Sesquiterpenoids and Their Anti-Inflammatory Activity: Evaluation of Ainsliaea yunnanensis
Abstract
:1. Introduction
2. Results
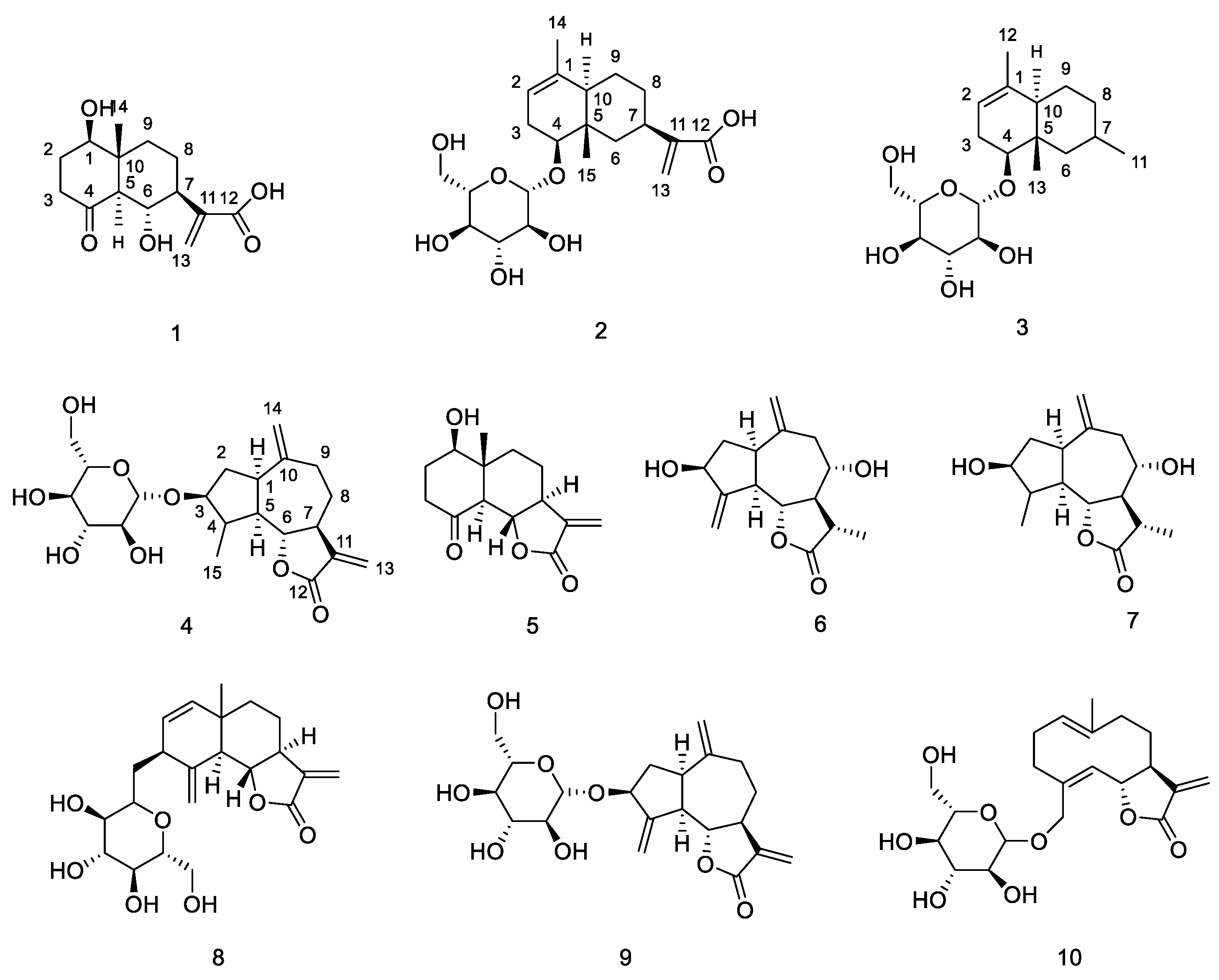
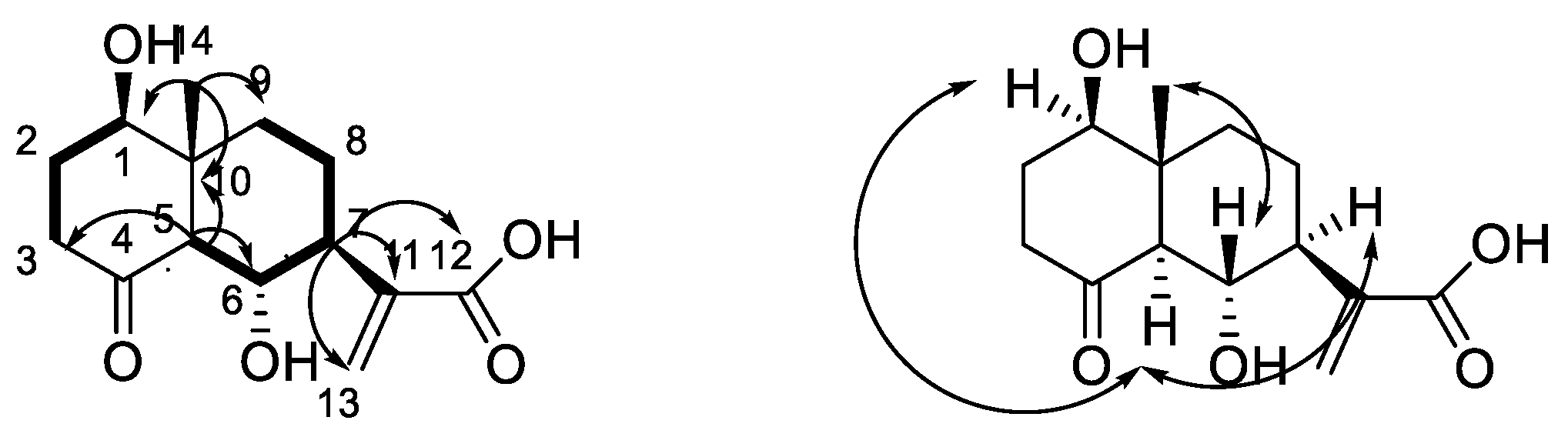
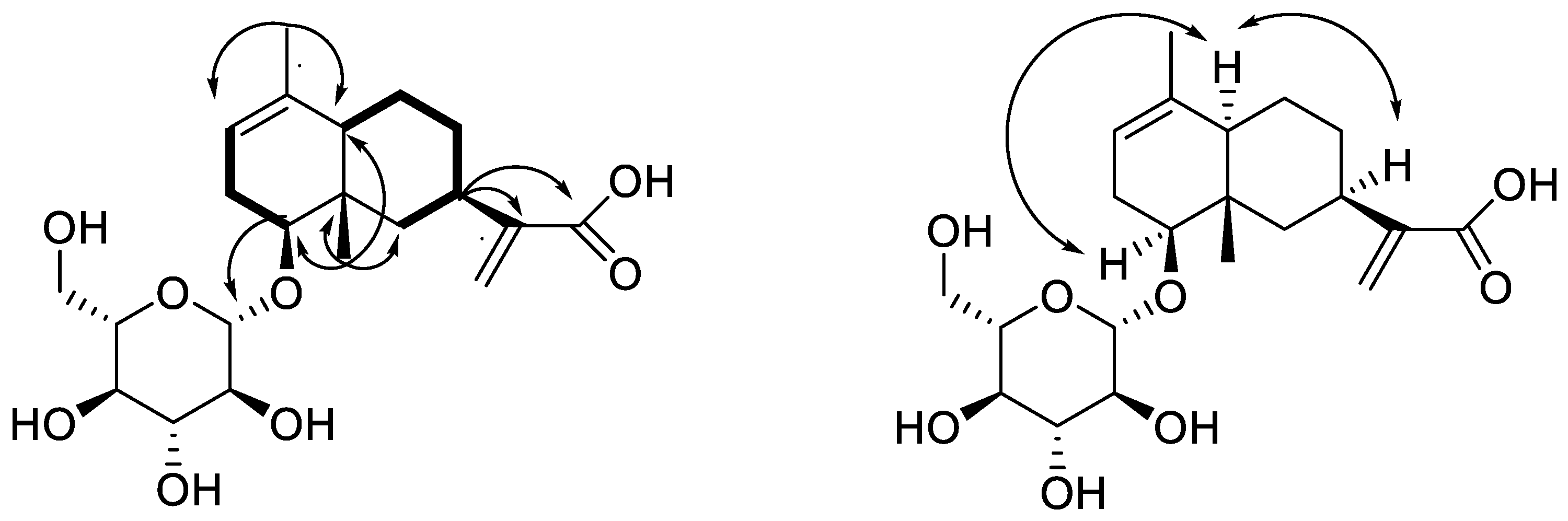
2.1. Structural Elucidation of New Compounds
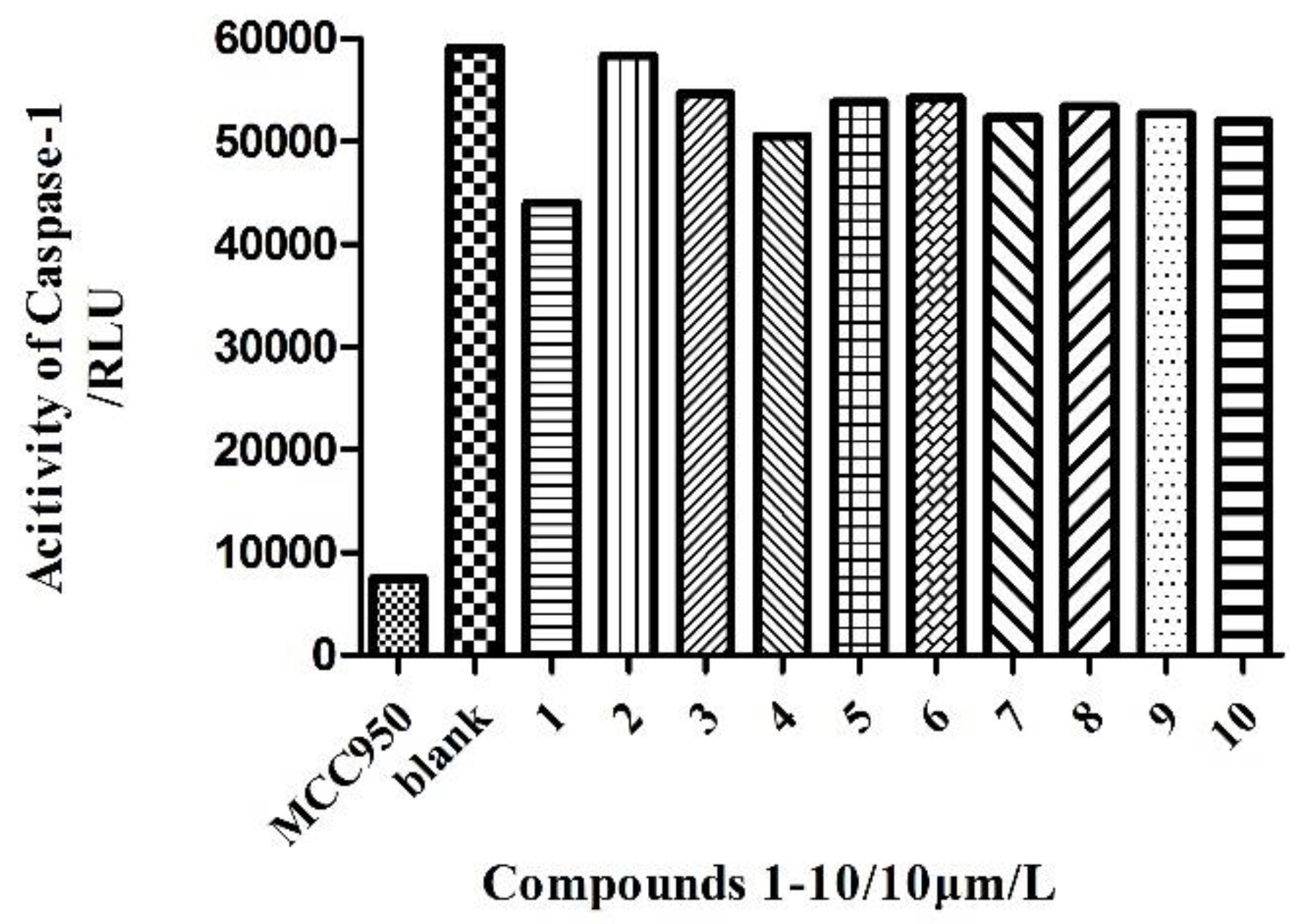
2.2. Anti-Inflammatory Activity Assay of Compounds
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Experimental Material
4.3. Extraction and Isolation
4.4. Anti-Inflammatory Activity Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, R.; Tang, Y.X.; Shang, X.Y. Research progress on chemical compositions and pharmacological activity of Ainsliaea. Zhongyaocai 2012, 35, 1171–1175. [Google Scholar]
- Jiangsu New Medical College. Dictionary Traditional Drugs; Shanghai People’s Publishing House: Shanghai, China, 1977; p. 1410. [Google Scholar]
- Choi, Z.C.; Yang, M.C.; Choi, S.U.; Lee, K.R. Cytotoxic terpenes and lignans from the roots of Ainsliaea acerifolia. Arch. Pharm. Res. 2006, 29, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Miyase, T.; Fukushima, S. Sesquiterpene lactones from Ainsliaea acerifolia SCH. BIP. and A. dissecta FRANCH. et SAV. Chem. Pharm. Bull. 1984, 32, 3043–3046. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Z.; Wang, A.L.; Yuan, Z.Z.; Li, J.J.; Shang, X.Y. GC-MS analysis of low polar components from Ainsliaea yunnanensis. Zhongyaocai 2013, 36, 61–64. [Google Scholar] [PubMed]
- Wu, X.L.; Xiong, X.J.; Lu, W.Q.; Huang, H.; Shen, Y.H.; Wu, Z.J.; Chen, W.S. New sesquiterpenenoids from Ainsliaea yunnanensis. Molecules 2016, 21, 1031. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xu, X.K.; Wang, G.W.; Zeng, R.T.; Tian, X.H.; Shi, Z.R.; Zhuo, Z.G.; Shen, Y.H.; Zhang, W.D. Guaianolide sesquiterpenoids from Ainsliaea yunnanensis. Phytochemistry 2017, 139, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhang, B.; Liu, H.L.; Zhang, X.; Shang, X.Y.; Zhao, C.Q. Triterpenoids from Ainsliaea yunnanensis Franch. and their biological activities. Molecules 2016, 21, 1481. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, A.L.; Yuan, Z.Z.; Wu, C.Y.; Yang, L.H.; Shang, X.Y. Triterpene compounds of Ainsliaea yunnanensis. China J. Chin. Mater. Med. 2013, 38, 56–60. [Google Scholar]
- Wu, X.L.; Xiong, X.J.; Wu, Z.J.; Shen, Y.T.; Huang, H. The pentacyclic triterpene of Ainsliaea yunnanensis. Guihaia 2015, 35, 109–114. [Google Scholar]
- Fang, X.; Zhuo, Z.G.; Xu, X.K.; Ye, J.; Li, H.L.; Shen, Y.H.; Zhang, W.D. Cytotoxic isovaleryl sucrose esters from Ainsliaea yunnanensis: Reduction of mitochondrial membrane potential and increase of reactive oxygen species levels in A549 cells. RSC Adv. 2017, 7, 20865–20873. [Google Scholar] [CrossRef]
- Jing, Y.Y.; Li, C.G.; Yan, L. Influences of scutellarin on ATP-inflammasome activation and pyroptosis in J774A.1 macrophages. Chin. Pharmacol. Bull. 2018, 34, 174–180. [Google Scholar]
- Yan, W.; Ming, L.X.; Hui, Z.J.; Jian, J.F.; Xiao, J.H.; Jiang, J.Q.; Shi, K.Y.; Yun, H.S.; Wei, D.Z. A new nor-sesquiterpene lactone from Ainsliaea fulvioides. Chin. Chem.l Lett. 2009, 20, 586–588. [Google Scholar]
- Wang, G.C.; Li, G.Q.; Geng, H.W.; Li, T.; Xu, J.J.; Ma, F.; Wu, X.; Ye, W.C.; Li, Y.L. Eudesmane-type sesquiterpene derivatives from Laggera alata. Phytochemistry 2013, 96, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Zhang, M.X.; Zhou, X.P.; Yang, X.H. Chemical constituents of whole plant of Scorzonera radiata Fisch. Chin. J. Med. Chem. 2010, 2, 527–530. [Google Scholar]
Sample Availability: Samples of the compounds 2 are available from the authors. |
| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Pos. | δH | δC | δH | δC | δH | δC | δH | δC |
| 1 | 3.80, dd, (10.0, 5.0) | 77.3 | - | 136.2 | - | 136.3 | 2.76, m | 42.1 |
| 2 | 1.84, m 2.08, m | 31.6 | 5.31, br s | 120.6 | 5.30, br s | 120.6 | 1.69, dd, (12, 10) 2.14, dd, (13.5, 7.0) | 37.3 |
| 3 | 2.21, m 2.53, m | 40.6 | 2.03, m 2.41, m | 29.9 | 2.00, m 2.40, m | 29.0 | 3.56, dd, (8.0, 15.5) | 86.2 |
| 4 | - | 212.6 | 3.72, dd, (10.0. 6.5) | 82.2 | 3.67, dd, (10.0, 6.5) | 82.2 | 1.85, m | 44.1 |
| 5 | 1.64, m | 62.2 | - | 37.9 | - | 37.9 | 1.91, m | 49.5 |
| 6 | 4,14, t, (10.0) | 67.6 | 1.26, m 2.40, m | 36.5 | 1.25, m 2.37, m | 36.3 | 3.93, t, (10) | 86.2 |
| 7 | 2.41, m | 48.4 | 2.02, m | 41.3 | 1.95, m | 42.5 | 2.78, m | 46.8 |
| 8 | 1.64, m | 28.1 | 1.50, m 1.67, m | 28.1 | 1.57, m 1.72, m | 25.3 | 1.24, m; 2.25, m | 30.6 |
| 9 | 1.36, m 1.81, m | 37.5 | 1.26, m 1.84, m | 30.2 | 1.25, m 1.72, m | 29.8 | 1.97, m; 2.54, m | 35.7 |
| 10 | - | 45.7 | 2.04, m | 37.9 | 2.02, m | 48.3 | - | 149.5 |
| 11 | 5.67, s 6.23, s | 125.9 | - | 147.9 | 1.13, d, (7.0) | 14.7 | - | 140.1 |
| 12 | - | 170.5 | 170.9 | 1.58, s | 21.0 | - | 169.6 | |
| 13 | - | 144.1 | 5.58, s 6.14, s | 123.0 | 0.81, s | 10.7 | 5.59, d, (2) 6.00, d, (2.5) | 119.5 |
| 14 | 0.76, s | 12.2 | 1.58 | 21.0 | - | - | 4.95, d, (5) 4.99, d, (5) | 112.7 |
| 15 | - | - | 0.85 | 10.9 | - | - | 1.15, d, (10) | 18.1 |
| Glu-1 | - | - | 4.30, d, (8.0) | 101.5 | 4.30, d, (7.5) | 101.5 | 4.19, d, (7.5) | 104.1 |
| Glu-2 | - | - | 3.14, m | 75.2 | 3.14, m | 75.2 | 2.95, m | 73.6 |
| Glu-3 | - | - | 3.30, m | 78.2 | 3.31, m | 78.2 | 3.16, m | 76.8 |
| Glu-4 | - | - | 3.27, m | 71.9 | 3.27, m | 71.9 | 3.04, m | 70.2 |
| Glu-5 | - | - | 3.25, m | 77.8 | 3.22, m | 77.8 | 3.08, m | 76.8 |
| Glu-6 | - | - | 3.65, dd, (12.0, 6.5) 3.85, dd, (11.5, 2.0) | 63.0 | 3.67, dd, (12.0, 6.5) 3.85, dd, (11.5, 2.0) | 63.0 | 3.42, dd, (11.5, 6.0) 3.65, d, (9.5, 4.5) | 61.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, X.; Wang, X.; Zhong, X.; Ji, L.; Guo, Z.; Liu, Y.; Shang, X. Sesquiterpenoids and Their Anti-Inflammatory Activity: Evaluation of Ainsliaea yunnanensis. Molecules 2019, 24, 1701. https://doi.org/10.3390/molecules24091701
Li J, Li X, Wang X, Zhong X, Ji L, Guo Z, Liu Y, Shang X. Sesquiterpenoids and Their Anti-Inflammatory Activity: Evaluation of Ainsliaea yunnanensis. Molecules. 2019; 24(9):1701. https://doi.org/10.3390/molecules24091701
Chicago/Turabian StyleLi, Jinjie, Xiuting Li, Xin Wang, Xiangjian Zhong, Linlin Ji, Zihan Guo, Yiran Liu, and Xiaoya Shang. 2019. "Sesquiterpenoids and Their Anti-Inflammatory Activity: Evaluation of Ainsliaea yunnanensis" Molecules 24, no. 9: 1701. https://doi.org/10.3390/molecules24091701
APA StyleLi, J., Li, X., Wang, X., Zhong, X., Ji, L., Guo, Z., Liu, Y., & Shang, X. (2019). Sesquiterpenoids and Their Anti-Inflammatory Activity: Evaluation of Ainsliaea yunnanensis. Molecules, 24(9), 1701. https://doi.org/10.3390/molecules24091701






