Detection of Pirimiphos-Methyl in Wheat Using Surface-Enhanced Raman Spectroscopy and Chemometric Methods
Abstract
:1. Introduction
2. Results and Discussion
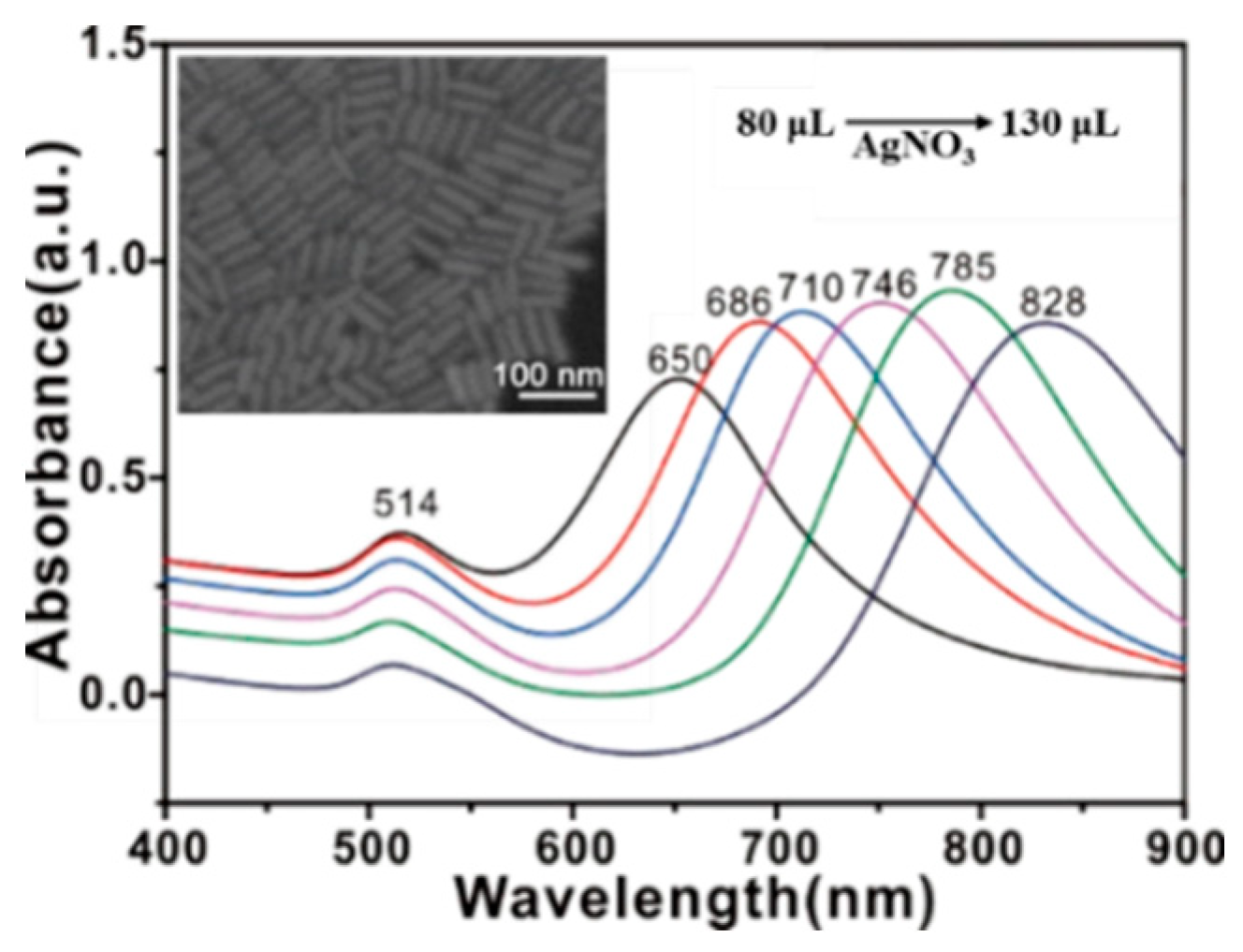
2.1. SERS Substrate
2.2. SERS Spectra of Pirimiphos-Methyl
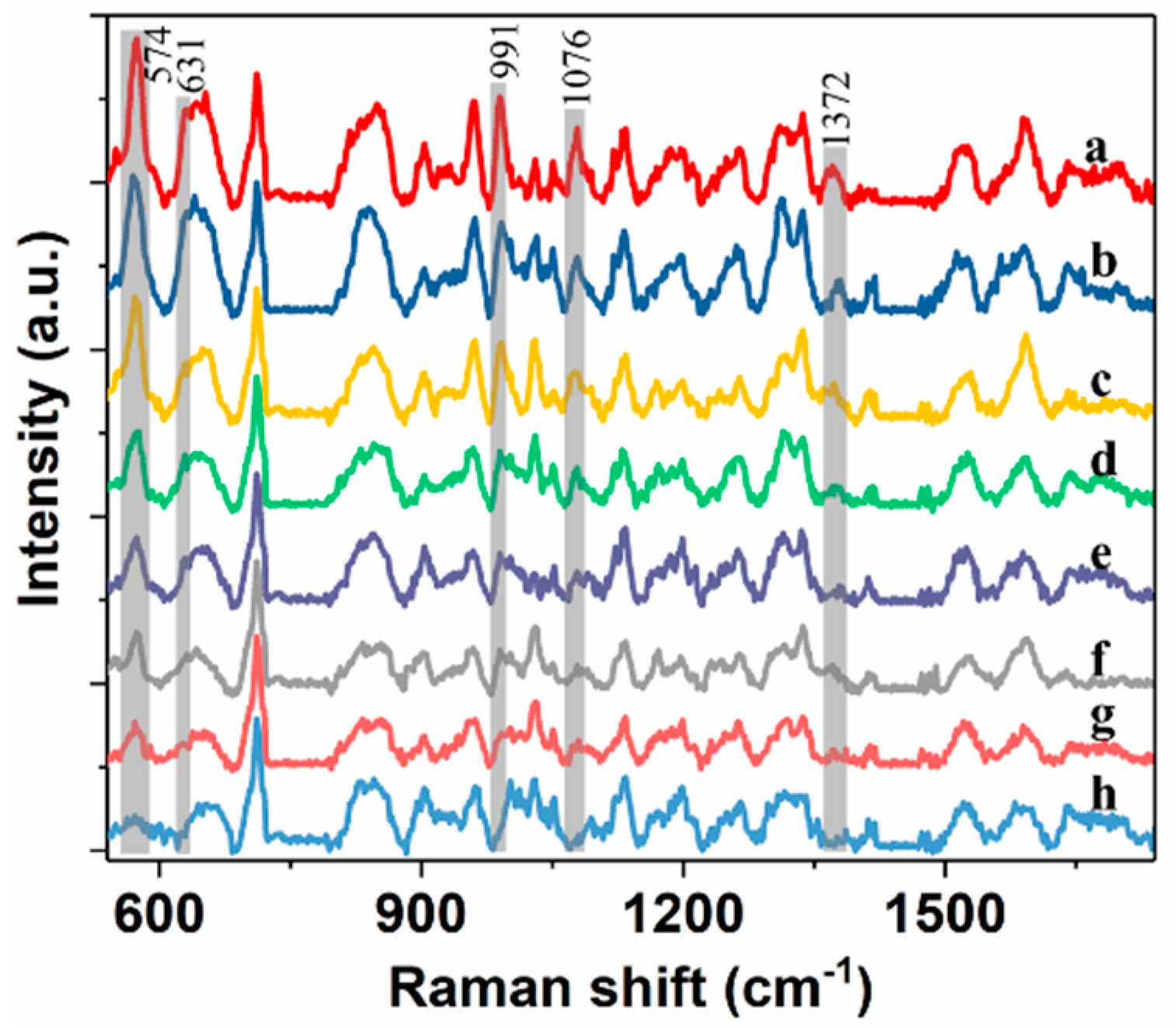
2.3. Comparison of Spectral Analysis Using Different Chemometric Methods
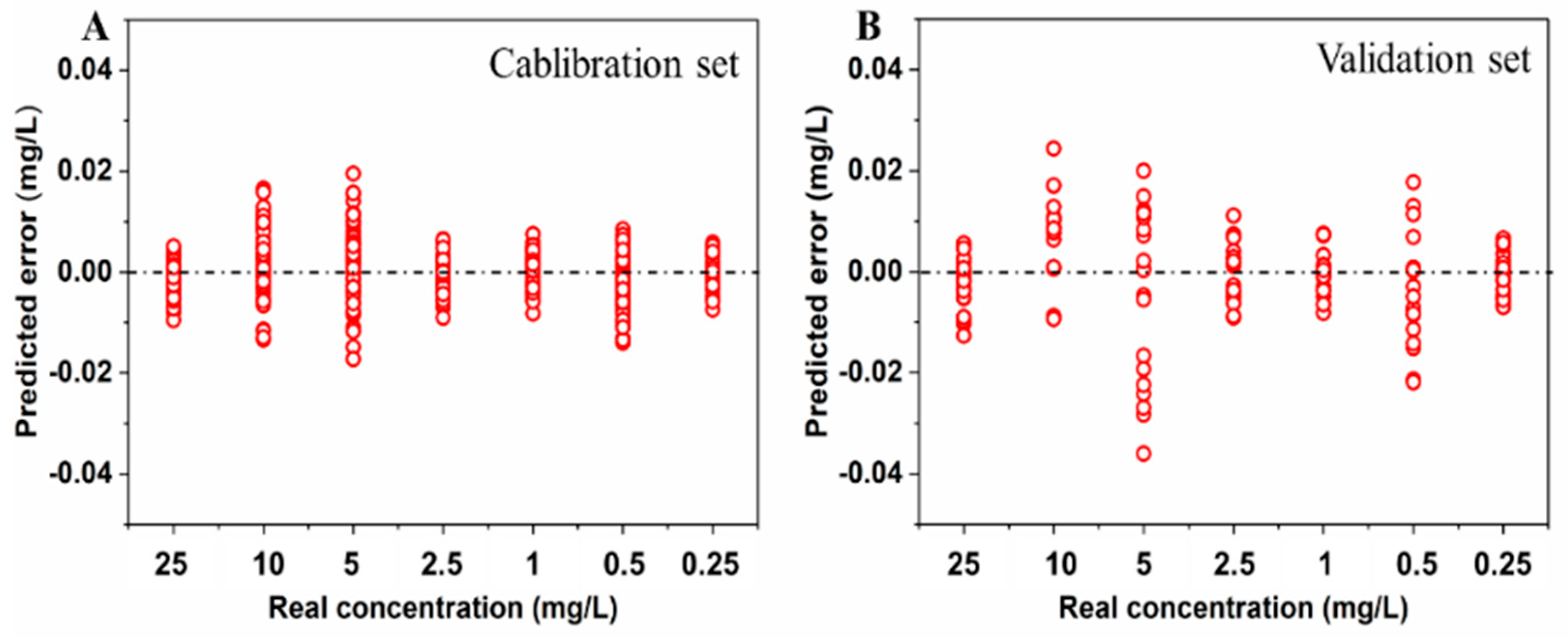
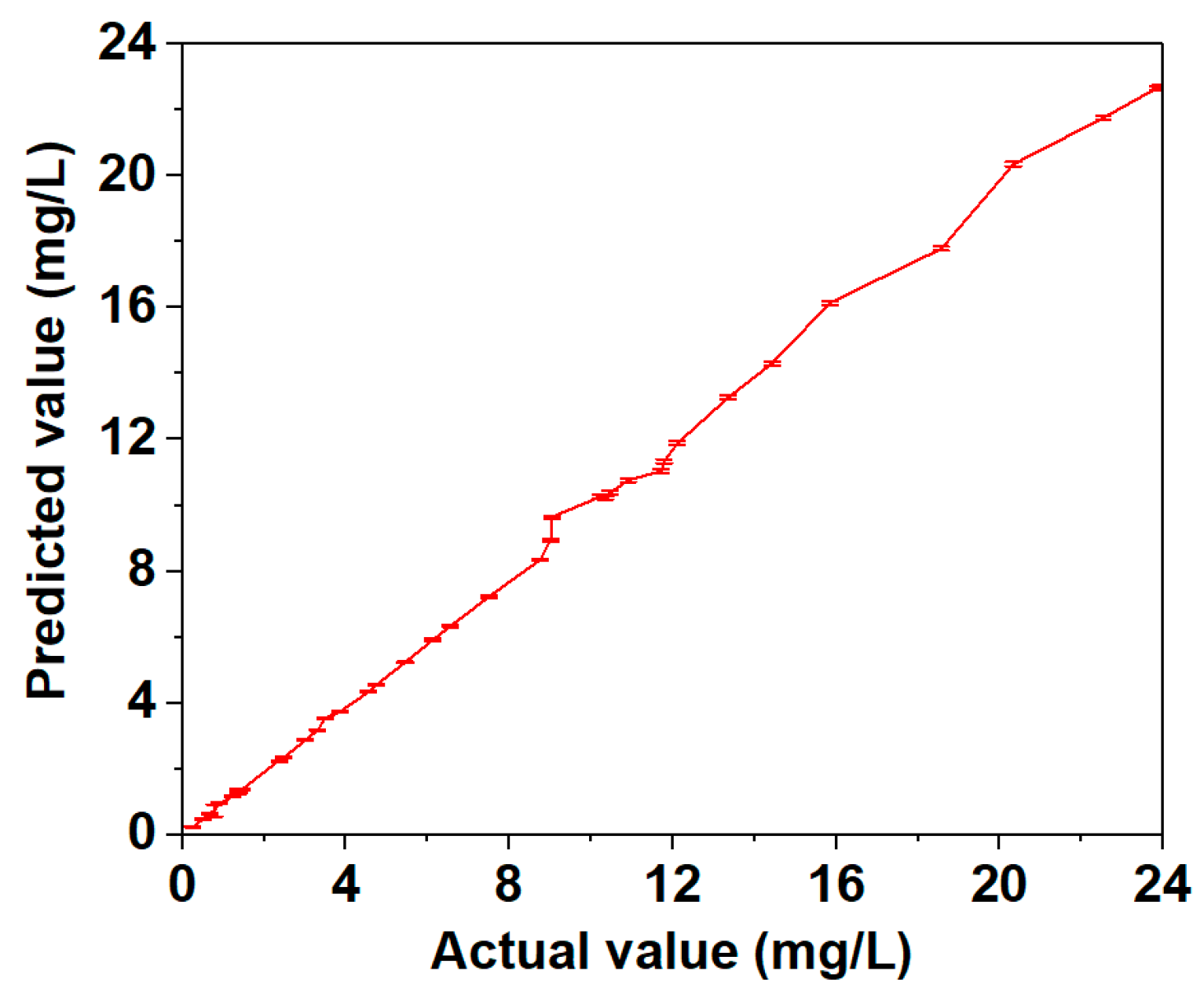
2.4. Quantification of Pirimiphos-Methyl Residue in Wheat
3. Materials and Methods
3.1. Reagents
3.2. DFT Calculation
3.3. Sample Preparation
3.4. SERS Measurement
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mhadhbi, L.; Beiras, R. Acute toxicity of seven selected pesticides (alachlor, atrazine, dieldrin, diuron, pirimiphos-methyl, chlorpyrifos, diazinon) to the marine fish (Turbot, Psetta maxima). Water Air Soil Pollut. 2012, 223, 5917–5930. [Google Scholar] [CrossRef]
- Stepić, S.; Hackenberger, B.K.; Velki, M.; Lončarić, Ž.; Hackenberger, D.K. Effects of individual and binary-combined commercial insecticides endosulfan, temephos, malathion and pirimiphos-methyl on biomarker responses in earthworm Eisenia andrei. Environ. Toxicol. Phar. 2013, 36, 715–723. [Google Scholar] [CrossRef]
- Qu, L.J.; Zhang, H.; Zhu, J.H.; Yang, G.S.; Aboul-Enein, H.Y. Rapid determination of organophosphorous pesticides in leeks by gas chromatography–triple quadrupole mass spectrometry. Food Chem. 2010, 122, 327–332. [Google Scholar] [CrossRef]
- Hou, X.; Han, M.; Dai, X.; Yang, X.; Yi, S. A multi-residue method for the determination of 124 pesticides in rice by modified QuEChERS extraction and gas chromatography–tandem mass spectrometry. Food Chem. 2013, 138, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, C.; Martínez-Bueno, M.J.; Lozano, A.; Fernández-Alba, A.R. Pesticide residue analysis of fruit juices by LC–MS/MS direct injection. One year pilot survey. Talanta 2011, 83, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, S.; Shin, J.Y.; Kim, M.; Kim, J.H. Development and verification for analysis of pesticides in eggs and egg products using QuEChERS and LC–MS/MS. Food Chem. 2015, 173, 1236–1242. [Google Scholar] [CrossRef]
- Lee, K.M.; Herrman, T.J.; Bisrat, Y.; Murray, S.C. Feasibility of surface-enhanced raman spectroscopy for rapid detection of aflatoxins in maize. J. Agr. Food Chem. 2014, 62, 4466–4474. [Google Scholar] [CrossRef]
- Dong, R.; Weng, S.; Yang, L.; Liu, J. Detection and direct readout of drugs in human urine using dynamic surface-enhanced Raman spectroscopy and support vector machines. Anal. Bioanal. Chem. 2015, 87, 2937–2944. [Google Scholar] [CrossRef]
- Alsammarraie, F.K.; Lin, M. Using Standing Gold Nanorod Arrays as Surface-Enhanced Raman Spectroscopy (SERS) Substrates for Detection of Carbaryl Residues in Fruit Juice and Milk. J. Agr. Food Chem. 2017, 65, 666–674. [Google Scholar] [CrossRef]
- Schlücker, S. Surface-Enhanced raman spectroscopy, Concepts and chemical applications. Angew. Chem. Int. Edit. 2014, 53, 4756–4795. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Wang, Z.L. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, M.H.; Wang, C.Y.; Chang, Y.M.; Gwo, S. Large-scale hot spot engineering for quantitative SERS at the single-molecule scale. J. Am. Chem. Soc. 2015, 137, 13698–13705. [Google Scholar] [CrossRef]
- Huang, S.; Hu, J.; Guo, P.; Liu, M.; Wu, R. Rapid detection of chlorpyriphos residue in rice by surface-enhanced Raman scattering. Anal. Method 2015, 7, 4334–4339. [Google Scholar] [CrossRef]
- Liu, D.; Han, Y.; Zhu, L.; Chen, W.; Zhou, Y.; Chen, J.; Dou, Z. Quantitative Detection of Isofenphos-Methyl in Corns Using Surface-Enhanced Raman Spectroscopy (SERS) with Chemometric Methods. Food Anal. Method 2017, 10, 1202–1208. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Yu, Z.; Yang, T. Surface-enhanced Raman spectroscopic analysis of phorate and fenthion pesticide in apple skin using silver nanoparticles. Appl. Spectrosc. 2014, 68, 483–487. [Google Scholar] [CrossRef]
- Lane, L.A.; Qian, X.; Nie, S. SERS nanoparticles in medicine, from label-free detection to spectroscopic tagging. Chem. Rev. 2015, 115, 10489–10529. [Google Scholar] [CrossRef]
- Seifert, S.; Merk, V.; Kneipp, J. Identification of aqueous pollen extracts using surface enhanced Raman scattering (SERS) and pattern recognition methods. J. Biophotonics 2016, 9, 181–189. [Google Scholar] [CrossRef]
- Domingo, E.; Tirelli, A.A.; Nunes, C.A.; Guerreiro, M.C.; Pinto, S.M. Melamine detection in milk using vibrational spectroscopy and chemometrics analysis, a review. Food Res. Int. 2014, 60, 131–139. [Google Scholar] [CrossRef]
- Shi, J.Y.; Zou, X.B.; Huang, X.W.; Zhao, J.W.; Li, Y.X.; Hao, L.M.; Zhang, J.C. Rapid detecting total acid content and classifying different types of vinegar based on near infrared spectroscopy and least-squares support vector machine. Food. Chem. 2013, 138, 192–199. [Google Scholar]
- Liu, M.; Wang, M.; Wang, J.; Li, D. Comparison of random forest, support vector machine and back propagation neural network for electronic tongue data classification, Application to the recognition of orange beverage and Chinese vinegar. Sensor Actuat. B-Chem. 2013, 177, 970–980. [Google Scholar] [CrossRef]
- Chen, H.; Lin, Z.; Wu, H.; Wang, L.; Wu, T.; Tan, C. Diagnosis of colorectal cancer by near-infrared optical fiber spectroscopy and random forest. Spectrochim. Acta A 2015, 135, 185–191. [Google Scholar] [CrossRef]
- Luce, R.; Hildebrandt, P.; Kuhlmann, U.; Liesen, J. Using separable nonnegative matrix factorization techniques for the analysis of time-resolved raman spectra. Appl. Spectrosc. 2016, 70, 1464–1475. [Google Scholar] [CrossRef]
- Hoonejani, M.R.; Pallaoro, A.; Braun, G.B.; Moskovits, M.; Meinhart, C.D. Quantitative multiplexed simulated-cell identification by SERS in microfluidic devices. Nanoscale 2015, 7, 16834–16840. [Google Scholar] [CrossRef]
- Witkowska, E.; Jagielski, T.; Kamińska, A.; Kowalska, A.; Hryncewicz-Gwóźdź, A.; Waluk, J. Detection and identification of human fungal pathogens using surface-enhanced Raman spectroscopy and principal component analysis. Anal. Methods-UK 2016, 8, 8427–8434. [Google Scholar] [CrossRef]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC-Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar]
- Chen, D.; Chen, Y.Q.; Xue, D. 1-D and 2-D digital fractional-order Savitzky–Golay differentiator. Signal Image Video P. 2012, 6, 503–511. [Google Scholar] [CrossRef]
- Tavassoli, N.; Chen, Z.; Bain, A.; Melo, L.; Chen, D.; Grant, E.R. Template-oriented genetic algorithm feature selection of analyte wavelets in the Raman spectrum of a complex mixture. Anal. Chem. 2014, 86, 10591–10599. [Google Scholar] [CrossRef]
- He, S.; Xie, W.; Zhang, W.; Zhang, L.; Wang, Y.; Liu, X.; Du, C. Multivariate qualitative analysis of banned additives in food safety using surface enhanced Raman scattering spectroscopy. Spectrochim. Acta Part A 2015, 137, 1092–1099. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Weng, S.Z.; Wang, F.; Dong, R.; Qiu, M.Q.; Zhao, J.L.; Huang, L.S.; Zhang, D.Y. Fast and Quantitative Analysis of Ediphenphos Residue in Rice Using Surface-Enhanced Raman Spectroscopy. J. Food Sci. 2018, 83, 1179–1185. [Google Scholar] [CrossRef]
- Dong, T.; Lin, L.; He, Y.; Nie, P.C.; Qu, F.F.; Xiao, S.P. Density Functional Theory Analysis of Deltamethrin and Its Determination in Strawberry by Surface Enhanced Raman Spectroscopy. Molecules 2018, 23, 1458. [Google Scholar] [CrossRef]
- Huang, S.G.; Hu, J.P.; Liu, M.H.; Wu, R.M.; Wang, X.B. Density Functional Theory Calculation and Raman Spectroscopy Studies of Carbamate Pesticides. J. Clin. Otolaryngol. Head Neck Surg. 2017, 37, 766–771. [Google Scholar]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, F.; Liu, H.; Yang, L.; Liu, J. Assembly of polymer–gold nanostructures with high reproducibility into a monolayer film SERS substrate with 5 nm gaps for pesticide trace detection. Analyst 2013, 138, 5832–5838. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
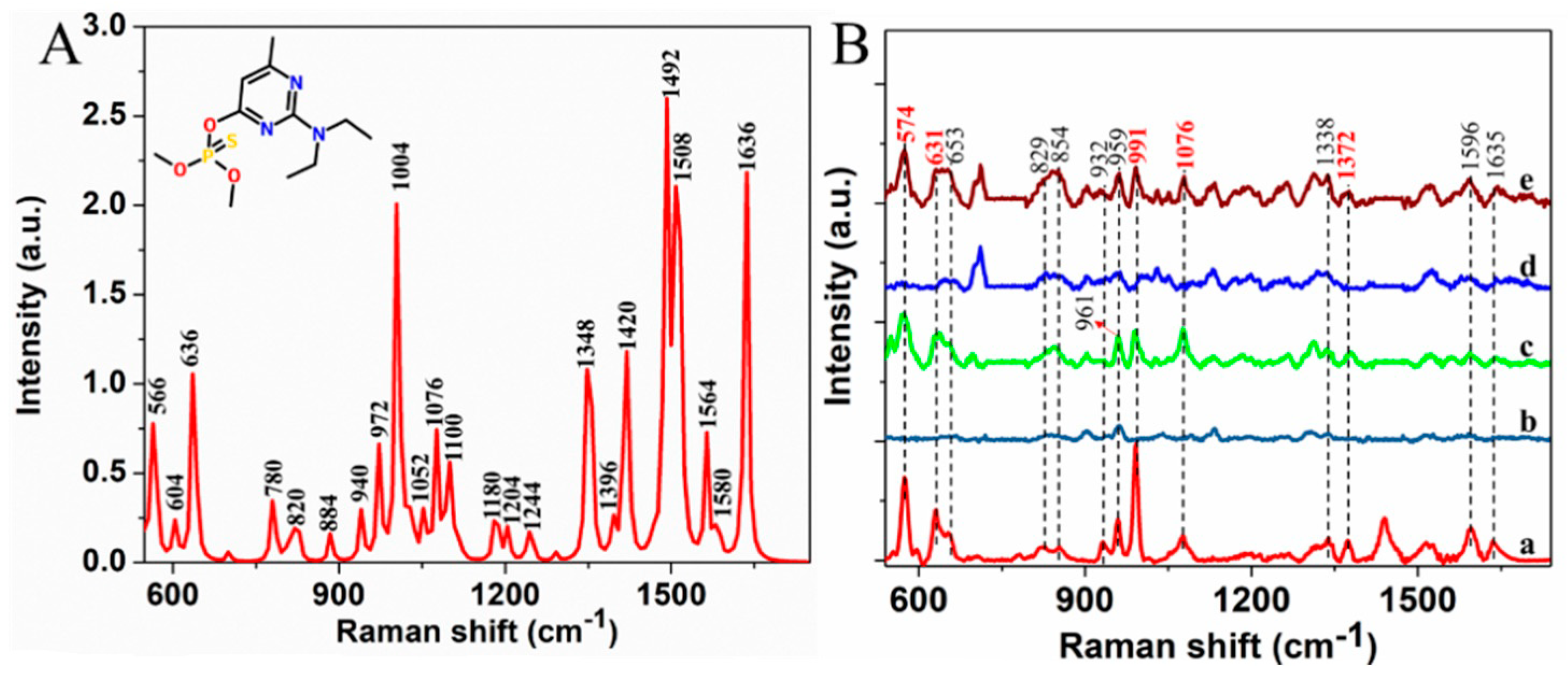

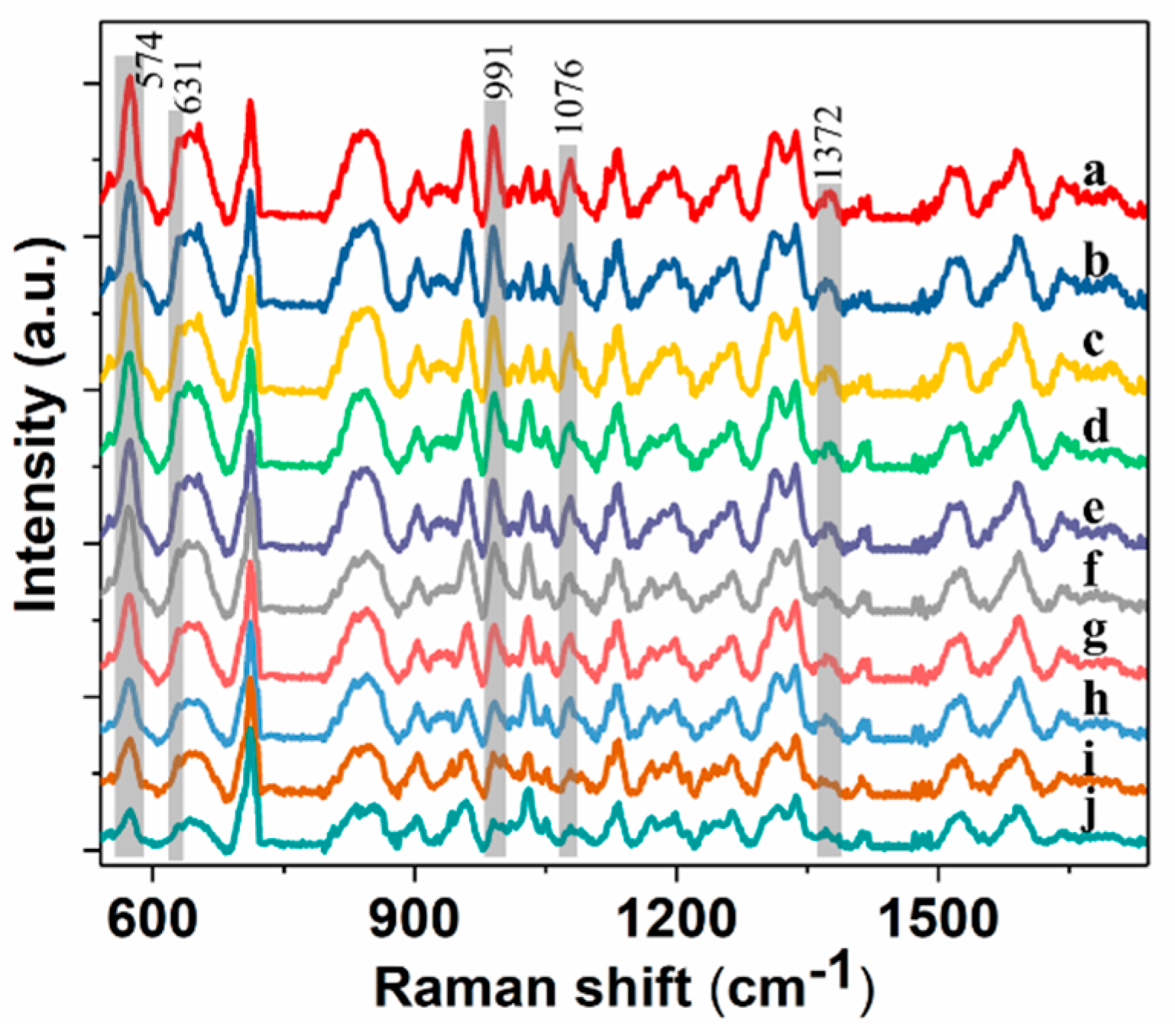
| Calculated Raman Peaks | Experimental Raman Peaks | Assignment |
|---|---|---|
| 566, 604, 780, 820, 884, 972, 1004, 1100, 1180,1348, 1564, 1580, 1636, 1396, 1420 | 574, 653, 829, 854, 959, 992, 1339, 1596, 1635, 1372, 1439 | stretching vibration of pyrimidine ring |
| 636 | 631 | stretching vibration of P=S |
| 940 | 932 | stretching vibration of C−C |
| 1052 | stretching vibration of P−O−CH3 | |
| 1076 | 1076 | stretching vibration of P−O−CH3 |
| 1204 | formation vibration of CH3 | |
| 1244 | asymmetrical stretching vibration of N−CH2 | |
| 1492 | stretching vibration of C−N | |
| 1508 | 1515 | formation vibration of CH3 |
| Methods | Number of Latent Variables | Original Spectra | 1st Derivative | 2nd Derivative | |||
|---|---|---|---|---|---|---|---|
| RMSEC | RMSEP | RMSEC | RMSEP | RMSEC | RMSEP | ||
| PLSR | 23 | 0.3067 | 0.4012 | 0.2502 | 0.3221 | 0.3333 | 0.4109 |
| SVR | 0.0084 | 0.0272 | 0.0086 | 0.0193 | 0.0092 | 0.0359 | |
| RF | 0.1581 | 0.1906 | 0.2192 | 0.3833 | 0.3679 | 0.8653 | |
| PCA+PLSR | 9 | 0.0051 | 0.0096 | 0.0092 | 0.0221 | 0.0053 | 0.0142 |
| PCA+SVR | 0.0094 | 0.0147 | 0.0095 | 0.0157 | 0.0092 | 0.0160 | |
| PCA+RF | 0.6737 | 1.6748 | 0.6225 | 1.6801 | 0.6410 | 1.6641 | |
| Actual Value by GC-MS (mg/L) | Predicted Values by SERS | Relative Deviation (%) | Recovery (%) | Error (mg/L) | |
|---|---|---|---|---|---|
| Mean Value (mg/L) | Standard Deviation (mg/L) | ||||
| 23.93 | 22.71 | 0.112 | 5.10 | 94.90 | 1.22 |
| 23.86 | 22.67 | 0.111 | 4.99 | 95.01 | 1.19 |
| 22.54 | 21.76 | 0.102 | 3.46 | 96.54 | 0.78 |
| 20.34 | 20.36 | 0.105 | 0.10 | 100.10 | −0.02 |
| 18.57 | 17.78 | 0.103 | 4.25 | 95.75 | 0.79 |
| 15.85 | 16.13 | 0.105 | 1.77 | 101.77 | −0.28 |
| 14.43 | 14.31 | 0.103 | 0.83 | 99.17 | 0.12 |
| 13.35 | 13.27 | 0.099 | 0.60 | 99.40 | 0.08 |
| 12.11 | 11.89 | 0.097 | 1.82 | 98.18 | 0.22 |
| 11.79 | 11.34 | 0.095 | 3.82 | 96.18 | 0.45 |
| 11.72 | 11.04 | 0.093 | 5.80 | 94.20 | 0.68 |
| 10.91 | 10.76 | 0.091 | 1.37 | 98.63 | 0.15 |
| 10.47 | 10.37 | 0.090 | 0.96 | 99.04 | 0.1 |
| 10.35 | 10.23 | 0.087 | 1.16 | 98.84 | 0.12 |
| 10.23 | 10.27 | 0.079 | 0.39 | 100.39 | −0.04 |
| 9.05 | 9.65 | 0.074 | 6.63 | 106.63 | −0.6 |
| 9.03 | 8.95 | 0.071 | 0.89 | 99.11 | 0.08 |
| 9.01 | 8.96 | 0.072 | 0.55 | 99.45 | 0.05 |
| 8.76 | 8.35 | 0.068 | 4.68 | 95.32 | 0.41 |
| 7.50 | 7.23 | 0.069 | 3.60 | 96.40 | 0.27 |
| 6.56 | 6.34 | 0.058 | 3.35 | 96.65 | 0.22 |
| 6.13 | 5.92 | 0.059 | 3.43 | 96.57 | 0.21 |
| 5.45 | 5.24 | 0.048 | 3.85 | 96.15 | 0.21 |
| 4.75 | 4.55 | 0.042 | 4.21 | 95.79 | 0.2 |
| 4.56 | 4.34 | 0.041 | 4.82 | 95.18 | 0.22 |
| 3.87 | 3.75 | 0.041 | 3.10 | 96.90 | 0.12 |
| 3.49 | 3.54 | 0.043 | 1.43 | 101.43 | −0.05 |
| 3.31 | 3.18 | 0.042 | 3.93 | 96.07 | 0.13 |
| 3.01 | 2.88 | 0.039 | 4.32 | 95.68 | 0.13 |
| 2.37 | 2.23 | 0.037 | 5.91 | 94.09 | 0.14 |
| 2.47 | 2.36 | 0.036 | 4.45 | 95.55 | 0.11 |
| 1.45 | 1.37 | 0.033 | 5.52 | 94.48 | 0.08 |
| 1.38 | 1.27 | 0.029 | 7.97 | 92.03 | 0.11 |
| 1.36 | 1.37 | 0.031 | 0.74 | 100.74 | −0.01 |
| 1.23 | 1.17 | 0.028 | 4.88 | 95.12 | 0.06 |
| 0.91 | 0.96 | 0.027 | 5.49 | 105.49 | −0.05 |
| 0.79 | 0.75 | 0.34 | 5.06 | 94.94 | 0.04 |
| 0.67 | 0.66 | 0.027 | 1.49 | 98.51 | 0.01 |
| 0.51 | 0.48 | 0.010 | 5.88 | 94.12 | 0.03 |
| 0.25 | 0.24 | 0.011 | 4.00 | 96.00 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, S.; Yu, S.; Dong, R.; Zhao, J.; Liang, D. Detection of Pirimiphos-Methyl in Wheat Using Surface-Enhanced Raman Spectroscopy and Chemometric Methods. Molecules 2019, 24, 1691. https://doi.org/10.3390/molecules24091691
Weng S, Yu S, Dong R, Zhao J, Liang D. Detection of Pirimiphos-Methyl in Wheat Using Surface-Enhanced Raman Spectroscopy and Chemometric Methods. Molecules. 2019; 24(9):1691. https://doi.org/10.3390/molecules24091691
Chicago/Turabian StyleWeng, Shizhuang, Shuan Yu, Ronglu Dong, Jinling Zhao, and Dong Liang. 2019. "Detection of Pirimiphos-Methyl in Wheat Using Surface-Enhanced Raman Spectroscopy and Chemometric Methods" Molecules 24, no. 9: 1691. https://doi.org/10.3390/molecules24091691
APA StyleWeng, S., Yu, S., Dong, R., Zhao, J., & Liang, D. (2019). Detection of Pirimiphos-Methyl in Wheat Using Surface-Enhanced Raman Spectroscopy and Chemometric Methods. Molecules, 24(9), 1691. https://doi.org/10.3390/molecules24091691






