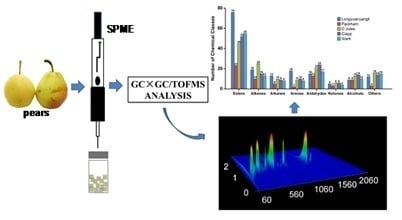
Analysis of Volatile Compounds in Pears by HS-SPME-GC×GC-TOFMS
Abstract
1. Introduction
2. Results and Discussion
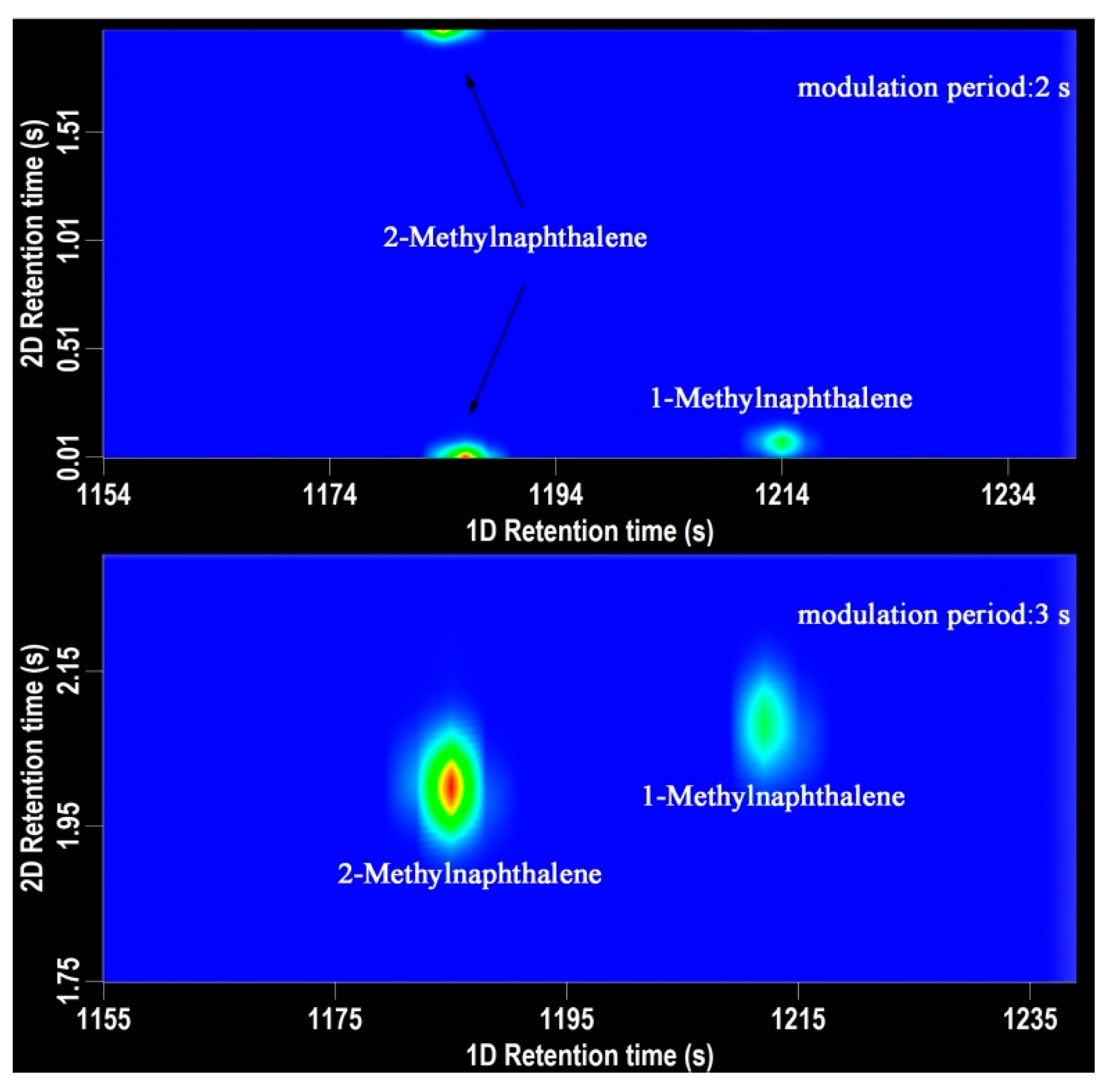
2.1. Optimization of the Modulation Period
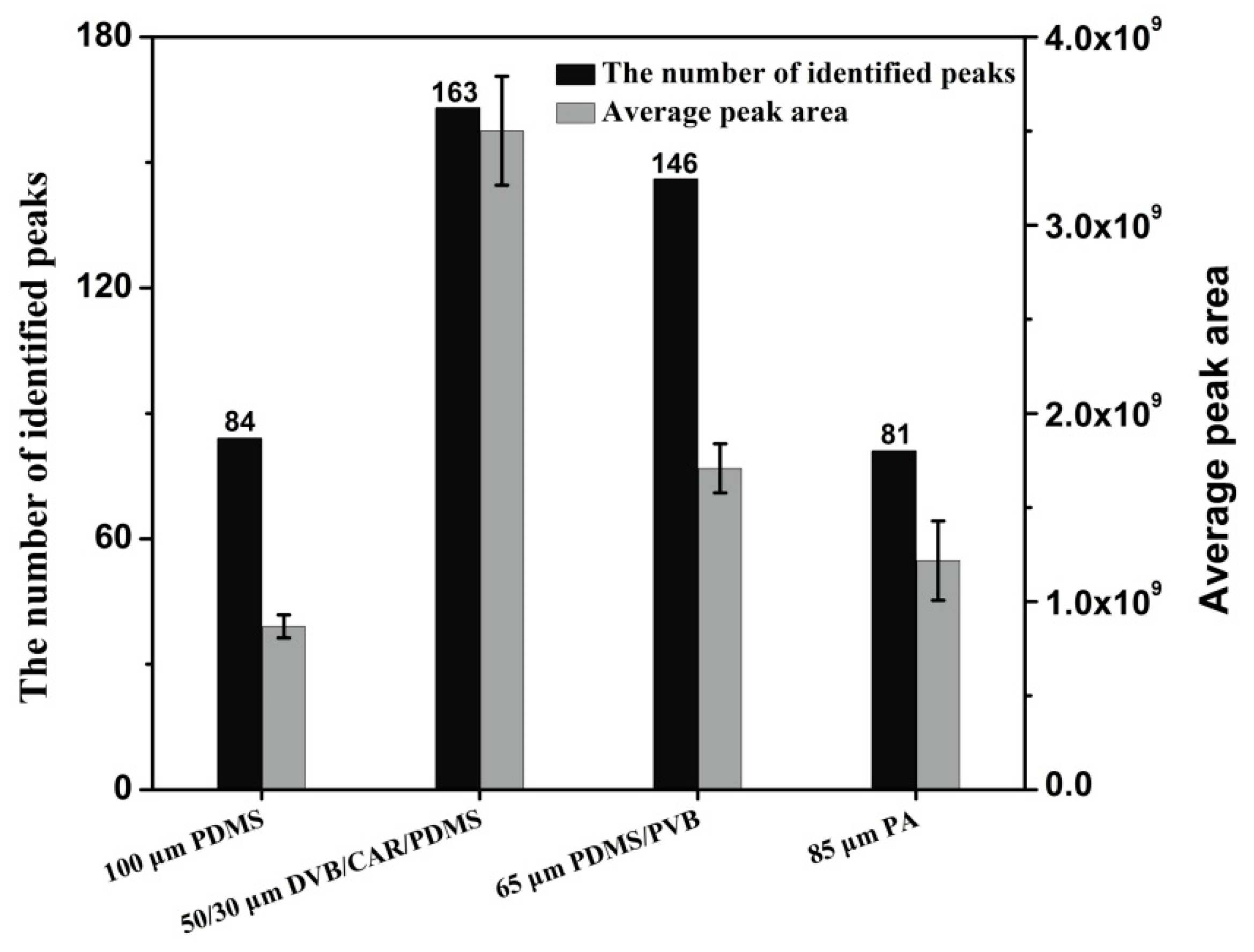
2.2. SPME Fibre Selection
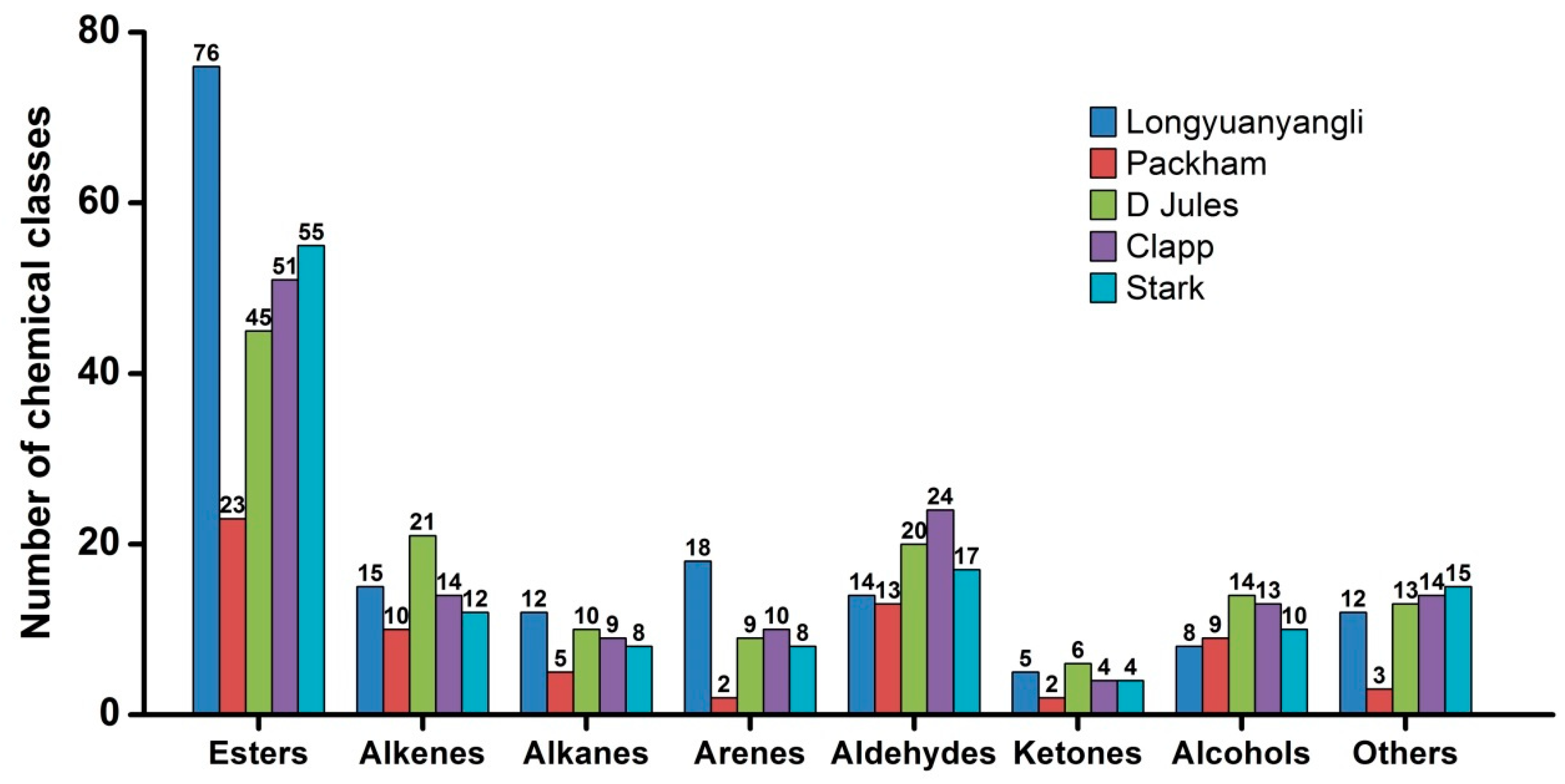
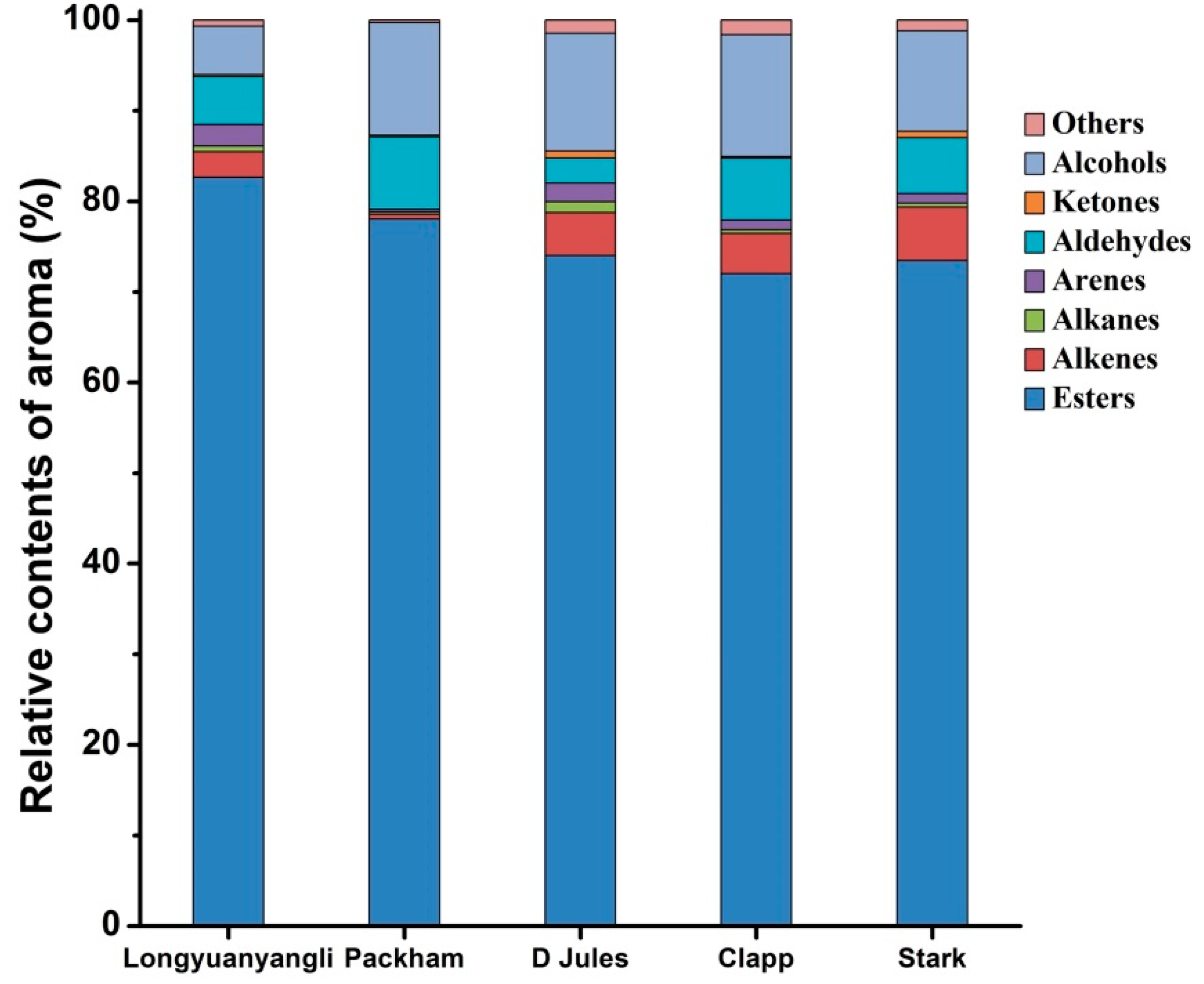
2.3. Volatile Compounds
2.3.1. Esters
2.3.2. Alcohols
2.3.3. Aldehydes and Ketones
2.3.4. Hydrocarbons
2.3.5. Others
2.4. Cluster Analysis (CA)
3. Materials and Methods
3.1. Materials
3.2. Volatiles Extraction
3.3. GC×GC-TOFMS Conditions
3.4. Data Processing and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO Statistical Database. Available online: http://www.fao.org/home/en/ (accessed on 20 December 2018).
- Rapparini, F.; Predieris, S. Pear fruit volatiles. Hort. Rev. 2003, 28, 237–324. [Google Scholar]
- Chen, Y.Y.; Yin, H.; Wu, X.; Shi, X.J.; Qi, K.J.; Zhang, S.L. Comparative analysis of the volatile organic compounds in mature fruits of 12 Occidental pear (Pyrus communis L.) cultivars. Sci. Hortic. 2018, 240, 239–248. [Google Scholar] [CrossRef]
- Qin, G.H.; Tao, S.T.; Cao, Y.F.; Wu, J.Y.; Zhang, H.P.; Huang, W.J.; Zhang, S.L. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC-MS. Food Chem. 2012, 134, 2367–2382. [Google Scholar] [CrossRef]
- Yi, X.K.; Liu, G.F.; Rana, M.M.; Zhu, L.W.; Jiang, S.L.; Huang, Y.F.; Lu, W.M.; Wei, S. Volatile profiling of two pear genotypes with different potential for white pear aroma improvement. Sci. Hortic. 2016, 209, 221–228. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Buttery, R.G.; Flath, R.A. Volatile constituents of asian pear (Pyrus serotina). J. Agric. Food chem. 1992, 40, 1925–1929. [Google Scholar] [CrossRef]
- Katayama, H.; Ohe, M.; Sugawara, E. Diversity of odor-active compounds from local cultivars and wild accessions of Iwateyamanashi (Pyrus ussuriensis var. aromatica) revealed by aroma extract dilution analysis (AEDA). Breed. Sci. 2013, 63, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Wu, J.H.; Wang, Q.; Deng, H.; Hu, X.S. Changes in the volatile compounds and chemical and physical properties of Kuerle fragrant pear (Pyrus serotina Reld) during Storage. J. Agric. Food Chem. 2006, 54, 8842–8847. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Yan, S.; Feng, Z.; Xiao, L.; Hu, X.S. Changes in the volatile compounds and chemical and physical properties of Yali pear (Pyrus bertschneideri Reld) during storage. Food Chem. 2006, 97, 248–255. [Google Scholar] [CrossRef]
- Zlatić, E.; Zadnik, V.; Fellman, J.; Demšar, L.; Hribar, J.; Čejić, Ž.; Vidrih, R. Comparative analysis of aroma compounds in ‘Bartlett’ pear in relation to harvest date, storage conditions, and shelf-life. Postharvest Biol. Technol. 2016, 117, 71–80. [Google Scholar] [CrossRef]
- Hendges, M.V.; Neuwald, D.A.; Steffens, C.A.; Vidrih, R.; Zlatić, E.; do Amarante, C.V.T. 1-MCP and storage conditions on the ripening and production of aromatic compounds in Conference and Alexander Lucas pears harvested at different maturity stages. Postharvest Biol. Technol. 2018, 146, 18–25. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, L.; Li, R.; Zhou, Q.; Wang, J.W.; Ji, S.J. Low temperature conditioning prevents loss of aroma-related esters from ‘Nanguo’ pears during ripening at room temperature. Postharvest Biol. Technol. 2015, 100, 23–32. [Google Scholar] [CrossRef]
- Wei, S.W.; Qin, G.H.; Zhang, H.P.; Tao, S.T.; Wu, J.; Wang, S.M.; Zhang, S.L. Calcium treatments promote the aroma volatiles emission of pear (Pyrus ussuriensis ‘Nanguoli’) fruit during post-harvest ripening process. Sci. Hortic. 2017, 215, 102–111. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yang, C.X.; Li, S.H.; Yang, L.; Wang, Y.N.; Zhao, J.B.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP-SPME with GC-MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Yang, C.X.; Wang, Y.J.; Liang, Z.C.; Fan, P.G.; Wu, B.H.; Yang, L.; Wang, Y.N.; Li, S.H. Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with GC-MS. Food Chem. 2009, 114, 1106–1114. [Google Scholar] [CrossRef]
- Gokbulut, I.; Karabulut, I. SPME-GC-MS detection of volatile compounds in apricot varieties. Food Chem. 2012, 132, 1098–1102. [Google Scholar] [CrossRef]
- Xu, C.H.; Chen, G.S.; Xiong, Z.H.; Fan, Y.X.; Wang, X.C.; Liu, Y. Applications of solid-phase microextraction in food analysis. TrAC-Trends Anal. Chem. 2016, 80, 12–29. [Google Scholar] [CrossRef]
- Murray, J.A. Qualitative and quantitative approaches in comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2012, 1261, 58–68. [Google Scholar] [CrossRef]
- Freye, C.E.; Bahaghighat, H.D.; Synovec, R.E. Comprehensive two-dimensional gas chromatography using partial modulation via a pulsed flow valve with a short modulation period. Talanta 2018, 177, 142–149. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Pulgati, F.H.; Zini, C.A. Main differences between volatiles of sparkling and base wines accessed through comprehensive two dimensional gas chromatography with time-of-flight mass spectrometric detection and chemometric tools. Food Chem. 2014, 164, 427–437. [Google Scholar]
- Lebedev, A.T.; Polyakova, O.V.; Mazur, D.M.; Artaev, V.B.; Canet, I.; Lallement, A.; Vaitilingom, M.; Deguillaume, L.; Delort, A.M. Detection of semi-volatile compounds in cloud waters by GC×GC-TOF-MS. Evidence of phenols and phthalates as priority pollutants. Environ. Pollut. 2018, 241, 616–625. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.P.; Shao, C.Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.D.; Tan, J.F.; Peng, Q.H.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef]
- Cannon, R.J.; Ho, C.T. Volatile sulfur compounds in tropical fruits. J. Food Drug Anal. 2018, 26, 445–468. [Google Scholar] [CrossRef]
- Feng, S.; Huang, M.Y.; Crane, J.H.; Wang, Y. Characterization of key aroma-active compounds in lychee (Litchi chinensis Sonn.). J. Food Drug Anal. 2018, 26, 497–503. [Google Scholar] [CrossRef]
- Mir, N.A.; Beaudry, R. Effect of superficial scald suppression by diphenylamine application on volatile evolution by stored cortland apple fruit. J. Agric. Food Chem. 1999, 47, 7–11. [Google Scholar] [CrossRef]
- Hui, W.; Niu, J.P.; Xu, X.Y.; Guan, J.P. Evidence supporting the involvement of MHO in the formation of superficial scald in ‘Dangshansuli’ pears. Postharvest Biol. Technol. 2016, 121, 43–50. [Google Scholar] [CrossRef][Green Version]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/index.html (accessed on 5 January 2019).
- Cheng, H.; Chen, J.L.; Li, X.; Pan, J.X.; Xue, S.J.; Liu, D.H.; Ye, X.Q. Differentiation of the volatile profiles of Chinese bayberry cultivars during storage by HS-SPME–GC/MS combined with principal component analysis. Postharvest Biol. Technol. 2015, 100, 59–72. [Google Scholar] [CrossRef]
- Mannucci, A.; Serra, A.; Remorini, D.; Castagna, A.; Mele, M.; Scartazza, A.; Ranieri, A. Aroma profile of Fuji apples treated with gelatin edible coating during their storage. LWT Food Sci. Technol. 2017, 85, 28–36. [Google Scholar] [CrossRef]
- Amanpour, A.; Guclu, G.; Kelebek, H.; Selli, S. Characterization of key aroma compounds in fresh and roasted terebinth fruits using aroma extract dilution analysis and GC–MS-Olfactometry. Microchem. J. 2019, 145, 96–104. [Google Scholar] [CrossRef]
- Zakaria, S.R.; Saim, N.; Osman, R.; Abdul Haiyee, Z.; Juahir, H. Combination of sensory, chromatographic, and chemometrics analysis of volatile organic compounds for the discrimination of authentic and unauthentic harumanis mangoes. Molecules 2018, 23, 2365. [Google Scholar] [CrossRef]
- Kjeldsen, F.; Christensen, L.P.; Edelenbos, M. Changes in volatile compounds of carrots (Daucus carota L.) during refrigerated and frozen storage. J. Agric. Food Chem. 2003, 51, 5400–5407. [Google Scholar] [CrossRef]
- Kjeldsen, F.; Christensen, L.P.; Edelenbos, M. Quantitative analysis of aroma compounds in carrot (Daucus carota L.) cultivars by capillary gas chromatography using large-volume injection technique. J. Agric. Food Chem. 2001, 49, 4342–4348. [Google Scholar] [CrossRef] [PubMed]
- Fariña, L.; Boido, E.; Carrau, F.; Versini, G.; Dellacassa, E. Terpene compounds as possible precursors of 1,8-cineole in red grapes and wines. J. Agric. Food Chem. 2005, 53, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Heuskin, S.; Godin, B.; Leroy, P.; Capella, Q.; Wathelet, J.P.; Verheggen, F.; Haubruge, E.; Lognay, G. Fast gas chromatography characterisation of purified semiochemicals from essential oils of Matricaria chamomilla L. (Asteraceae) and Nepeta cataria L. (Lamiaceae). J. Chromatogr. A 2009, 1216, 2768–2775. [Google Scholar] [CrossRef] [PubMed]
- Jalali-Heravi, M.; Parastar, H.; Sereshti, H. Development of a method for analysis of Iranian damask rose oil: combination of gas chromatography-mass spectrometry with Chemometric techniques. Anal. Chim. Acta 2008, 623, 11–21. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Pear Cultivars | Abbreviation | Producing Area | Sampling Time |
|---|---|---|---|
| Longyuanyangli | longyuanyangli | Qiqihaer city of Heilongjiang province | 07 September 2018 |
| Packham’s Triumph | Packham | Weihai city of Shandong Province | 27 September 2018 |
| Docteur Jules Guyot | D Jules | Yantai city of Shandong Province | 01 August 2018 |
| Clapp’s Favorite | Clapp | Yantai city of Shandong Province | 07 August 2018 |
| Starkrimson | Stark | Yantai city of Shandong Province | 01 August 2018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, W.; Li, H.; Mao, J.; Guo, C.; Ding, R.; Wang, Y.; Fang, L.; Chen, Z.; Yang, G. Analysis of Volatile Compounds in Pears by HS-SPME-GC×GC-TOFMS. Molecules 2019, 24, 1795. https://doi.org/10.3390/molecules24091795
Wang C, Zhang W, Li H, Mao J, Guo C, Ding R, Wang Y, Fang L, Chen Z, Yang G. Analysis of Volatile Compounds in Pears by HS-SPME-GC×GC-TOFMS. Molecules. 2019; 24(9):1795. https://doi.org/10.3390/molecules24091795
Chicago/Turabian StyleWang, Chenchen, Wenjun Zhang, Huidong Li, Jiangsheng Mao, Changying Guo, Ruiyan Ding, Ying Wang, Liping Fang, Zilei Chen, and Guosheng Yang. 2019. "Analysis of Volatile Compounds in Pears by HS-SPME-GC×GC-TOFMS" Molecules 24, no. 9: 1795. https://doi.org/10.3390/molecules24091795
APA StyleWang, C., Zhang, W., Li, H., Mao, J., Guo, C., Ding, R., Wang, Y., Fang, L., Chen, Z., & Yang, G. (2019). Analysis of Volatile Compounds in Pears by HS-SPME-GC×GC-TOFMS. Molecules, 24(9), 1795. https://doi.org/10.3390/molecules24091795