α-Ionone Protects Against UVB-Induced Photoaging in Human Dermal Fibroblasts
Abstract
1. Introduction
2. Results
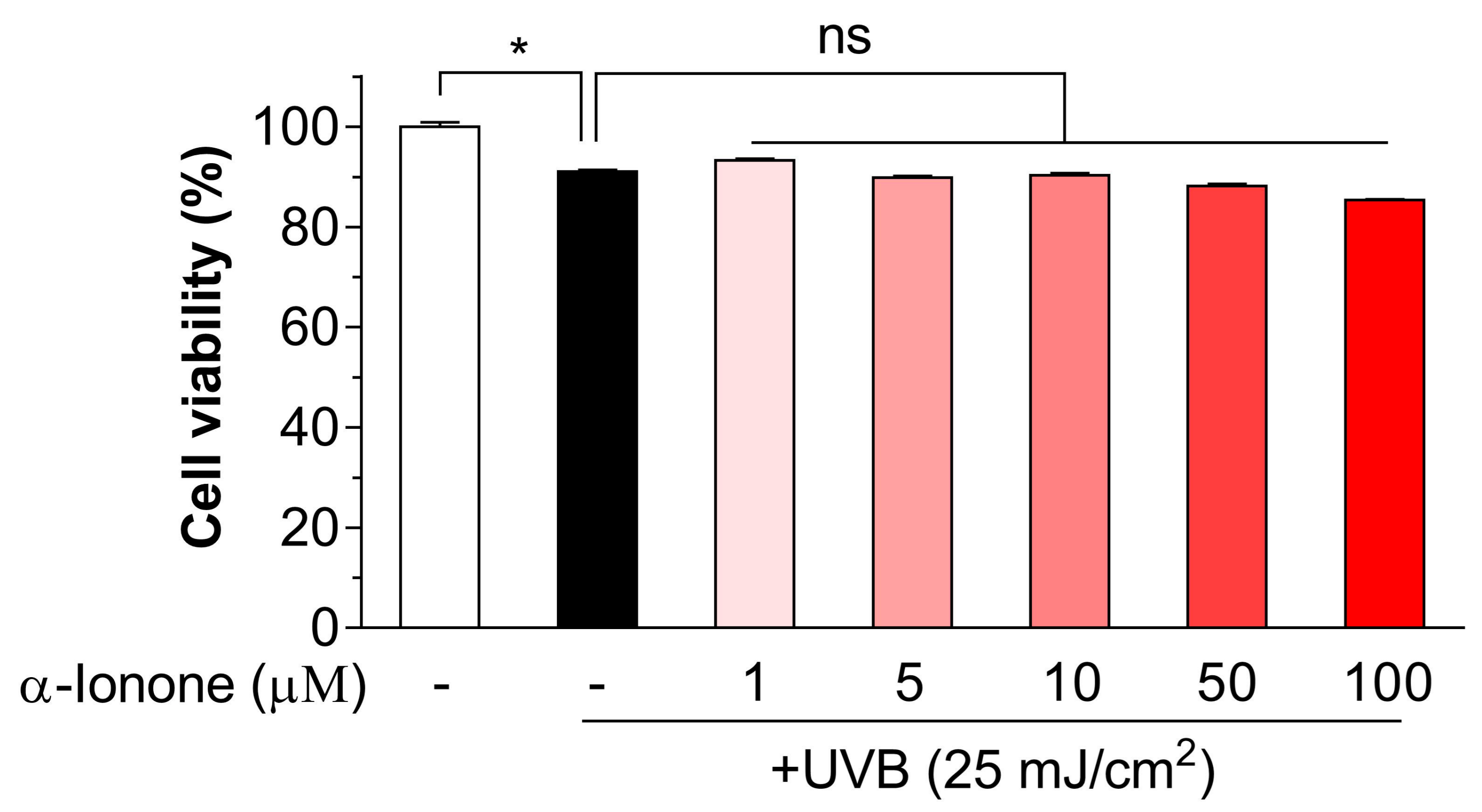
2.1. α-Ionone Has No Toxicity to UVB-Exposed Human Hs68 Dermal Fibroblasts
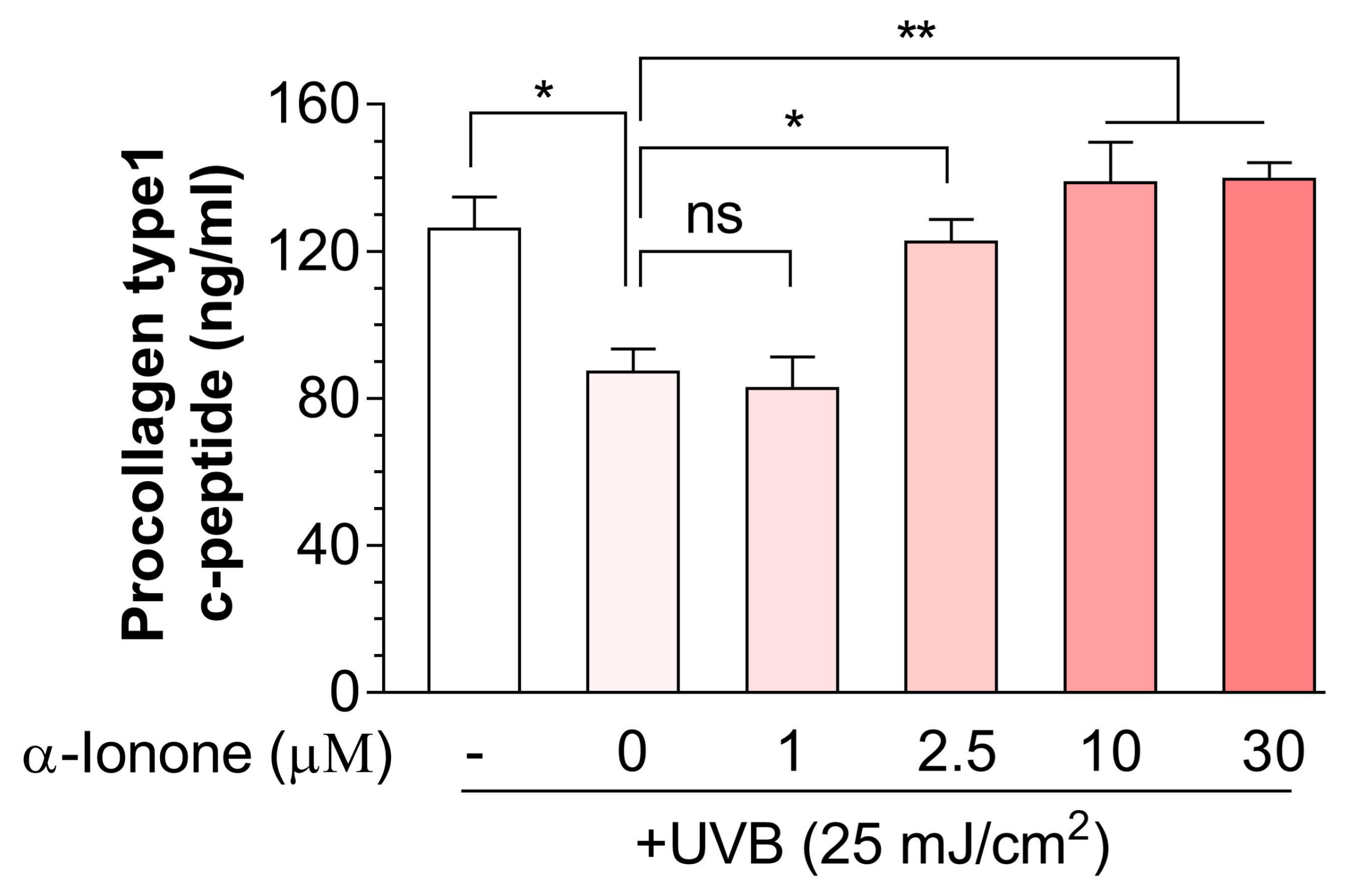
2.2. α-Ionone Attenuates UVB-Induced Loss of Collagen in Human Hs68 Dermal Fibroblasts
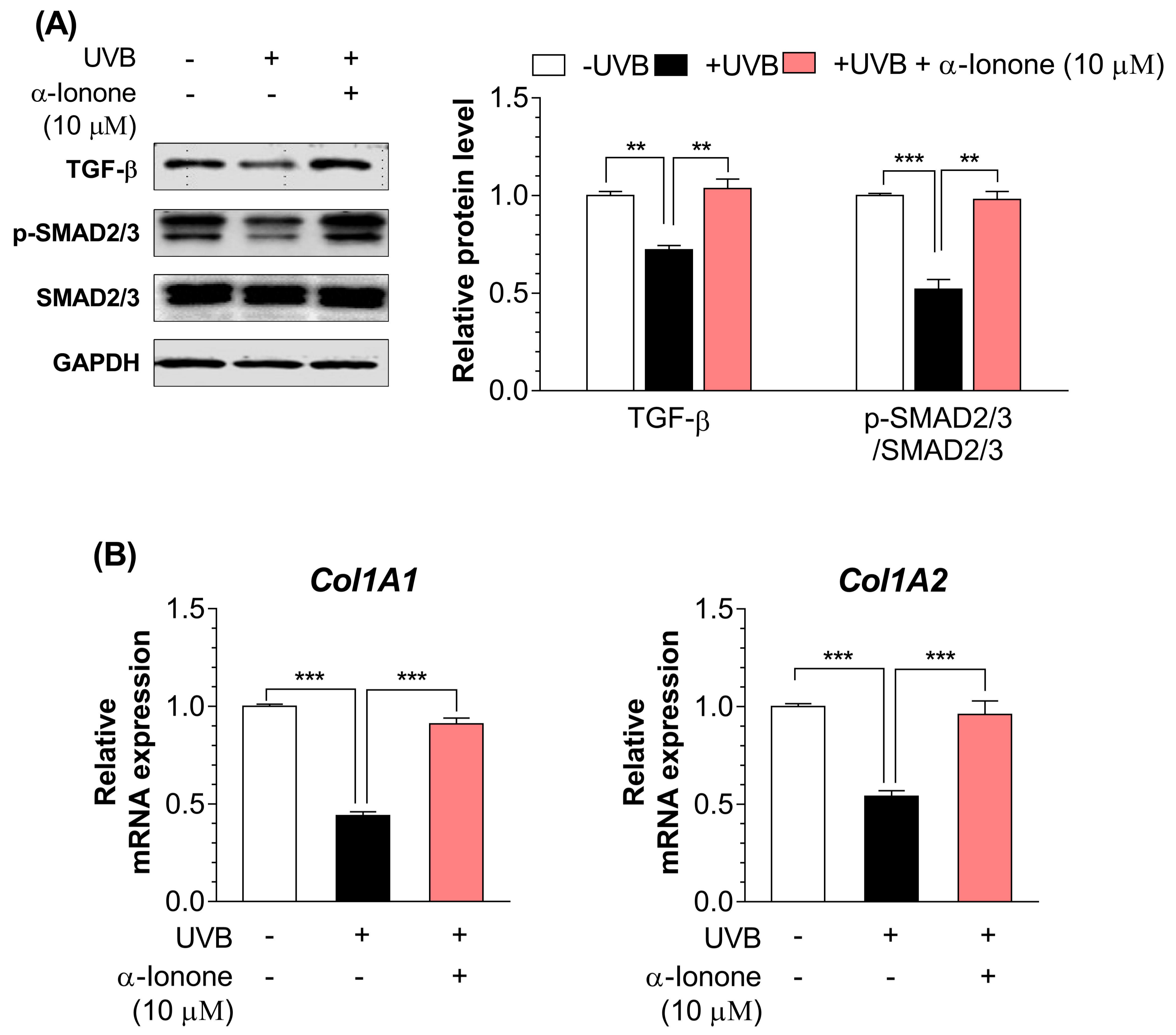
2.3. α-Ionone Upregulates the Expression of Molecules Related to Collagen Synthesis in Human Hs68 Dermal Fibroblasts
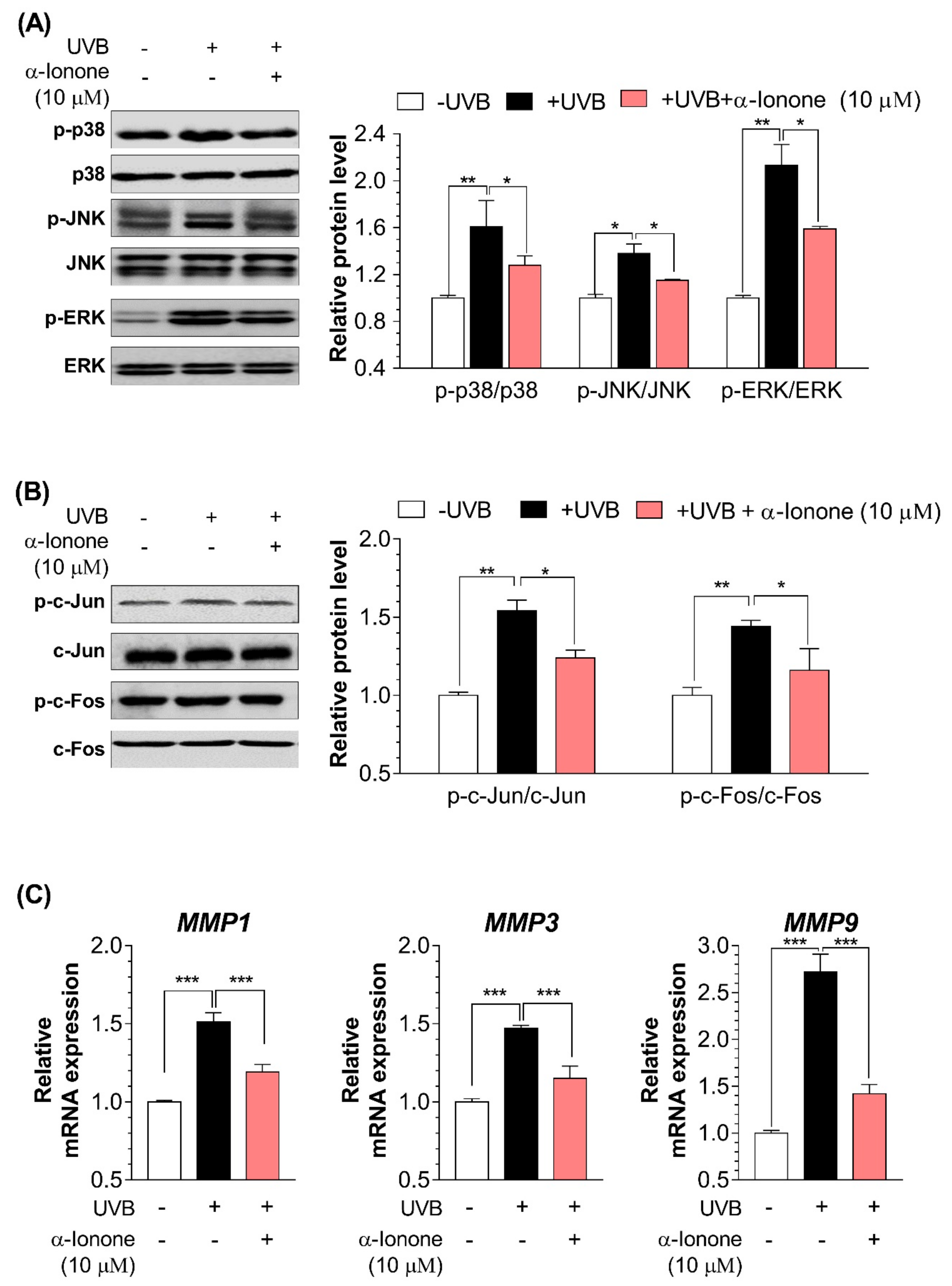
2.4. α-Ionone Suppresses the Expression of Molecules Related to Collagen Degradation in Human Hs68 Dermal Fibroblasts
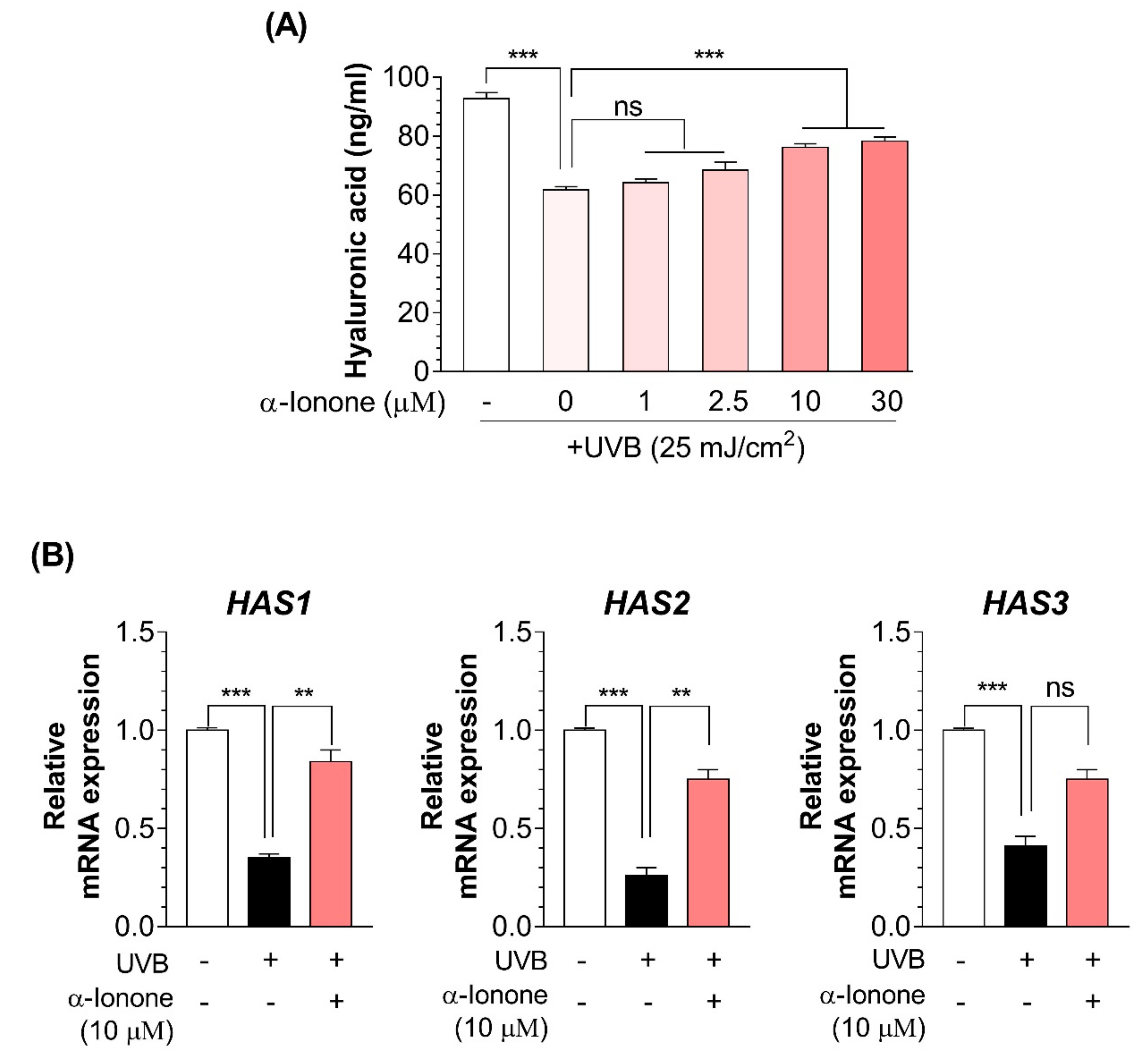
2.5. α-Ionone Attenuates UVB-Induced Loss of Hyaluronic Acid (HA) in Human Hs68 Dermal Fibroblasts
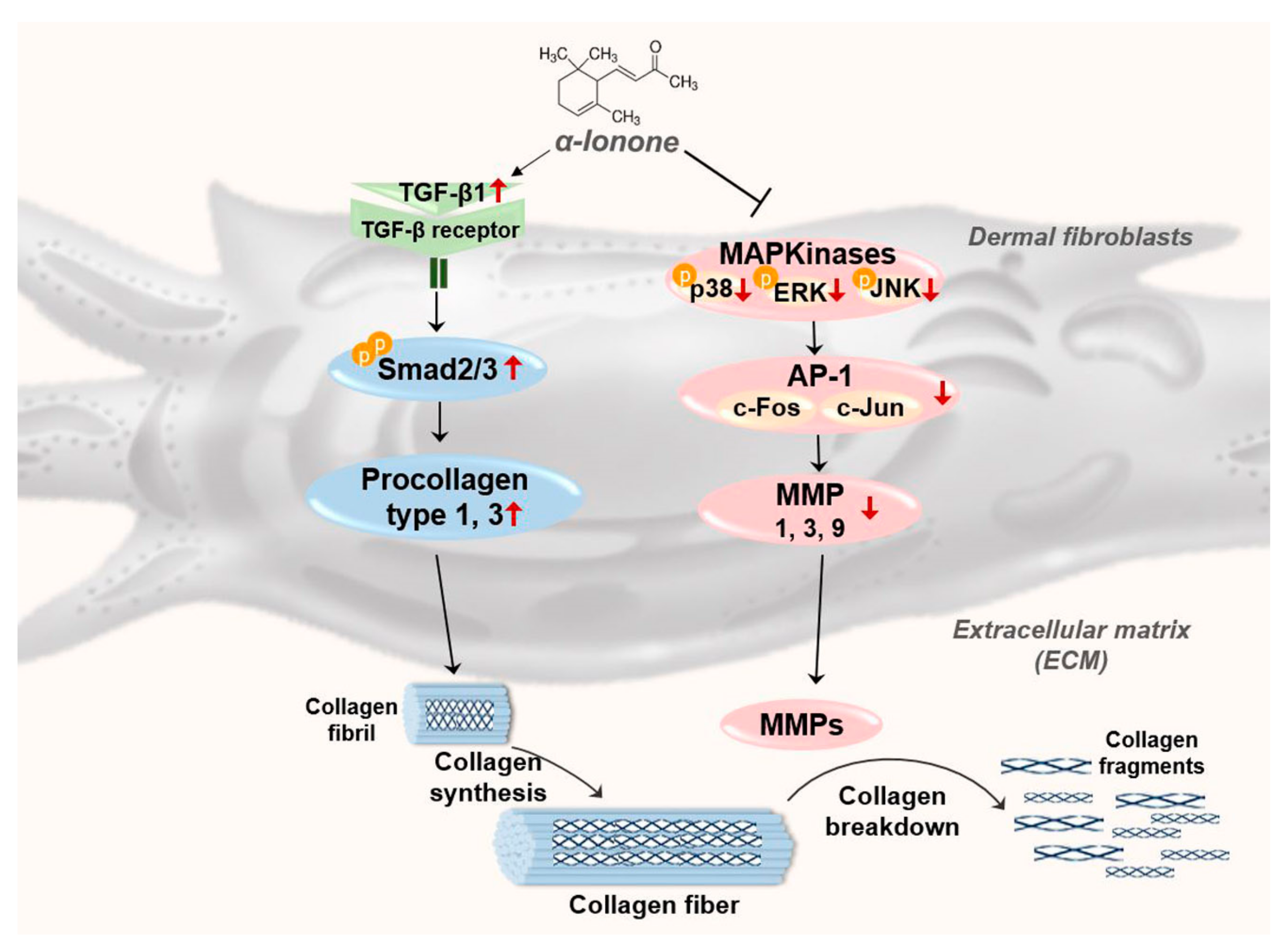
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. The Cell Viability Assay
4.4. Determination of Procollagen I C-Terminal Peptide and HA Secretion
4.5. RNA Extraction and PCR
4.6. Protein Extraction and Western Blotting
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ricard-Blum, S. The collagen family. CSH Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J. Connective-tissue biochemistry of the aging dermis—Age-related alterations in collagen and elastin. Dermatol. Clin. 1986, 4, 433–446. [Google Scholar] [CrossRef]
- Quan, T.H.; Qin, Z.P.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.H.; He, T.Y.; Shao, Y.; Lin, L.; Kang, S.W.; Voorhees, J.J.; Fisher, G.J. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am. J. Pathol. 2006, 169, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Gil, C.H.; Kim, Y.R.; Shin, H.K.; Choi, B.T. Anti-photoaging properties of the phosphodiesterase 3 inhibitor cilostazol in ultraviolet b-irradiated hairless mice. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Rhie, G.E.; Park, C.H.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Modulation of collagen metabolism by the topical application of dehydroepiandrosterone to human skin. J. Investig. Dermatol. 2005, 124, 315–323. [Google Scholar] [CrossRef]
- Schiller, M.; Javelaud, D.; Mauviel, A. Tgf-beta-induced smad signaling and gene regulation: Consequences for extracellular matrix remodeling and wound healing. J. Dermatol. Sci. 2004, 35, 83–92. [Google Scholar] [PubMed]
- Martinez-Ferrer, M.; Afshar-Sherif, A.R.; Uwamariya, C.; de Crombrugghe, B.; Davidson, J.M.; Bhowmick, N.A. Dermal transforming growth factor-beta responsiveness mediates wound contraction and epithelial closure. Am. J. Pathol. 2010, 176, 98–107. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Pathological roles of mapk signaling pathways in human diseases. BBA-Mol. Basis. Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Jansen-Durr, P. Molecular mechanisms of uvb-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [PubMed]
- Gao, W.; Wang, Y.S.; Hwang, E.; Lin, P.; Bae, J.; Seo, S.A.; Yan, Z.; Yi, T.H. Rubus idaeus L. (red raspberry) blocks uvb-induced mmp production and promotes type i procollagen synthesis via inhibition of mapk/ap-1, nf-kappabeta and stimulation of tgf-beta/smad, nrf2 in normal human dermal fibroblasts. J. Photochem. Photobiol. B 2018, 185, 241–253. [Google Scholar] [CrossRef]
- Li, L.; Hwang, E.; Ngo, H.T.T.; Lin, P.; Gao, W.; Liu, Y.; Yi, T.H. Antiphotoaging effect of prunus yeonesis blossom extract via inhibition of mapk/ap-1 and regulation of the tgf-betai/smad and nrf2/are signaling pathways. Photochem. Photobiol. 2018, 94, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, Y.T.; Kuo, H.C.; Chiou, W.F.; Lee, M.H. Compounds isolated from eriobotrya deflexa leaves protect against ultraviolet radiation b-induced photoaging in human fibroblasts. J. Photochem. Photobiol. B 2017, 175, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Tagami, H.; et al. A toxicologic and dermatologic assessment of ionones when used as fragrance ingredients. Food Chem. Toxicol. 2007, 45, S130–S167. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Kim, M.; Park, T. Alpha-ionone attenuates high-fat diet-induced skeletal muscle wasting in mice via activation of camp signaling. Food Funct. 2019, 10, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.L.; Dekant, W.; Fryer, A.D.; Kromidas, L.; La Cava, S.; et al. Rifm fragrance ingredient safety assessment, alpha-ionone, cas registry number 127-41-3. Food Chem. Toxicol. 2016, 97S, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. Uv-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Hiraku, Y.; Ito, K.; Hirakawa, K.; Kawanishi, S. Photosensitized DNA damage and its protection via a novel mechanism. Photochem. Photobiol. 2007, 83, 205–212. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Short-term and long-term cellular and molecular events following uv irradiation of skin: Implications for molecular medicine. Expert. Rev. Mol. Med. 2002, 4, 1–22. [Google Scholar] [CrossRef]
- Dai, G.; Freudenberger, T.; Zipper, P.; Melchior, A.; Grether-Beck, S.; Rabausch, B.; de Groot, J.; Twarock, S.; Hanenberg, H.; Homey, B.; et al. Chronic ultraviolet b irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007, 171, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massague, J. Mechanisms of tgf-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Piek, E.; Heldin, C.H.; Ten Dijke, P. Specificity, diversity, and regulation in tgf-beta superfamily signaling. FASEB J. 1999, 13, 2105–2124. [Google Scholar] [CrossRef]
- Quan, T.H.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. Ap-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef] [PubMed]
- Angel, P.; Karin, M. The role of jun, fos and the ap-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1991, 1072, 129–157. [Google Scholar] [CrossRef]
- Clark, I.A.; Swingler, T.E.; Sampieri, C.L.; Edwards, D.R. The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 2008, 40, 1362–1378. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Yi, B.R.; Choi, K.C. Molecular mechanisms and in vivo mouse models of skin aging associated with dermal matrix alterations. Lab. Anim. Res. 2011, 27, 1–8. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Behav. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.W.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.S.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Manuskiatti, W.; Maibach, H.I. Hyaluronic acid and skin: Wound healing and aging. Int. J. Dermatol. 1996, 35, 539–544. [Google Scholar] [CrossRef]
- Itano, N.; Kimata, K. Molecular cloning of human hyaluronan synthase. Biochem. Biophys. Res. Commun. 1996, 222, 816–820. [Google Scholar] [CrossRef]
- Rock, K.; Grandoch, M.; Majora, M.; Krutmann, J.; Fischer, J.W. Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet b (uvb): New insights into mechanisms of matrix remodeling. J. Biol. Chem. 2011, 286, 18268–18276. [Google Scholar] [CrossRef]
- Margelin, D.; Medaisko, C.; Lombard, D.; Picard, J.; Fourtanier, A. Hyaluronic acid and dermatan sulfate are selectively stimulated by retinoic acid in irradiated and nonirradiated hairless mouse skin. J. Investig. Dermatol. 1996, 106, 505–509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, Y.; Ishikawa, O.; Okada, K.; Ohnishi, K.; Miyachi, Y. Disaccharide analysis of the skin glycosaminoglycans in chronically ultraviolet light-irradiated hairless mice. J. Dermatol. Sci. 1995, 10, 139–144. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Tong, T.; Yu, R.; Park, T. Alpha-cedrene protects rodents from high-fat diet-induced adiposity via adenylyl cyclase 3. Int. J. Obes. (Lond.) 2019, 43, 202–216. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound α-ionone are available from the authors. |
| Type | Gene Description | Sequences (5′→3) |
|---|---|---|
| Human | >Collagen type I alpha 1 chain (Col1A1) | F: TTGCTCCCCAGCTGTCTTAT |
| R: AGACCACGAGGACCAGAGG | ||
| Collagen type I alpha 2 chain (Col1A2) | F: CGGAGGTATGCAGACAACGA | |
| R: CTAGGGTGCCTCCAAAAGGG | ||
| Matrix metalloproteinase 3 (MMP1) | F: ATTCTACTGATATCGGGGCTTTGA | |
| R: ATGTCCTTGGGGTATCCGTGTAG | ||
| Matrix metalloproteinase 3 (MMP3) | F: TGAGGACACCAGCATGAACC | |
| R: ACTTCGGATGCCCAGGAAAG | ||
| Matrix metalloproteinase 3 (MMP9) | F: TTGACAGCGACAAGAAGTGG | |
| R: GCCATTCACGTCGTCCTTAT | ||
| Hyaluronan synthase 1 (HAS1) | F: TACAACCAGAAGTTCCTGGG | |
| R: CTGGAGGTGTACTTGGTAGC | ||
| Hyaluronan synthase 2 (HAS2) | F: GTGGATTATGTACAGGTTTGTGA | |
| R: TCCAACCATGGGATCTTCTT | ||
| Hyaluronan synthase 3 (HAS3) | F: GAGATGTCCAGATCCTCAACAA | |
| R: CCCACTAATACACTGCACAC | ||
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | F: GTGAAGGTCGGAGTCAACG | |
| R: TGAGGTCAATGAAGGGGTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, T.; Park, J.; Moon, Y.; Kang, W.; Park, T. α-Ionone Protects Against UVB-Induced Photoaging in Human Dermal Fibroblasts. Molecules 2019, 24, 1804. https://doi.org/10.3390/molecules24091804
Tong T, Park J, Moon Y, Kang W, Park T. α-Ionone Protects Against UVB-Induced Photoaging in Human Dermal Fibroblasts. Molecules. 2019; 24(9):1804. https://doi.org/10.3390/molecules24091804
Chicago/Turabian StyleTong, Tao, Jinju Park, Youna Moon, Wesuk Kang, and Taesun Park. 2019. "α-Ionone Protects Against UVB-Induced Photoaging in Human Dermal Fibroblasts" Molecules 24, no. 9: 1804. https://doi.org/10.3390/molecules24091804
APA StyleTong, T., Park, J., Moon, Y., Kang, W., & Park, T. (2019). α-Ionone Protects Against UVB-Induced Photoaging in Human Dermal Fibroblasts. Molecules, 24(9), 1804. https://doi.org/10.3390/molecules24091804







