman, and Noha M. Hilmy Elnagdi. 2019. "Glycerol and Q-Tubes: Green Catalyst and Technique for Synthesis of Polyfunctionally Substituted Heteroaromatics and Anilines" Molecules 24, no. 9: 1806. https://doi.org/10.3390/molecules24091806
APA StyleAlMarzouq, D. S., & Elnagdi, N. M. H. (2019). Glycerol and Q-Tubes: Green Catalyst and Technique for Synthesis of Polyfunctionally Substituted Heteroaromatics and Anilines. Molecules, 24(9), 1806. https://doi.org/10.3390/molecules24091806





