Preparation of Large-Size, Superparamagnetic, and Highly Magnetic Fe3O4@PDA Core–Shell Submicrosphere-Supported Nano-Palladium Catalyst and Its Application to Aldehyde Preparation through Oxidative Dehydrogenation of Benzyl Alcohols
Abstract
:1. Introduction
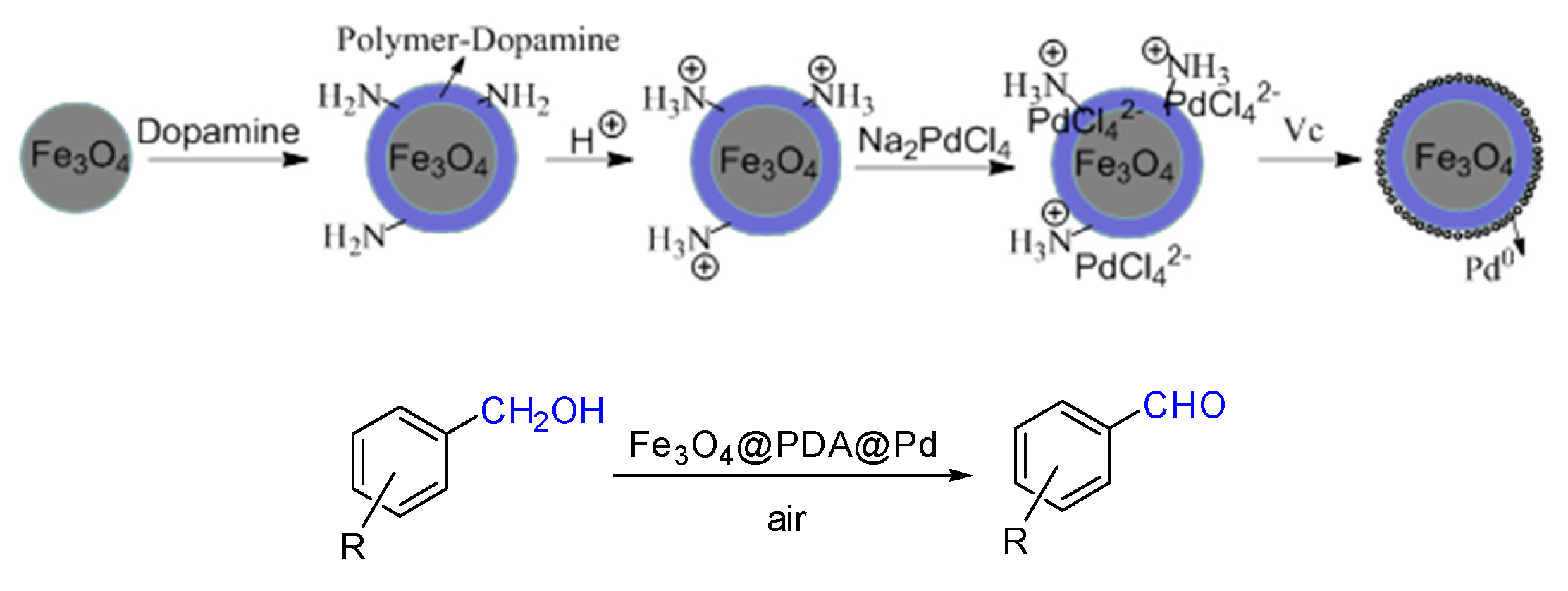
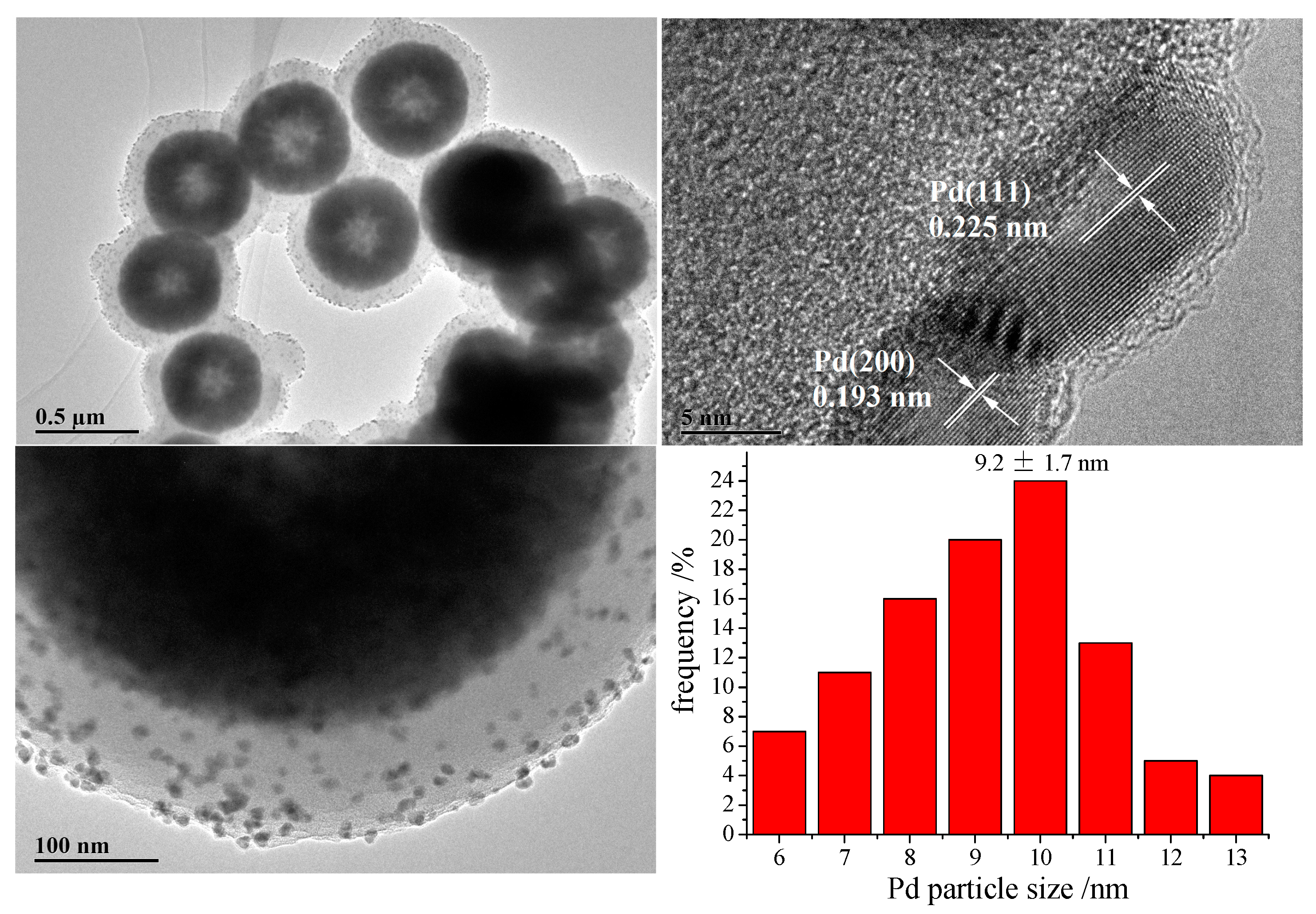
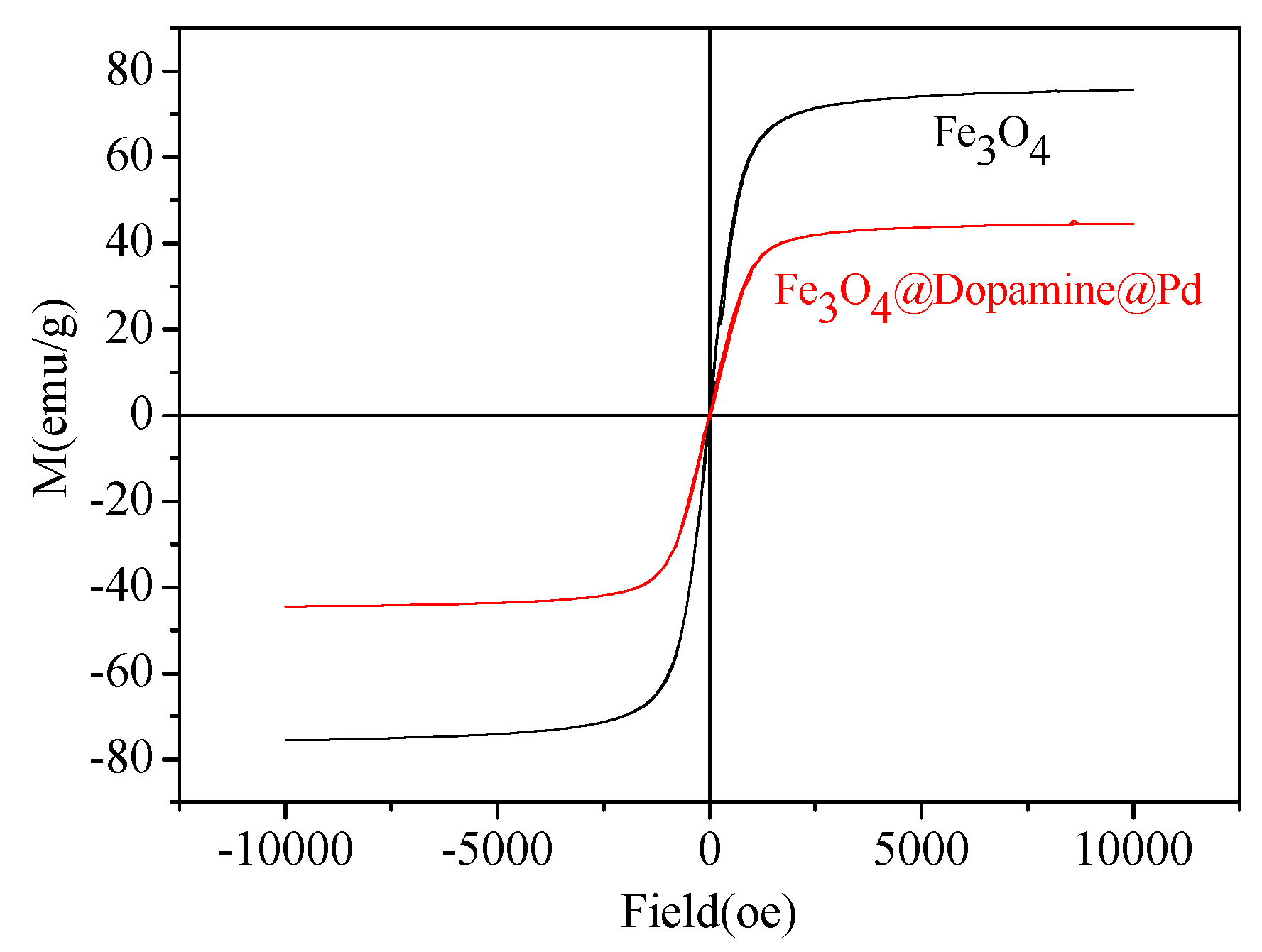
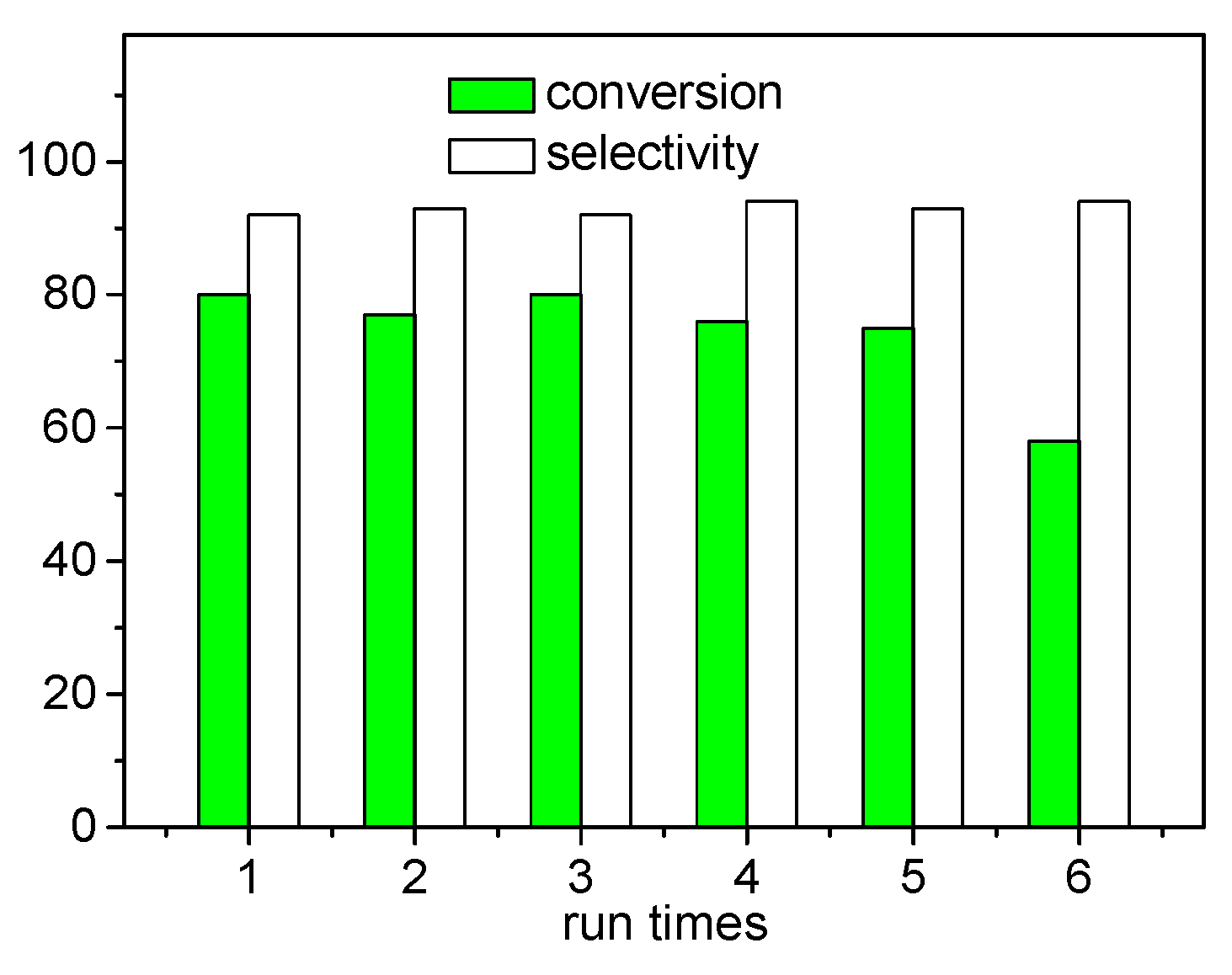
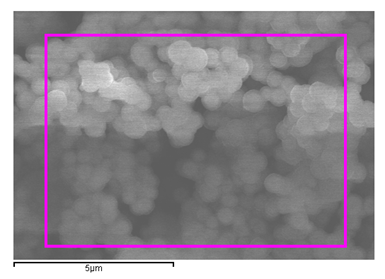
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, H.; Cui, L.L.; Tong, N.H.; Gu, H.C. Development of high magnetization Fe3O4/polystyrene/silica nanospheres via combined miniemulsion/emulsion polymerization. J. Am. Chem. Soc. 2006, 128, 15582–15583. [Google Scholar] [CrossRef]
- Lima, E.; Brandl, A.L.; Arelaro, A.D.; Goya, G.F. Spin disorder and magnetic anisotropy in Fe3O4 nanoparticles. J. Appl. Phys. 2006, 99, 083908. [Google Scholar] [CrossRef]
- Allia, P.; Barrera, G.; Tiberto, P.; Nardi, T.; Leterrier, Y.; Sangermano, M. Fe3O4 nanoparticles and nanocomposites with potential application in biomedicine and in communication technologies: Nanoparticle aggregation, interaction, and effective magnetic anisotropy. J. Appl. Phys. 2014, 116, 113903. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, X.Q.; Row, H.K. Magnetic solid-phase extraction with Fe3O4/molecularly imprinted polymers modified by deep eutectic solvents and ionic liquids for the rapid purification of alkaloid isomers (theobromine and theophylline) from green tea. Molecules 2017, 22, 1061. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.M.; Nivetha, A.; Prabha, I. Superparamagnetic Properties and Significant Applications of Iron Oxide Nanoparticles for Astonishing Efficacy-a Review. J. Supercond. Nov. Magn. 2018, 3, 1–18. [Google Scholar] [CrossRef]
- Peelamedu, R.; Grimes, C.; Cheng, J.; Agrawal, D. Major phase transformations and magnetic property changes caused by electromagnetic fields at microwave frequencies. J. Mater. Res. 2002, 17, 3008–3011. [Google Scholar]
- Qiang, C.; Xu, J.; Zhang, Z.; Tian, L.; Xiao, S.; Liu, Y.; Xu, P. Magnetic properties and microwave absorption properties of carbon fibers coated by Fe3O4 nanoparticles. J. Alloys Compd. 2010, 506, 93–97. [Google Scholar] [CrossRef]
- Mordina, B.; Kumar, R.; Tiwari, R.K.; Setua, D.K.; Sharma, A. Fe3O4 Nanoparticles Embedded Hollow Mesoporous Carbon Nanofibers and Polydimethylsiloxane-Based Nanocomposites as Efficient Microwave Absorber. J. Phys. Chem. C 2017, 121, 7810–7820. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, X.; Zhang, Q.; Chen, H.; Chen, X. Fe3O4 nanoparticles decorated MWCNTs @ C ferrite nanocomposites and their enhanced microwave absorption properties. J. Magn. Magn. Mater. 2018, 452, 55–63. [Google Scholar] [CrossRef]
- Tartaj, P.; Serna, C.J. Synthesis of monodisperse superparamagnetic Fe/silica nanospherical composites. J. Am. Chem. Soc. 2003, 125, 15754–15755. [Google Scholar] [PubMed]
- Khosroshahi, M.E.; Ghazanfari, L. Amino surface modification of Fe3O4/SiO2 nanoparticles for bioengineering applications. Surf. Eng. 2013, 27, 508–573. [Google Scholar] [CrossRef]
- Ding, C.F.; Sun, Y.L.; Wang, Y.H.; Li, J.B.; Lin, Y.N.; Sun, W.Y.; Luo, C.N. Adsorbent for resorcinol removal based on cellulose functionalized with magnetic poly(dopamine). Int. J. Biol. Macromol. 2017, 99, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, T.; Chakraborty, A.; Dasgupta, S.; Dutta, A.; Menéndez, M.I.; Zangrando, E. A route to magnetically separable nanocatalysts: Combined experimental and theoretical investigation of alkyl substituent role in ligand backbone towards epoxidation ability. Appl. Organomet. Chem. 2016, 31, e3663. [Google Scholar] [CrossRef]
- Dam, B.; Patil, R.A.; Ma, Y.; Pal, A.K. Preparation, characterization and catalytic application of nano-Fe3O4-DOPA-SnO2 having high TON and TOF for non-toxic and sustainablesynthesis of dihydroquinazolinone derivatives. New J. Chem. 2017, 41, 6553–6563. [Google Scholar] [CrossRef]
- Gao, F.; Qu, H.; Duan, Y.Y.; Wang, J.; Song, X.; Ji, T.J.; Cao, L.X.; Nie, G.G.; Sun, S.G. Dopamine coating as a general and facile route to biofunctionalization of superparamagnetic Fe3O4 nanoparticles for magnetic separation of proteins. RSC Adv. 2014, 4, 6657–6663. [Google Scholar] [CrossRef]
- Li, X.N.; Wen, F.; Creran, B.; Jeong, Y.; Zhang, X.R.; Rotello, V.M. Colorimetric protein sensing using catalytically amplified sensor arrays. Small 2012, 8, 3589–3592. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Si, H.L.; Niu, J.Z.; Shen, H.B.; Zhou, C.G.; Yuan, H. Size-controlled syntheses and hydrophilic surface modification of Fe3O4, Ag, and Fe3O4/Ag heterodimer nanocrystals. Dalton Trans. 2010, 39, 10984–10989. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Vand, W.H.; Shumbula, P.M. Engineered Inorganic/Organic-Core/Shell Magnetic FexOy Nanoparticles with Oleic Acid and/or Oleylamine As Capping Agents. Curr. Pharm. Des. 2015, 21, 5369–5388. [Google Scholar] [CrossRef]
- Bagga, K.; Brougham, D.F.; Keyes, T.E.; Brabazon, D. Magnetic and noble metal nanocomposites for separation and optical detection of biological species. Phys. Chem. Chem. Phys. 2015, 17, 27968–27980. [Google Scholar] [CrossRef]
- Batalha, Í.L.; Roque, A.C. Phosphopeptide Enrichment Using Various Magnetic Nanocomposites: An Overview. Methods Mol. Biol. 2016, 1355, 193–209. [Google Scholar]
- Gujrati, V.; Mishra, A.; Ntziachristos, V. Molecular imaging probes for multi-spectral optoacoustic tomography. Chem. Commun. 2017, 53, 4653–4672. [Google Scholar] [CrossRef]
- Li, J.C.; Wang, S.G.; Shi, X.Y.; Shen, M.W. Aqueous-phase synthesis of iron oxide nanoparticles and composites for cancer diagnosis and therapy. Adv. Colloid Interface Sci. 2017, 249, 374–385. [Google Scholar] [CrossRef]
- Wu, M.; Deng, H.Y.; Fan, Y.J.; Hu, Y.C. Rapid Colorimetric Detection of Cartap Residues by AgNP Sensor with Magnetic Molecularly Imprinted Microspheres as Recognition Elements. Molecules 2018, 23, 1443. [Google Scholar] [CrossRef]
- Wang, L.; Yue, S.Y.; Zhang, Q.; Zhang, Y.M. Morphological and Chemical Tuning of High-Energy-Density Metal Oxides for Lithium Ion Battery Electrode Applications. ACS Energy Lett. 2017, 2, 1465–1478. [Google Scholar] [CrossRef]
- Schacht, E.; Desmarets, G.; Bogaert, Y. Polymeric pesticides, 4. Synthesis of polyamides and a polyester containing 2,6-dichlorobenzaldehyde. Macromol. Chem. Phys. 1978, 179, 837–840. [Google Scholar] [CrossRef]
- Derya, O.; Serkan, L.; Abdullah, B.K.; Sinem, I. Synthesis of New Benzothiazole Acylhydrazones as Anticancer Agents. Molecules 2018, 23, 1054. [Google Scholar]
- Chen, J.J.; Lin, Y.H.; Day, S.H.; Hwang, T.L.; Chen, I.S. New benzenoids and anti-inflammatory constituents from Zanthoxylum nitidum. Food Chem. 2011, 125, 282–287. [Google Scholar] [CrossRef]
- Bello, K.A.; Martins, C.M.O.A.; Adamu, I.K. Methine dyes formed by condensation of indane-1,3-dione and cyanovinyl analogues with benzaldehydes. Color. Technol. 2010, 110, 238–240. [Google Scholar]
- Young, Y.H.; Hyun, K.D.; Sujin, S.; Sultan, U.; Jin, K.; Young, Y.; Ryong, M.H. Design, synthesis, and anti-melanogenic effects of (E)-2-benzoyl-3-(substituted phenyl)acrylonitriles. Drug Des. Dev. Ther. 2015, 9, 4259–4268. [Google Scholar]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, N.M.R.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Catalytic Oxidation of Alcohols: Recent Advances. ChemInform 2016, 47, 91–174. [Google Scholar] [CrossRef]
- Majumdar, B.; Sarma, D.; Jain, S.; Sarma, T.K. One-Pot Magnetic Iron Oxide-Carbon Nanodot CompositeCatalyzed Cyclooxidative Aqueous Tandem Synthesis of Quinazolinones in the Presence of tert-Butyl Hydroperoxide. ACS Omega 2018, 3, 13711–13719. [Google Scholar] [CrossRef]
- Zamani, F.; Hosseini, S.M. Palladium nanoparticles supported on Fe3O4/amino acid nanocomposite: Highly active magnetic catalyst for solvent-free aerobic oxidation of alcohols. Catal. Commun. 2014, 43, 164–168. [Google Scholar] [CrossRef]
- Dadras, A.; Naimi-Jamal, M.R.; Moghaddam, F.M.; Ayati, S.E. Green and selective oxidation of alcohols by immobilized Pd onto triazole functionalized Fe3O4 magnetic nanoparticles. J. Chem. Sci. 2018, 130, 162. [Google Scholar] [CrossRef]
- Zohreh, N.; Hosseini, S.H.; Tavakolizadeh, M.; Busuioc, C.; Negrea, R. Palladium pincer complex incorporation onto the Fe3O4-entrapped crosslinked multilayered polymer as a high loaded nanocatalyst for oxidation. J. Mol. Liq. 2018, 266, 393–404. [Google Scholar] [CrossRef]
- Movahed, S.K.; Lehi, N.F.; Dabiri, M. Palladium nanoparticles supported on core-shell and yolk-shell Fe3O4@nitrogen doped carbon cubes as a highly efficient, magnetically separable catalyst for the reduction of nitroarenes and the oxidation of alcohols. J. Catal. 2018, 364, 69–79. [Google Scholar] [CrossRef]
- Ramazani, A.; Khoobi, M.; Sadri, F.; Tarasi, R.; Shafiee, A.; Aghahosseini, H.; Joo, S.W. Efficient and selective oxidation of alcohols in water employing palladium supported nanomagnetic Fe3O4@hyperbranched polyethylenimine (Fe3O4@HPEI.Pd) as a new organic-inorganic hybrid nanocatalyst. Appl. Organomet. Chem. 2018, 32, e3908. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Carbon-coated magnetic palladium: Applications in partial oxidation of alcohols and coupling reactions. Green Chem. 2014, 16, 4333–4338. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Shin, K.Y.; Lee, J.S.; Hackett, M.J.; Jun, S.W.; Oh, M.H.; Jang, J.; Hyeon, T. Magnetically recyclable core-shell nanocatalysts for efficient heterogeneous oxidation of alcohols. J. Mater. Chem. A 2014, 2, 7593–7599. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Fe3O4, 4-methoxybenzaldehyde, 4-chlorobenzaldehyde and 4-biphenylcarboxaldehyde are available from the authors. |

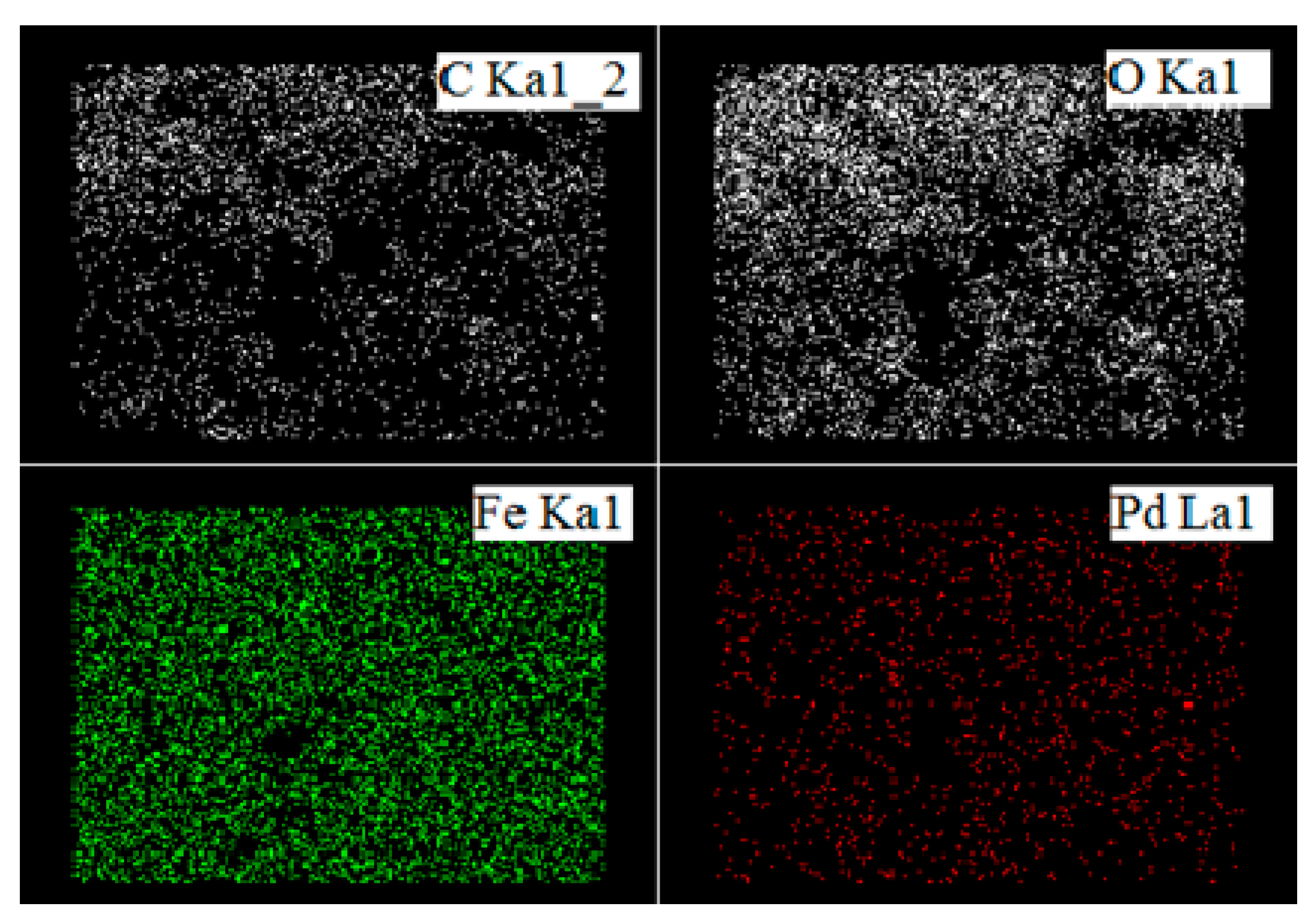
| Element | wt% |
|---|---|
| C K | 13.43 |
| O K | 33.67 |
| Fe K | 48.66 |
| Pd L | 4.24 |
| Total | 100.00 |

| Entry | Temperature (°C) | Time (h) | Oxidant | Conversion a (%) | Selectivity a (%) |
|---|---|---|---|---|---|
| 1 | 80 | 24 | Air | 0 | – |
| 2 | 100 | 24 | Air | <5 | >99 |
| 3 | 120 | 12 | Air | 62 | 95 |
| 4 | 120 | 24 | Air | 80 | 92 |
| 5 | 120 | 48 | Air | 87 | 79 |
| 6 | 140 | 24 | Air | 100 | 42 b |
| 7 | 120 | 24 | O2 | 86 | 70 |
| 8 | 120 | 48 | None c | 31 | >99 |

| Entry | Substituted Benzylic Alcohol | Aldehyde | Yield a (%) |
|---|---|---|---|
| 1 | 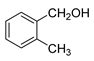 |  | 57 |
| 2 | 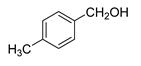 | 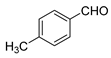 | 76 |
| 3 |  | 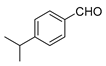 | 77 |
| 4 | 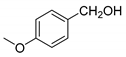 | 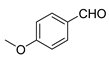 | 84 |
| 5 | 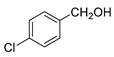 |  | 73 |
| 6 |  | 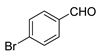 | 71 |
| 7 | 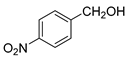 | 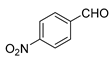 | 60 |
| 8 | 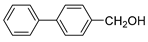 | 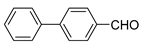 | 86 |
| 9 | 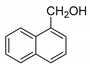 | 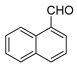 | 69 |
| 10 | 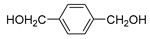 | 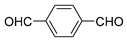 | 53 |
| 11 | 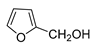 | 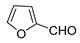 | 65 b |
| 12 | 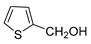 |  | 72 b |
| 13 |  | 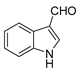 | 72 |
| 14 |  | 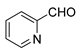 | 74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Zheng, R.; Jiang, H.; Xu, Z.; Xia, A. Preparation of Large-Size, Superparamagnetic, and Highly Magnetic Fe3O4@PDA Core–Shell Submicrosphere-Supported Nano-Palladium Catalyst and Its Application to Aldehyde Preparation through Oxidative Dehydrogenation of Benzyl Alcohols. Molecules 2019, 24, 1730. https://doi.org/10.3390/molecules24091730
Guo H, Zheng R, Jiang H, Xu Z, Xia A. Preparation of Large-Size, Superparamagnetic, and Highly Magnetic Fe3O4@PDA Core–Shell Submicrosphere-Supported Nano-Palladium Catalyst and Its Application to Aldehyde Preparation through Oxidative Dehydrogenation of Benzyl Alcohols. Molecules. 2019; 24(9):1730. https://doi.org/10.3390/molecules24091730
Chicago/Turabian StyleGuo, Haichang, Renhua Zheng, Huajiang Jiang, Zhenyuan Xu, and Aibao Xia. 2019. "Preparation of Large-Size, Superparamagnetic, and Highly Magnetic Fe3O4@PDA Core–Shell Submicrosphere-Supported Nano-Palladium Catalyst and Its Application to Aldehyde Preparation through Oxidative Dehydrogenation of Benzyl Alcohols" Molecules 24, no. 9: 1730. https://doi.org/10.3390/molecules24091730
APA StyleGuo, H., Zheng, R., Jiang, H., Xu, Z., & Xia, A. (2019). Preparation of Large-Size, Superparamagnetic, and Highly Magnetic Fe3O4@PDA Core–Shell Submicrosphere-Supported Nano-Palladium Catalyst and Its Application to Aldehyde Preparation through Oxidative Dehydrogenation of Benzyl Alcohols. Molecules, 24(9), 1730. https://doi.org/10.3390/molecules24091730





