Carbon and Tin-Based Polyacrylonitrile Hybrid Architecture Solid Phase Microextraction Fiber for the Detection and Quantification of Antibiotic Compounds in Aqueous Environmental Systems
Abstract
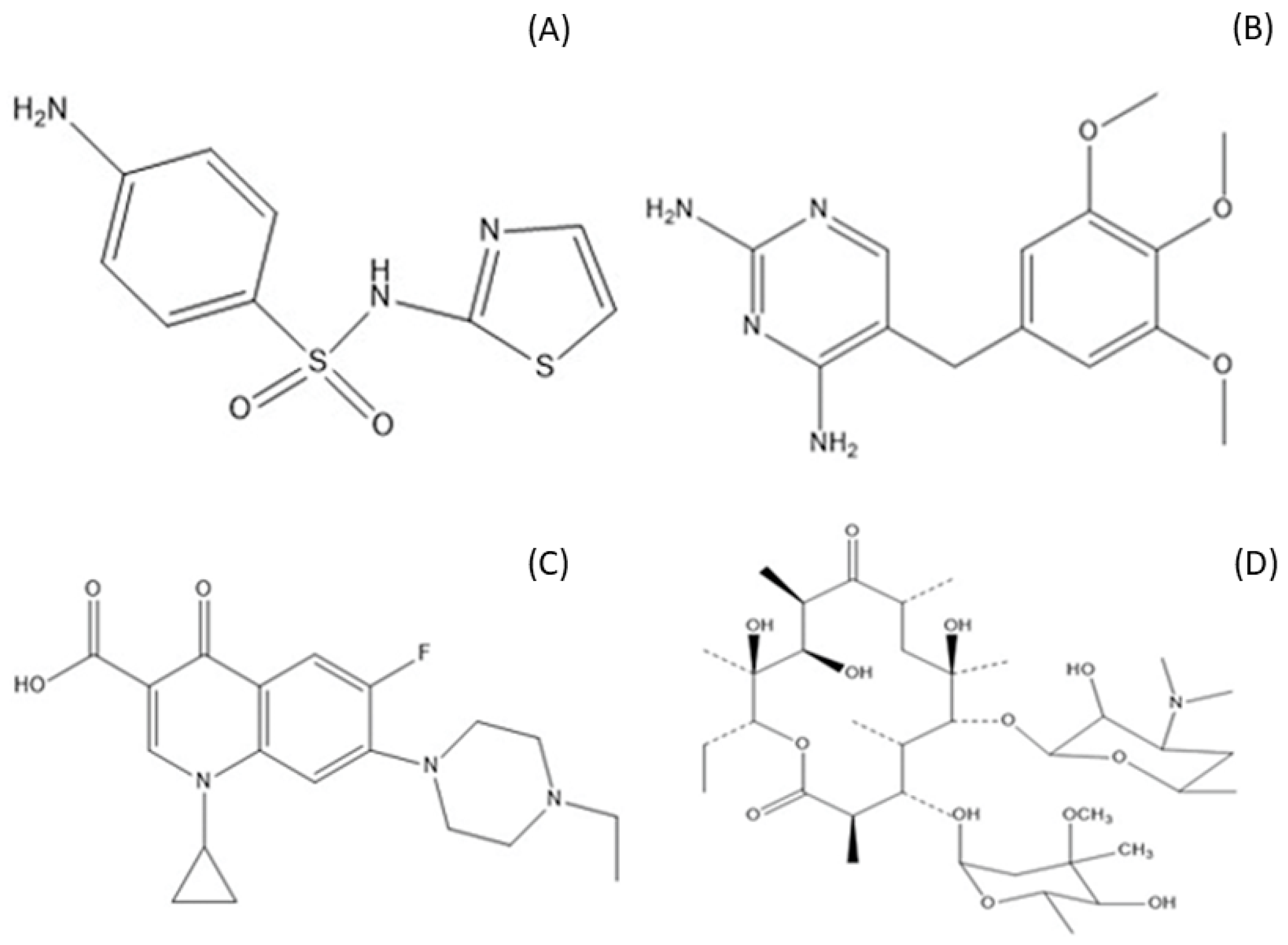
:1. Introduction
2. Results and Discussion
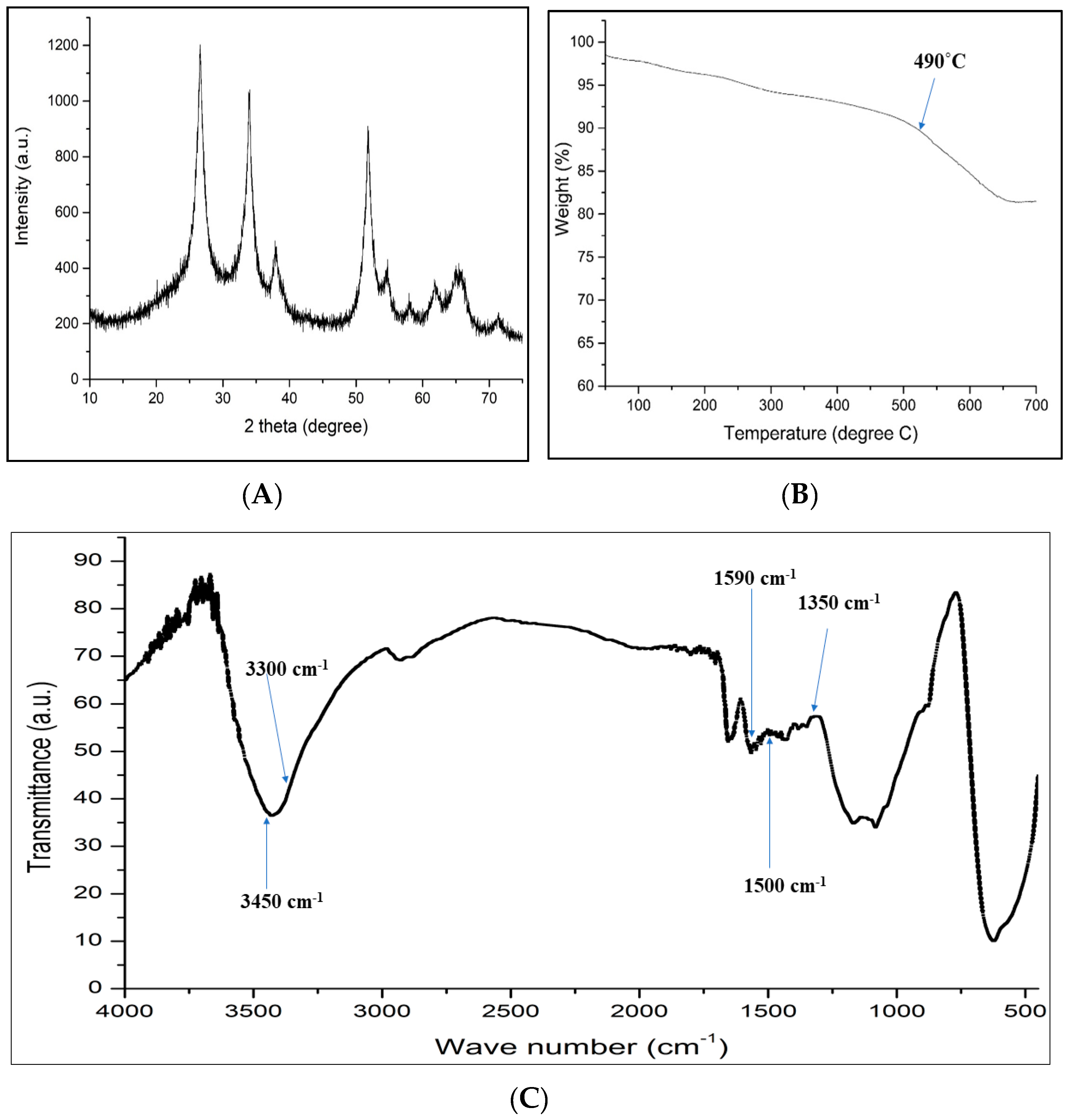
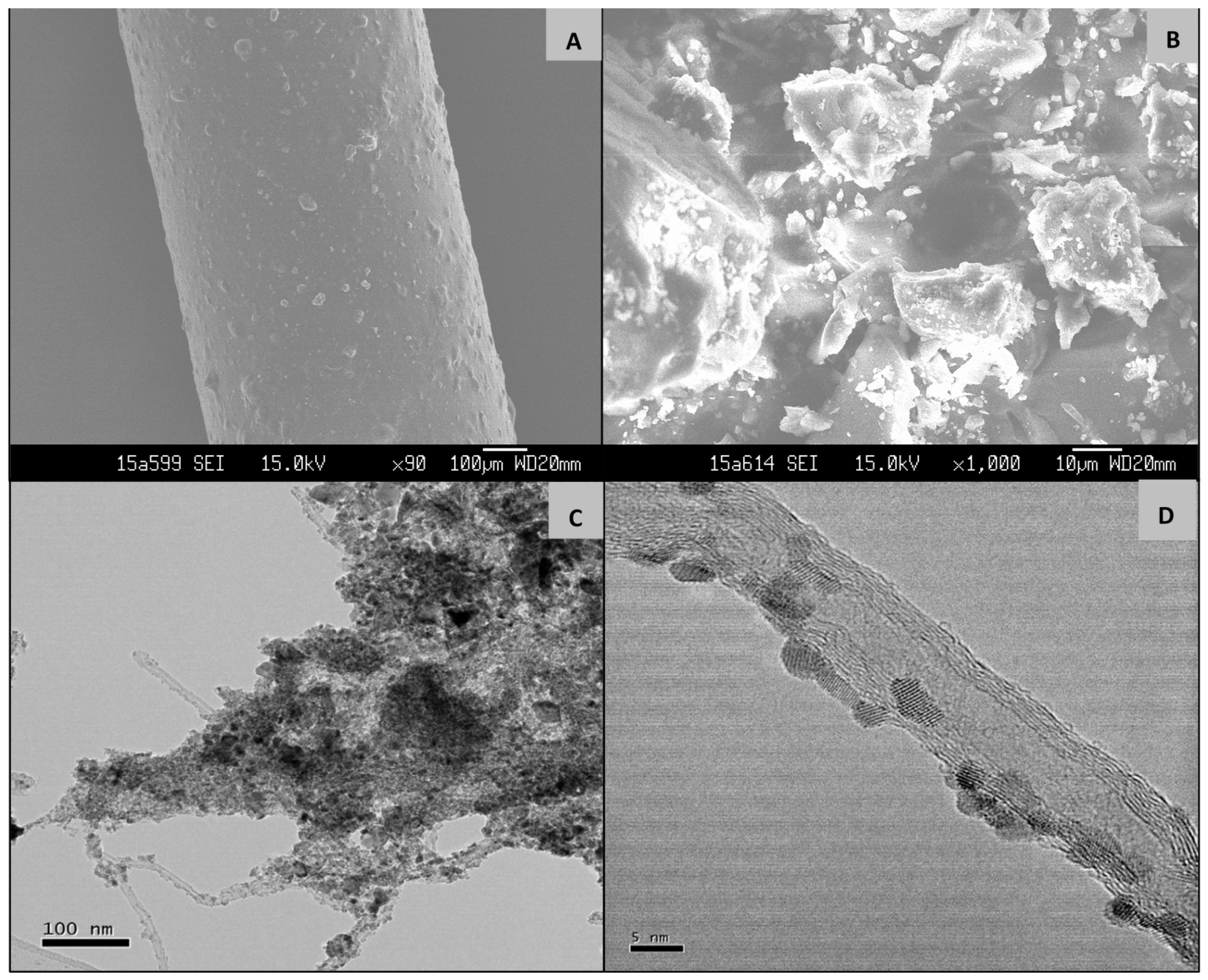
2.1. Characterization of GCT Hybrid Architectures
2.2. Cost Analysis of SPME Fiber Preparation
2.3. Optimization of the GCT-PAN Hybrid Architecture Fiber Coating in Water for Antibiotics (ABs) Extraction
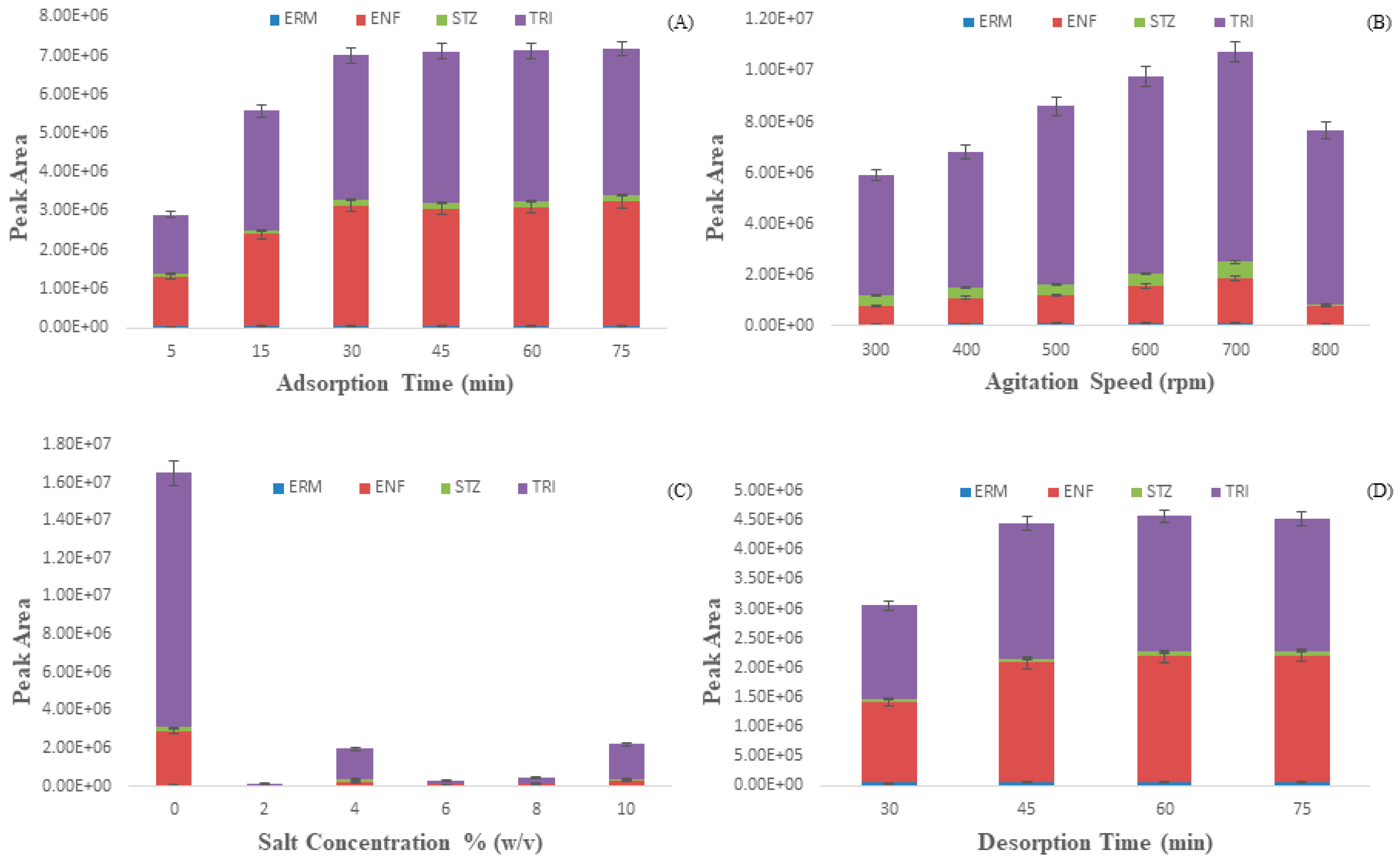
2.3.1. Extraction Time
2.3.2. Agitation Speed
2.3.3. Salt Concentration
2.3.4. Desorption Time and Solvent System
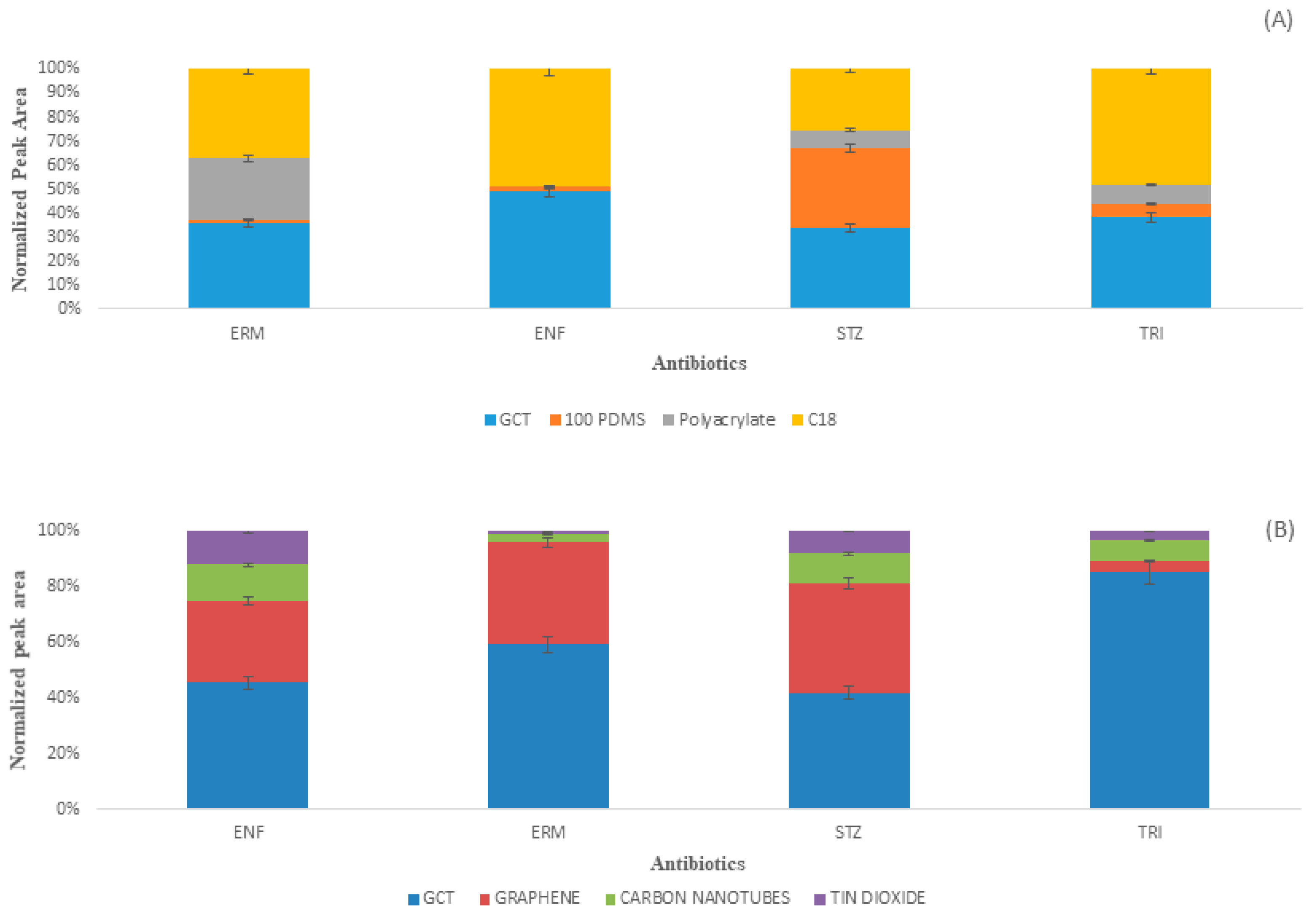
2.4. Comparison with Commercial Fibers
2.5. Evaluation of Method Performance
2.6. Application in Real Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of GCT Hybrid Architectures
3.3. SPME Fiber Preparation Methods
3.4. Instrumentation
3.5. Instrumental Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, B.; Patra, S. Chapter 1-Antimicrobials: Meeting the Challenges of Antibiotic Resistance Through Nanotechnology. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Atlanta, GA, USA, 2017; pp. 1–22. [Google Scholar]
- Aarestrup, F.M. The livestock reservoir for antimicrobial resistance: A personal view on changing patterns of risks, effects of interventions and the way forward. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Zhang, L.; Song, X.; Liu, X.; Yang, L.; Pan, F.; Lv, J. Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem. Eng. J. 2011, 178, 26–33. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Han, S. Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 2014, 4, 5326. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.Y.C.; Peake, B.M.; Braund, R. Disposal practices for unused medications around the world. Environ. Int. 2011, 37, 292–298. [Google Scholar] [CrossRef]
- Ji, K.; Kho, Y.; Park, C.; Paek, D.; Ryu, P.; Paek, D.; Kim, M.; Kim, P.; Choi, K. Influence of water and food consumption on inadvertent antibiotics intake among general population. Environ. Res. 2010, 110, 641–649. [Google Scholar] [CrossRef]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of Persistent Tetracycline Residues in Soil Fertilized with Liquid Manure by High-Performance Liquid Chromatography with Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pal, D. Antibiotic resistance and wastewater: Correlation, impact and critical human health challenges. J. Environ. Chem. Eng. 2018, 6, 52–58. [Google Scholar] [CrossRef]
- Félix, L.M.; Serafim, C.; Valentim, A.M.; Antunes, L.M.; Campos, S.; Matos, M.; Coimbra, A.M. Embryonic Stage-Dependent Teratogenicity of Ketamine in Zebrafish (Danio rerio). Chem. Res. Toxicol. 2016, 29, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.-C.; Hong, Y.-T.; Lee, T.-W.; Huang, J.-F. Facile fabrication of ascorbic acid reduced graphene oxide-modified electrodes toward electroanalytical determination of sulfamethoxazole in aqueous environments. Chem. Eng. J. 2018, 352, 188–197. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, K.Y.; Hamm, S.-Y.; Kim, M.; Kim, H.K.; Oh, J.-E. Occurrence and distribution of pharmaceutical and personal care products, artificial sweeteners, and pesticides in groundwater from an agricultural area in Korea. Sci. Total Environ. 2019, 659, 168–176. [Google Scholar] [CrossRef]
- Xie, H.; Wang, X.; Chen, J.; Li, X.; Jia, G.; Zou, Y.; Zhang, Y.; Cui, Y. Occurrence, distribution and ecological risks of antibiotics and pesticides in coastal waters around Liaodong Peninsula, China. Sci. Total Environ. 2019, 656, 946–951. [Google Scholar] [CrossRef]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.-L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Jia, W.; Chu, X.; Chang, J.; Wang, P.G.; Chen, Y.; Zhang, F. High-throughput untargeted screening of veterinary drug residues and metabolites in tilapia using high resolution orbitrap mass spectrometry. Anal. Chim. Acta 2017, 957, 29–39. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to effective antimicrobials: a worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Igarashi, K.; Ishitsuka, H.; Kaji, A. Comparative studies on the mechanism of action of lincomycin, streptomycin, and erythromycin. Biochem. Biophys. Res. Commun. 1969, 37, 499–504. [Google Scholar] [CrossRef]
- Wisell, K.T.; Kahlmeter, G.; Giske, C.G. Trimethoprim and enterococci in urinary tract infections: New perspectives on an old issue. J. Antimicrob. Chemoth. 2008, 62, 35–40. [Google Scholar] [CrossRef]
- Papich, M.G. Enrofloxacin. In Saunders Handbook of Veterinary Drugs, 4th ed.; Papich, M.G., Ed.; W.B. Saunders: St. Louis, MO, USA, 2016; pp. 287–289. [Google Scholar]
- Leston, S.; Nebot, C.; Nunes, M.; Cepeda, A.; Pardal, M.Â.; Ramos, F. Sulfathiazole: Analytical methods for quantification in seawater and macroalgae. Environ. Toxicol. Pharmacol. 2015, 39, 77–84. [Google Scholar] [CrossRef]
- Ide, A.H.; Ahmad, S.M.; Neng, N.R.; Nogueira, J.M.F. Enhancement for trace analysis of sulfonamide antibiotics in water matrices using bar adsorptive microextraction (BAμE). J. Pharm. Biomed. Anal. 2016, 129, 593–599. [Google Scholar] [CrossRef]
- Moema, D.; Nindi, M.M.; Dube, S. Development of a dispersive liquid–liquid microextraction method for the determination of fluoroquinolones in chicken liver by high performance liquid chromatography. Anal. Chimi. Acta 2012, 730, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Leung, D.; Lenz, S.P. Determination of Five Macrolide Antibiotic Residues in Raw Milk Using Liquid Chromatography−Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, C.; Wang, Y.; Guo, Y.; Huang, P.; Zhao, L. Simultaneous determination of 12 pharmaceuticals in water samples by ultrasound-assisted dispersive liquid–liquid microextraction coupled with ultra-high performance liquid chromatography with tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 8099–8109. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ying, J.; Chen, H.; Huang, J.; Liao, L. LC Determination of Chloramphenicol in Honey Using Dispersive Liquid–Liquid Microextraction. Chromatographia 2008, 68, 629–634. [Google Scholar] [CrossRef]
- Choi, K.-J.; Kim, S.-G.; Kim, C.-W.; Kim, S.-H. Determination of antibiotic compounds in water by on-line SPE-LC/MSD. Chemosphere 2007, 66, 977–984. [Google Scholar] [CrossRef]
- Xu, J.-J.; An, M.; Yang, R.; Tan, Z.; Hao, J.; Cao, J.; Peng, L.-Q.; Cao, W. Determination of Tetracycline Antibiotic Residues in Honey and Milk by Miniaturized Solid Phase Extraction Using Chitosan-Modified Graphitized Multiwalled Carbon Nanotubes. J. Agric. Food Chem. 2016, 64, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Xu, J.; Chen, G.; Huang, S.; Huang, C.; Yin, L.; Ouyang, G. Solid-phase microextraction of antibiotics from fish muscle by using MIL-101(Cr)NH2-polyacrylonitrile fiber and their identification by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2019, 1047, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, V.K.; Terry, K.A.; Toito, J. Determination of sulfonamide antibiotics in wastewater: A comparison of solid phase microextraction and solid phase extraction methods. J. Chromatogr. A 2006, 1131, 1–10. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, S.; Chen, H. Determination of tetracycline antibiotics in fatty food samples by selective pressurized liquid extraction coupled with high-performance liquid chromatography and tandem mass spectrometry. J. Sep. Sci. 2015, 38, 115–120. [Google Scholar] [CrossRef]
- Juan, C.; Moltó, J.C.; Mañes, J.; Font, G. Determination of macrolide and lincosamide antibiotics by pressurised liquid extraction and liquid chromatography-tandem mass spectrometry in meat and milk. Food Control 2010, 21, 1703–1709. [Google Scholar] [CrossRef]
- Li, M.; Sun, Q.; Li, Y.; Lv, M.; Lin, L.; Wu, Y.; Ashfaq, M.; Yu, C.-P. Simultaneous analysis of 45 pharmaceuticals and personal care products in sludge by matrix solid-phase dispersion and liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 4953–4964. [Google Scholar] [CrossRef]
- García-Mayor, M.A.; Gallego-Picó, A.; Garcinuño, R.M.; Fernández-Hernando, P.; Durand-Alegría, J.S. Matrix solid-phase dispersion method for the determination of macrolide antibiotics in sheep’s milk. Food Chem. 2012, 134, 553–558. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Pu, C.; Chen, J.; Sun, Y.; Hu, L. Determination of multiple antibiotics in leafy vegetables using QuEChERS–UHPLC–MS/MS. J. Sep. Sci. 2018, 41, 713–722. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, Y.; Xu, Y.; Liu, X. Determination of Antibiotics in Vegetables Using QuEChERS-Based Method and Liquid Chromatography-Quadrupole Linear Ion Trap Mass Spectrometry. Food Anal. Methods 2018, 11, 2857–2864. [Google Scholar] [CrossRef]
- Katsnelson, M.I. Graphene: carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Gaskov, A. Selectivity of Catalytically Modified Tin Dioxide to CO and NH3 Gas Mixtures. Chemosensors 2015, 3, 241–252. [Google Scholar]
- Zhao, H.; Liu, X.; Cao, Z.; Zhan, Y.; Shi, X.; Yang, Y.; Zhou, J.; Xu, J. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J. Hazard. Mater. 2016, 310, 235–245. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, J.; Xu, J.; Fang, X.a.; Liu, Y.; Liu, S.; Wei, S.; Jiang, R.; Luan, T.; Zeng, F.; et al. A novel probe based on phenylboronic acid functionalized carbon nanotubes for ultrasensitive carbohydrate determination in biofluids and semi-solid biotissues. Chem. Sci. 2016, 7, 1487–1495. [Google Scholar] [CrossRef]
- Vuckovic, D.; Risticevic, S.; Pawliszyn, J. In Vivo Solid-Phase Microextraction in Metabolomics: Opportunities for the Direct Investigation of Biological Systems. Angew. Chem. Int. Ed. 2011, 50, 5618–5628. [Google Scholar] [CrossRef]
- Vuckovic, D.; Pawliszyn, J. Systematic Evaluation of Solid-Phase Microextraction Coatings for Untargeted Metabolomic Profiling of Biological Fluids by Liquid Chromatography−Mass Spectrometry. Anal. Chem. 2011, 83, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Cudjoe, E.; Bojko, B.; de Lannoy, I.; Saldivia, V.; Pawliszyn, J. Solid-Phase Microextraction: A Complementary In Vivo Sampling Method to Microdialysis. Angew. Chem. Int. Ed. 2013, 52, 12124–12126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, L.; Xiao, J.; Xiao, F.; Wang, S. One-Pot Synthesis of Three-Dimensional Graphene/Carbon Nanotube/SnO2 Hybrid Architectures with Enhanced Lithium Storage Properties. ACS Appl. Mater. Interfaces 2015, 7, 17963–17968. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Aikat, K.; Halder, G. Biosorptive uptake of ibuprofen by chemically modified Parthenium hysterophorus derived biochar: Equilibrium, kinetics, thermodynamics and modeling. Ecol. Eng. 2016, 92, 158–172. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, W.-R.; Jiao, X.-Y.; Zhao, R.-S.; Wang, M.-L.; Lin, J.-M. Zinc(II)-based metal–organic nanotubes coating for high sensitive solid phase microextraction of nitro-polycyclic aromatic hydrocarbons. Talanta 2018, 186, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Huang, P.; Ma, X.; Lu, X.; Du, X. Preparation of three-dimensional mesoporous polymer in situ polymerization solid phase microextraction fiber and its application to the determination of seven chlorophenols. J. Chromatogr. A 2017, 1479, 40–47. [Google Scholar] [CrossRef]
- Grundl, G.; Müller, M.; Touraud, D.; Kunz, W. Salting-out and salting-in effects of organic compounds and applications of the salting-out effect of Pentasodium phytate in different extraction processes. J. Mol. Liq. 2017, 236, 368–375. [Google Scholar] [CrossRef]
- Gao, J.; Huang, C.; Lin, Y.; Tong, P.; Zhang, L. In situ solvothermal synthesis of metal–organic framework coated fiber for highly sensitive solid-phase microextraction of polycyclic aromatic hydrocarbons. J. Chromatogr. A 2016, 1436, 1–8. [Google Scholar] [CrossRef]
- Chen, J.; Pawliszyn, J.B. Solid Phase Microextraction Coupled to High-Performance Liquid Chromatography. Anal. Chem. 1995, 67, 2530–2533. [Google Scholar] [CrossRef]
- Jinno, K.; Taniguchi, M.; Hayashida, M. Solid phase micro extraction coupled with semi-microcolumn high performance liquid chromatography for the analysis of benzodiazepines in human urine. J. Pharm. Biomed. Anal. 1998, 17, 1081–1091. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Chen, L.; Qiu, J.; Liu, Y.; Ouyang, G. Rapid in vivo determination of fluoroquinolones in cultured puffer fish (Takifugu obscurus) muscle by solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. Talanta 2017, 175, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ben Aissa, S.; Sherazi, A.T.; Catanante, G.; Hayat, A.; Marty, L.J. Development of an Impedimetric Aptasensor for Label Free Detection of Patulin in Apple Juice. Molecules 2019, 24, 1017. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds GCT are available from the authors. |

| Antibiotics | Linear Range (ng L−1) | R2 | LOD (ng L−1) S/N = 3 | LOQ (ng L−1) S/N = 10 | Intra-Fiber RSD% | Inter-Fiber (n = 6) RSD% |
|---|---|---|---|---|---|---|
| ENF | 20–50000 | 0.999 | 6.6 | 12.2 | 5.1 | 6.1 |
| STZ | 20–50000 | 0.997 | 7.69 | 15.6 | 6.8 | 8.8 |
| ETM | 10–50000 | 0.996 | 1.36 | 4.54 | 2.8 | 4.6 |
| TMP | 10–50000 | 0.998 | 0.9 | 3.02 | 1.8 | 4.5 |
| River Water | ||||
| Antibiotics | Detected Conc. (ng/L) | Spiked Conc. (ng L−1) | Recovery | RSD% |
| ENF | 46 | 100 200 | 92.14 89.38 | 9.8 8.2 |
| STZ | 19.3 | 100 200 | 89.3 82.1 | 7.3 4.6 |
| ERM | 47.2 | 100 200 | 113.3 104.5 | 5.8 4.3 |
| TMP | 12.93 | 100 200 | 89.4 89.6 | 4.6 4.8 |
| Pond Water | ||||
| Detected Conc. (ng/L) | Spiked Conc. (ng L−1) | Recovery | RSD% | |
| ENF | 9.2 | 100 200 | 85.57 87.3 | 7.9 6.9 |
| STZ | 13.42 | 100 200 | 82.7 81.5 | 4.9 6.3 |
| ERM | 33.2 | 100 200 | 94.45 91.68 | 7.9 7.2 |
| TMP | 7.29 | 100 200 | 90.7 90.4 | 4.1 5.2 |
| Tap Water | ||||
| Detected Conc. (ng/L) | Spiked Conc. (ng L−1) | Recovery | RSD% | |
| ENF | Not detected | 100 200 | 84.86 82.95 | 9.7 10.2 |
| STZ | Not detected | 100 200 | 84.40 80.34 | 8.6 8.3 |
| ERM | 29.5 | 100 200 | 95.16 89.39 | 4.2 4.6 |
| TMP | 6.26 | 100 200 | 91.46 90.24 | 3.6 4.3 |
| Antibiotics | Molecular Weight | Q1 Mass (Da) | Q3 Mass (Da) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Enrofloxacin | 359.39 | 360.1 | 244.8 | 116 | 11 | 33 | 17 |
| Sulfathiazole | 255.32 | 255.9 | 107.8 | 64 | 12 | 36 | 14 |
| Erythromycin | 733.93 | 734.6 | 576.3 | 164 | 12 | 29 | 30 |
| Trimethoprim | 290.32 | 291 | 230 | 70 | 7 | 34 | 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, S.; Jiang, J.; Li, Y.; Ouyang, G. Carbon and Tin-Based Polyacrylonitrile Hybrid Architecture Solid Phase Microextraction Fiber for the Detection and Quantification of Antibiotic Compounds in Aqueous Environmental Systems. Molecules 2019, 24, 1670. https://doi.org/10.3390/molecules24091670
Mondal S, Jiang J, Li Y, Ouyang G. Carbon and Tin-Based Polyacrylonitrile Hybrid Architecture Solid Phase Microextraction Fiber for the Detection and Quantification of Antibiotic Compounds in Aqueous Environmental Systems. Molecules. 2019; 24(9):1670. https://doi.org/10.3390/molecules24091670
Chicago/Turabian StyleMondal, Sandip, Jialing Jiang, Yin Li, and Gangfeng Ouyang. 2019. "Carbon and Tin-Based Polyacrylonitrile Hybrid Architecture Solid Phase Microextraction Fiber for the Detection and Quantification of Antibiotic Compounds in Aqueous Environmental Systems" Molecules 24, no. 9: 1670. https://doi.org/10.3390/molecules24091670
APA StyleMondal, S., Jiang, J., Li, Y., & Ouyang, G. (2019). Carbon and Tin-Based Polyacrylonitrile Hybrid Architecture Solid Phase Microextraction Fiber for the Detection and Quantification of Antibiotic Compounds in Aqueous Environmental Systems. Molecules, 24(9), 1670. https://doi.org/10.3390/molecules24091670





