…Fell Upas Sits, the Hydra-Tree of Death †, or the Phytotoxicity of Trees
Abstract
1. Introduction
2. Allelopathic Tree Species
2.1. Ailanthus Altissima
2.2. Eucalyptus
2.3. Fabaceae
2.4. Juglandaceae
2.5. Other Decidious Trees
2.6. Gymnosperm Species
3. Allelochemicals in Trees
3.1. Ailanthone
3.2. Juglone
3.3. Mimosine
3.4. Allelochemicals in the Acacia Species
3.5. Allelochemicals in the Eucalyptus Species
3.6. Allelochemicals in Other Deciduous Species
3.7. Allelochemicals in Conifers
4. Biosynthesis of Allelochemicals
4.1. Juglone Biosynthesis
4.2. Mimosine Biosynthesis
4.3. Terpenoid Biosynthesis
5. Natural Biopesticides Originated from Trees
5.1. Allelochemicals as Herbicides
5.2. Allelochemicals as Insecticides
5.3. Development of New Biopesticides
6. Transgenic Trees and Allelopathy
7. Conclusions
Funding
Conflicts of Interest
References
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Torres, A.; Oliva, R.M.; Castellano, D.; Cross, P. First World Congress on Allelopathy. A Science of the Future; SAI, University of Cadiz: Cadiz, Spain, 1996; p. 278. [Google Scholar]
- Reigosa, M.S.; Gonzalesy, L.; Souto, X.C.; Pastoriza, J.E. Allelopathy in forest ecosystem. In Allelopathy in Ecological Agriculture and Forestry; Narwal, S.S., Hoagland, R.E., Dilday, R.H., Reigosa, M.J., Eds.; Kluwer Academic Pubishersl: Dordrecht, The Netherlands, 2000; pp. 183–193. [Google Scholar]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate change effects on secondary compounds of forest trees in the Northern Hemisphere. Front. Plant Sci. 2018, 9, 1445. [Google Scholar] [CrossRef]
- Cummings, J.A.; Parker, I.M.; Gilbert, G.S. Allelopathy: A tool for weed management in forest restoration. Plant Ecol. 2012, 213, 1975–1989. [Google Scholar] [CrossRef]
- Reigosa, M.J. Estudio del Potencial Alelopático de Acacia dealbata Link. Ph.D. Thesis, University of Santiago de Compostela, Santiago de Compostela, Spain, October 1988. [Google Scholar]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowska, A. Allelochemicals as Bioherbicides—Present and Perspectives. In Herbicides—Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Pazos-Malvido, E. Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J. Chem. Ecol. 2007, 33, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Reigosa, M.J.; Sánchez-Moreiras, A.; González, L. Ecophysiological approach in allelopathy. Crit. Rev. Plant Sci. 1999, 18, 577–608. [Google Scholar] [CrossRef]
- Chick, T.A.; Kielbaso, J.J. Allelopathy as an inhibition factor in ornamental tree growth: Implications from the literature. J. Arboricult. 1998, 24, 274–279. [Google Scholar]
- Coder, K.D.; Warnell, B.D. Potential Allelopathy in Different Tree Species; University of Georgia School of Forest Resources: Athens, Greece, 1999. [Google Scholar]
- Sladonja, B.; Susek, M.; Guillermic, J. Review on invasive tree of heaven (Ailanthus altissima (Mill.) Swingle) conflicting values: Assessment of its ecosystem services and potential biological threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef]
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying threats to imperiled species in the United States. BioSci. 1998, 48, 607–615. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, M.; Chen, X.; Qu, B. Review on allelopathy of exotic invasive plants. Proc. Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: A biochemically based hypothesis for invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Rabotnov, T.A. On the allelopathy in the phytocenoses. Izv Akad Nauk SSSR Ser. Biol. 1974, 6, 811–820. [Google Scholar]
- Blanco, J.A. The representation of allelopathy in ecosystem-level forest models. Ecol. Modelling 2007, 209, 65–77. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Global Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Autotoxicity: Concept, organisms, and ecological significance. Crit. Rev. Plant Sci. 1999, 18, 757–772. [Google Scholar] [CrossRef]
- Chou, C.H. The role of allelopathy in phytochemical ecology. In Phytochemical Ecology: Allelochemicals, Mycotoxins and Insect Pheromones and Allomones; Chou, C.H., Waller, G.R., Eds.; Institute of Botany, Academia Sinica Monograph Series No. 9; Institute of Botany, Academia Sinica: Taipei, Taiwan, 1989; pp. 19–38. [Google Scholar]
- Dayan, F.E.; Duke, S.O. Biological activity of allelochemicals. In Plant-Derived Natural Products: Synthesis, Function, and Application; Osbourn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 361–384. [Google Scholar] [CrossRef]
- Cna’ani, A.; Shavit, R.; Ravid, J.; Aravena-Calvo, J.; Skaliter, O.; Masci, T.; Vainstein, A. Phenylpropanoid scent compounds in Petunia × hybrida are glycosylated and accumulate in vacuoles. Front. Plant Sci. 2017, 8, 1898. [Google Scholar] [CrossRef]
- Müller, W.-U.; Leistner, E. Metabolic relation between naphthalene derivatives in Juglans. Phytochemistry 1978, 17, 1735–1738. [Google Scholar] [CrossRef]
- Smith, I.K.; Fowden, L. A study of mimosine toxicity in plants. J. Exp. Bot. 1966, 17, 750–761. [Google Scholar] [CrossRef]
- Weston, L.A.; Inderjit. Allelopathy: A potential tool in the development of strategies for biorational weed management. In Non-Chemical Weed Management: Principles, Concepts, and Technology; Upadhyaya, M.K., Blackshaw, R.E., Eds.; CABI: Wallingford, UK, 2007; pp. 65–76. [Google Scholar]
- Orcutt, D.M.; Nilsen, E.T. The Physiology of Plants under Stress Soil and Biotic Factors; John Wiley and Sons Inc.: New York, NY, USA, 2000; p. 680. [Google Scholar]
- Metlen, K.L.; Aschehoug, E.T.; Callaway, R.M. Plant behavioural ecology: Dynamic plasticity in secondary metabolites. Plant Cell Environ. 2009, 32, 641–653. [Google Scholar] [CrossRef]
- Kovarik, I.; Säumel, I. Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. Evolut. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Knapp, L.B.; Canham, C.D. Invasion of an old-growth forest in New York by Ailanthus altissima: Sapling growth and recruitment in canopy gaps. J. Torrey Bot. Soc. 2000, 127, 307–315. [Google Scholar] [CrossRef]
- Landenberger, R.E.; Kota, N.L.; McGraw, J.B. Seed dispersal of the non-native invasive tree Ailanthus altissima into contrasting environments. Plant Ecol. 2007, 192, 55–70. [Google Scholar] [CrossRef]
- De Feo, V.; De Martino, L.; Quaranta, E.; Pizza, C. Isolation of phytotoxic compounds from tree-of-heaven (Ailanthus altissima Swingle). J. Agric. Food Chem. 2003, 51, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Mergen, F. A toxic principle in the leaves of Ailanthus. Bot. Gaz. 1959, 121, 32–36. [Google Scholar] [CrossRef]
- Heisey, R.M. Evidence for allelopathy by tree of heaven (Ailanthus altissima). J. Chem. Ecol. 1990, 16, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.G.; Colwell, A.; Sexton, O.J. The Ecological impact of allelopathy in Ailanthus altissima (Simaroubaceae). Am. J. Bot. 1991, 78, 948–958. [Google Scholar] [CrossRef]
- Novak, N.; Novak, M.; Barić, K.; Šćepanović, M.; Ivić, D. Allelopathic potential of segetal and ruderal invasive alien plants. J. Cent. Eur. Agric. 2018, 19, 408–422. [Google Scholar] [CrossRef]
- Albouchi, F.; Hassen, I.; Casabianca, H.; Hosni, K. Phytochemicals, antioxidant, antimicrobial and phytotoxic activities of Ailanthus altissima (Mill.) Swingle leaves. S. Afr. J. Bot. 2013, 87, 164–174. [Google Scholar] [CrossRef]
- Meng, P.; Pei, H.; Hu, W.; Liu, Z.; Li, X.; Xu, H. Allelopathic effects of Ailanthus altissima extracts on Microcystis aeruginosa growth, physiological changes and microcystins release. Chemosphere 2015, 141, 219–226. [Google Scholar] [CrossRef]
- Small, C.J.; White, D.C.; Breanna Hargbol, B. Allelopathic influences of the invasive Ailanthus altissima on a native and a non-native herb. J. Torrey Botan. Soc. 2010, 137, 366–372. [Google Scholar] [CrossRef]
- Gomez-Aparicio, L.; Canham, C.D. Neighbourhood analyses of the allelopathic effects of the invasive tree Ailanthus altissima in temperate forests. J. Ecol. 2008, 96, 447–458. [Google Scholar] [CrossRef]
- Motard, E.; Dusz, S.; Geslin, B.; Akpa-Vinceslas, M.; Hignard, C.; Babiar, O.; Clair-Maczulajtys, D.; Michel-Salzat, A. How invasion by Ailanthus altissima transforms soil and litter communities in a temperate forest ecosystem. Biol. Invasions 2015. [Google Scholar] [CrossRef]
- Wu, J.; Fan, H.; Liu, W.; Huang, G.; Tang, J.; Zeng, R. Should exotic Eucalyptus be planted in subtropical China: Insights from understory plant diversity in two contrasting Eucalyptus chronosequences. Environ. Manage. 2015, 56, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Lerner, H.R.; Evenari, M. The nature of the germination inhibitor present in the leaves of Eucalyptus rostrata. Physiologia Plantarum 1961, 14, 221–229. [Google Scholar] [CrossRef]
- Del Moral, R.; Muller, C.H. Fog drip: A mechanism for toxin transport in Eucalyptus globulus. Bullet. Torrey Botan. Club 1969, 96, 467–475. [Google Scholar] [CrossRef]
- May, E.F.; Ash, E.J. An assessment of the allelopathic potential of Eucalyptus. Aust. J. Bot. 1990, 38, 245–254. [Google Scholar] [CrossRef]
- Babu, R.C.; Kandasamy, O.S. Allelopathic effect of Eucalyptus globulus Lahill. on Cyperus rotundus L. and Cynodon dactylon L. Pers. J. Agron. Crop Sci. 1997, 179, 123–126. [Google Scholar] [CrossRef]
- Ahmed, R.; Rafiqul Hoque, A.T.M.; Hossain, M.K. Allelopathic effects of Leucaena leucocephala leaf litter on some forest and agricultural crops grown in nursery. J. Forestry Res. 2008, 19, 298–302. [Google Scholar] [CrossRef]
- Fang, B.; Yu, S.; Wang, Y.; Qiu, X.; Cai, C.; Liu, S. Allelopathic effects of Eucalyptus urophylla on ten tree species in south China. Agroforest Syst. 2009, 76, 401–408. [Google Scholar] [CrossRef]
- Ghnaya, A.D.; Hamrouni, L.; Amri, I.; Ahoues, H.; Hanana, M.; Romane, A. Study of allelopathic effects of Eucalyptus erythrocorys L. crude extracts against germination and seedling growth of weeds and wheat. Nat. Product Res. 2016, 30, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zheng, Z.; Zhang, J.; Roger, S.-F.; Luo, X. Evaluation of the use of eucalyptus to control algae bloom and improve water quality. Sci. Total Environ. 2019, 667, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, J.; Yang, W.; Wu, F. Potential allelopathic effect of Eucalyptus grandis across a range of plantation ages. Ecol. Res. 2010, 25, 13–23. [Google Scholar] [CrossRef]
- Padhy, B.; Pattnaik, P.K.; Tripathy, A.K. Allelopathic potential of Eucalyptus leaf litter- leachate on the germination and seedling growth of finger-millet. Allelopathy J. 2000, 7, 69–78. [Google Scholar]
- Niakan, M.; Saberi, K. Effects of Eucalyptus allelopathy on growth characters and antioxidant enzymes activity in Phalaris weed. Asian J. Plant Sci. 2009, 8, 440–446. [Google Scholar] [CrossRef][Green Version]
- Silva, E.R.; Lazarotto, D.C.; Schwambach, J.; Overbeck, G.E.; Geraldo, L.G.; Soares, G.L.G. Phytotoxic effects of extract and essential oil of Eucalyptus saligna (Myrtaceae) leaf litter on grassland species. Austral. J. Botan. 2017, 65, 172–182. [Google Scholar] [CrossRef]
- Morsi, M.M.; Abdelmigid, H.M. Allelopathic activity of Eucalyptus globulus leaf aqueous extract on Hordeum vulgare growth and cytogenetic behavior. AJCS 2016, 10, 1551–1556. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M. Cytotoxic and molecular impacts of allelopathic effects of leaf residues of Eucalyptus globulus on soybean (Glycine max). J. Genet. Engineer. Biotechnol. 2017, 15, 297–302. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Vanclay, J.K. Mixed-species plantations of Eucalyptus with nitrogen fixing trees: A review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, S. Allelopathic effects of eucalyptus and the establishment of mixed stands of eucalyptus and native species. For. Ecol. Manag. 2009, 258, 1391–1396. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Chen, Y.; Zhao, J.; Wan, S.; Lin, Y.; Fu, S. Effects of eucalyptus litter and roots on the establishment of native tree species in eucalyptus plantations in South China. For. Ecol. Manag. 2016, 375, 76–83. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, S. Allelopathic effects of leaf litter and live roots exudates of Eucalyptus species on crops. Allelopathy J. 2010, 26, 91–100. [Google Scholar]
- Ahmed, R.; Alam, M.S.; Ahmed, F.U.; Hossain, M.K. Assaying the allelopathic effects of Eucalyptus camaldulensis in a nursery bed incorporated with leaf litter. J. For. Res. 2017, 29. [Google Scholar] [CrossRef]
- Da Silva, E.R.; da Silveira, L.H.R.; Gerhard, E.; Overbeck, G.E.; Soares, G.L.G. Inhibitory effects of Eucalyptus saligna leaf litter on grassland species: Physical versus chemical factors. Plant Ecol. Div. 2018, 11, 55–67. [Google Scholar] [CrossRef]
- Chu, C.; Mortimer, P.E.; Wang, H.; Wanga, Y.; Liu, X.; Yu, S. Allelopathic effects of Eucalyptus on native and introduced tree species. For. Ecol. Manag. 2014, 323, 79–84. [Google Scholar] [CrossRef]
- Qin, F.; Liu, S.; Yu, S. Effects of allelopathy and competition for water and nutrients on survival and growth of tree species in Eucalyptus urophylla plantations. For. Ecol. Manag. 2018, 424, 387–395. [Google Scholar] [CrossRef]
- Lorenzo, P.; Gonzalez, L.; Reigosa, M.J. The genus Acacia as invader: The characteristic case of Acacia dealbata Link in Europe. Ann. For. Sci. 2010, 67, 101. [Google Scholar] [CrossRef]
- Sheppard, A.W.; Shaw, R.H.; Sforza, R. Top 20 environmental weeds for classical biological control in Europe: A review of opportunities, regulations and other barriers to adoption. Weed Res. 2006, 46, 93–117. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Casal, J.F.; Carballeira, A. Efectos alelopaticos de Acacia dealbata Link durante su floracibn. Stud. Oecol. 1984, 5, 135–150. [Google Scholar]
- Carballeira, A.; Reigosa, M.J. Effects of natural leachates of Acacia dealbata link in Galicia (NW Spain). Bot. Bull. Acad. Sinica. 1999, 40, 87–92. [Google Scholar]
- Lorenzo, P.; Pazos-Malvido, E.; Reigosa, M.J.; González, L. Differential responses to allelopathic compounds released by the invasive Acacia dealbata Link (Mimosaceae) indicate stimulation of its own seed. Austral. J. Botan. 2010, 58, 546–553. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Ocaña, A.C. Studies on the allelopathic potential of Acacia dealbata Link.: Allelopathic potential produced during laboratory decomposition of plant residues incorporated into soil. J. Allelochem. Interact. 2017, 3, 23–33. [Google Scholar]
- Lorenzo, P.; Palomera-Pérez, A.; Reigosa, M.J.; González, L. Allelopathic interference of invasive Acacia dealbata Link on the physiological parameters of native understory species. Plant Ecol. 2011, 212, 403–412. [Google Scholar] [CrossRef]
- Aguilera, N.; Becerra, J.; Villaseñor-Parada, C.; Lorenzo, P.; González, L.; Hernández, V. Effects and identification of chemical compounds released from the invasive Acacia dealbata Link. Chem. Ecol. 2015, 31, 479–493. [Google Scholar] [CrossRef]
- Lorenzo, P.; Rodrguez, J.; González, L.; Rodríguez-Echeverría, S. Changes in microhabitat, but not allelopathy, affect plant establishment after Acacia dealbata invasion. J. Plant Ecol. 2017, 10, 610–617. [Google Scholar] [CrossRef]
- González, L.; Souto, X.C.; Reigosa, M.J. Allelopathic effects of Acacia melanoxylon R. Br. Phyllodes during their decomposition. For. Ecol. Manag. 1995, 77, 53–63. [Google Scholar] [CrossRef]
- Hussain, M.I.; González, L.; Reigosa, M.J. Allelopathic potential of Acacia melanoxylon on the germination and root growth of native species. Weed Biol. Manag. 2011, 11, 18–28. [Google Scholar] [CrossRef]
- Ismail, N.A.N.; Metali, F. Allelopathic effects of invasive Acacia mangium on germination and growth of local paddy varieties. J. Agron. 2014, 13, 158–168. [Google Scholar] [CrossRef]
- Noumi, Z.; Dhaou, S.O.; Abdallah, F.; Touzard, B.; Chaieb, M. Acacia tortilis subsp. raddiana in the North African arid zone: The obstacles to natural regeneration. Acta Bot. Gallica 2010, 157, 231–240. [Google Scholar] [CrossRef]
- Peguero, G.; Lanuza, O.R.; Save, R.; Espelta, J.M. Allelopathic potential of the neotropical dry-forest tree Acacia pennatula Benth.: Inhibition of seedling establishment exceeds facilitation under tree canopies. Plant. Ecol. 2012, 213, 1945–1953. [Google Scholar] [CrossRef]
- John, J.; Narwal, S.S. AllelopathicPlants. 9. Leucaena leucocephulu (Lam.) de Wit. Allelophaty J. 2003, 2, 13–36. [Google Scholar]
- Ishihara, K.L.; Honda, M.D.H.; Bageel, A.; Borthakur, D. Leucaena leucocephala: A leguminous tree suitable for eroded habitats of Hawaiian islands. In Ravine Lands: Greening for Livelihood and Environmental Security; Dagar, J., Singh, A., Eds.; Springer: Singapore, 2018; pp. 413–431. [Google Scholar] [CrossRef]
- Garcia, G.W.; Ferguson, T.U.; Neckles, F.A.; Archibald, K.A.E. The nutritive value and forage productivity of Leucaena leucocephala. Anim. Feed Sci. Technol. 1996, 60, 29–41. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic effect of Leucaena leucocephala on Zea mays. J. Trop. For. Sci. 1999, 11, 801–808. [Google Scholar]
- Chai, T.-T.; Ooh, K.-F.; Ooi, P.-W.; Chue, P.-S.; Wong, F.-C. Leucaena leucocephala leachate compromised membrane integrity, respiration and antioxidative defence of water hyacinth leaf tissues. Bot. Stud. 2013, 54, 8. [Google Scholar] [CrossRef]
- Koutika, L.-S.; Richardson, D.M. Acacia mangium Willd: Benefits and threats associated with its increasing use around the world. For. Ecosyst. 2019, 6, 2. [Google Scholar] [CrossRef]
- Rietveld, W.J. Allelopathic effects of juglone on germination and growth of several herbaceous and woody species. J. Chem. Ecol. 1983, 9, 295–308. [Google Scholar] [CrossRef]
- Massey, A.B. Antagonism of the walnuts (Juglans nigra L. and Julgans cinerea L.) in certain plant associations. Phytopathology 1925, 15, 773–784. [Google Scholar]
- Kocacaliskan, I.; Terzi, I. Allelopathic effects of walnut leaf extracts and juglone on seed germination and seedling growth. J. Horticult. Sci. Biotechnol. 2001, 76, 436–440. [Google Scholar] [CrossRef]
- Ercisli, S.; Esitken, A.; Turkkal, C.; Orhan, E. The allelopathic effects of juglone and walnut leaf extractson yield, growth, chemical and PNE compositionsof strawberry cv. Fern. Plant Soil Environ. 2005, 51, 283–287. [Google Scholar] [CrossRef]
- Wang, Q.; Xuab, Z.; Hua, T.; Rehmanc, H.; Chena, H.; Lia, Z.; Dingd, B.; Hu, H. Allelopathic activity and chemical constituents of walnut (Juglans regia) leaf litter in walnut–winter vegetable agroforestry system. Nat. Product Res. 2014, 28, 2017–2020. [Google Scholar] [CrossRef]
- Shrestha, A. Potential of a black walnut (Juglans nigra) extract product (NatureCur®) as a pre- and post-emergence bioherbicide. J. Sustain. Agric. 2009, 33, 810–822. [Google Scholar] [CrossRef]
- Sunmonu, T.O.; van Staden, J. Phytotoxicity evaluation of six fast-growing tree species in South Africa. South African. J. Botan. 2014, 90, 101–106. [Google Scholar] [CrossRef]
- Uddin, M.B.; Ahmed, R.; Mukul, S.A.; Hossain, M.K. Inhibitory effects of Albizia lebbeck leaf extracts on germination and growth behavior of some popular agricultural crops. J. For. Res. 2007, 18, 128–132. [Google Scholar] [CrossRef]
- Wang, C.M.; Chen, H.T.; Li, T.C.; Weng, J.H.; Jhan, Y.L.; Lin, S.X.; Chou, C.H. The role of pentacyclic triterpenoids in the allelopathic effects of Alstonia scholaris. J. Chem. Ecol. 2014, 40, 90–98. [Google Scholar] [CrossRef]
- Javaid, A.; Shafique, S.; Bajwa, R.; Shafique, S. Parthenium management through aqueous extracts of Alstonia scholaris. Pak. J. Bot. 2010, 42, 3651–3657. [Google Scholar]
- Catalan, P.; Vazquez-de-Aldana, B.R.; de las Heras, P.; Fernandez-Seral, A.; Perez-Corona, M.E. Comparing the allelopathic potential of exotic and native plant species on understory plants: Are exotic plants better armed? Anales de Biologia 2013, 35, 65–74. [Google Scholar] [CrossRef]
- Medina-Villar, S.; Alonso, A.; Castro-Diez, C.; Perez-Corona, M.E. Allelopathic potentials of exotic invasive and native trees over coexisting understory species: The soil as modulator. Plant Ecol. 2017, 218, 579–594. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, R.; Huang, Y.; Feng, S.; Ma, X.; Zhang, Y.; Sikdar, A.; Roy, R. Allelopathic effects of aqueous leaf extracts from four shrub species on seed germination and initial growth of Amygdalus pedunculata Pall. Forests 2018, 9, 711. [Google Scholar] [CrossRef]
- Hisashi Kato-Noguchi, H.; Salam, M.A.; Ohno, O.; Suenaga, K. Nimbolide B and nimbic acid B, phytotoxic substances in neem leaves with allelopathic activity. Molecules 2014, 19, 6929–6940. [Google Scholar] [CrossRef]
- Xuan, T.; Eiji, T.; Hiroyuki, T.; Mitsuhiro, M.; Khanh, T.D.; Ill-Min Chung, I.-M. Evaluation on phytotoxicity of neem (Azadirachta indica. A. Juss) to cropsand weeds. Crop Protect. 2004, 23, 335–345. [Google Scholar] [CrossRef]
- Nebo, L.; Varela, R.M.; Molinillo, J.M.G.; Severino, V.G.P.; Sarria, A.L.F.; Cazal, C.M.; Fernandes, M.F.; Fernandes, J.B.; Dominguez, F.A.M. Phytotoxicity of triterpenes and limonoids from the Rutaceae and Meliaceae. 5α,6β,8α,12α-Tetrahydro-28-norisotoonafolin? A Potent Phytotoxin from Toona ciliata. Nat. Prod. Commun. 2015, 10, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Vandermasta, D.B.; van Learb, D.H.; Clinton, B.D. American chestnut as an allelopath in the southern Appalachians. For. Ecol. Manag. 2002, 165, 173–181. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Lin, L.J.; Yang, L.; Ruan, X.; Li, X.J.; Pan, C.D.; Zhang, H.; Wang, G.; Wang, Q. Comparative allelopathic potentials of American chestnut (Castanea dentata) and Chinese chestnut (C. mollissima). Allelopathy J. 2015, 36, 303–314. [Google Scholar]
- Batish, D.R.; Singh, H.P.; Kohli, R.K. Vegetation exclusion under Casuarina equisetifolia L.: Does allelopathy play a role? Commun. Ecol. 2001, 2, 93–100. [Google Scholar] [CrossRef]
- Xu, Z.X.; Zhang, Y.; Yao, Y.; Li, H.M.; Li, L. Allelopathic effects of Casuarina equisetifolia extracts on seed germination of native tree species. Allelopathy J. 2015, 36, 283–292. [Google Scholar]
- Long, F.; Xie, B.B.; Liang, A.J.; Liu, Y.; Lin, Y.M.; Chen, C.; Hong, T.; Hong, W.; Wu, C.Z.; Li, J. Replant problem in Casuarina equisetifolia L.: Isolation and identification of allelochemicals from its roots. Allelopathy J. 2018, 43, 73–82. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yamahi, K.; Kobayashi, K. Allelopathic activity of camphor released from camphor tree (Cinnamomum camphora). Allelopathy J. 2011, 27, 123–132. [Google Scholar]
- Satyal, P.; Paudel, P.; Poudel, A.; Dosoky, N.S.; Pokharel, K.K.; Setzer, W.N. Bioactivities and compositional analyses of Cinnamomum essential oils from Nepal: C. camphora, C. tamala, and C. glaucescens. Nat. Prod. Commun. 2013, 8, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hu, T.; Chen, H.; Wang, Q.; Hu, H.; Tu, L.; Jing, L. Impact of decomposing Cinnamomum septentrionale leaf litter on the growth of Eucalyptus grandis saplings. Plant Physiol. Biochem. 2013, 70, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hu, H.; Hu, T.; Wang, Q.; Ye, M.; Luo, J.; Peng, Y.; Zhang, R. Chemical constituents of Cinnamomum septentrionale leaf litter and its allelopathic activity on the growth of maize (Zea mays). Nat. Prod. Res. 2017, 31, 1314–1317. [Google Scholar] [CrossRef]
- Cui, C.; Liu, B.; Hou, L.; Zhang, S.H. Allelopathic effects of persimmon (Diospyros kaki) leaves extracts on germination, seedling growth and enzymatic activities of receptor plants. Allelopathy J. 2017, 42, 49–64. [Google Scholar] [CrossRef]
- Bauer, J.T.; Shannon, S.M.; Stoops, R.E.; Reynolds, H.L. Context dependency of the allelopathic effects of Lonicera maackii on seed germination. Plant Ecol. 2012, 213, 1907–1916. [Google Scholar] [CrossRef]
- Cipollini, D.; Randall Stevenson, R.; Enright, S.; Eyles, A.; Bonello, P. Phenolic metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential phytotoxic and anti-herbivore effects. J. Chem. Ecol. 2008, 34, 144–152. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Rubio-Rosas, E.; Ruiz-González, N.; Cruz-Duran, R.; Lozoya-Gloria, E.; Zurita-Vásquez, G.; Franco-Monsreal, J. Alkaloid profile, antibacterial and allelopathic activities of Lupinus jaimehintoniana, BL Turner. (Fabaceae). Arch. Biol. Sci. 2012, 64, 1065–1071. [Google Scholar] [CrossRef]
- Haq, R.A.; Hussain, M.; Cheema, Z.A.; Mushtaq, M.N.; Farooq, M. Mulberry leaf water extract inhibits bermudagrass and promotes wheat growth. Weed Biol. Manag. 2010, 10, 234–240. [Google Scholar] [CrossRef]
- Nekonam, M.S.; Razmjoo, J.; Sharifnabi, B.; Karimmojeni, H. Assessment of allelopathic plants for their herbicidal potential against field bindweed (Convolvulus arvensis). AJCS 2013, 7, 1654–1660. [Google Scholar]
- Zhang, S.; Shi, F.; Yang, W.; Xiang, Z.; Kang, H.; Duan, Z. Autotoxicity as a cause for natural regeneration failure in Nyssa yunnanensis and its implications for conservation. Israel J. Plant Sci. 2015, 62, 187–197. [Google Scholar] [CrossRef]
- Halarewicz, A.; Liszewski, M.; Babelewski, P.; Baczek, P. Allelopathic effects of Paulownia tomentosa and hybrid P. elongata × P. fortunei on Sinapis alba, Festuca pratensis and Poa pratensis. Allelopathy J. 2018, 43, 83–92. [Google Scholar] [CrossRef]
- Singh, H.P.; Kohli, R.K.; Batish, D.R. Allelopathic interference of Populus deltoides with some winter season crops. Agronomie 2001, 21, 139–146. [Google Scholar] [CrossRef]
- Alrababah, M.A.; Tadros, M.J.; Samarah, N.H.; Ghosheh, H.G. Allelopathic effects of Pinus halepensis and Quercus coccifera on the germination of Mediterranean crop seeds. New For. 2009, 38, 261–272. [Google Scholar] [CrossRef]
- Dabral, A.; Butola, B.S.; Khanduri, V.P.; Sharma, C.M.; Dhanush, C. Autotoxicity against seed germination, seedling emergence of Quercus leucotrichophora A. Camus Ex. Bahadur. J. Pharmacog. Phytochem. 2018, 7, 1813–1816. [Google Scholar]
- Klionsky, S.M.; Amatangelo, K.L.; Donald, M.; Waller, D.M. Above- and belowground impacts of European buckthorn (Rhamnus cathartica) on four native forbs. Restoration Ecol. 2011, 19, 728–737. [Google Scholar] [CrossRef]
- Warren II, R.J.; Adam Labatore, A.; Candeias, M. Allelopathic invasive tree (Rhamnus cathartica) alters native plant communities. Plant. Ecol. 2017, 218, 1233–1241. [Google Scholar] [CrossRef]
- Seltzner, S.; Eddy, T.L. Allelopathy in Rhamnus cathartica, European buckthorn. Michigan Botan. 2003, 42, 51–61. [Google Scholar]
- Chou, S.C.; Huang, C.H.; Hsu, T.W.; Wu, C.C.; Chou, C.H. Allelopathic potential of Rhododendron formosanum Hemsl in Taiwan. Allelopathy J. 2010, 25, 73–91. [Google Scholar]
- Wang, C.-M.; Li, T.-C.; Jhan, Y.-L.; Weng, J.-H.; Chou, C.-H. The Impact of Microbial Biotransformation of Catechin in Enhancing the Allelopathic Effects of Rhododendron formosanum. PLoS ONE 2013, 8, e85162. [Google Scholar] [CrossRef]
- Nasir, H.; Iqbal, Z.; Hiradate, S.; Fujii, Y. Allelopathic potential of Robinia pseudo-acacia L. J. Chem. Ecol. 2005, 31, 9. [Google Scholar] [CrossRef] [PubMed]
- Kole, R.K.; Karmakar, P.R.; Poi, R.; Mazumdar, D. Allelopathic inhibition of teak leaf extract: A potential pre-emergent herbicide. J. Crop Weed 2011, 7, 101–109. [Google Scholar]
- Lacret, R.; Varela, R.M.; Molinillo, J.M.G.; Nogueiras, C.; Macías, F.A. Anthratectone and naphthotectone, two quinones from bioactive extracts of Tectona grandis. J. Chem. Ecol. 2011, 37, 1341–1348. [Google Scholar] [CrossRef]
- Macías, F.A.; Lacret, R.; Rosa, M.; Varela, R.M.; Nogueiras, C.; Molinillo, J.M.G. Isolation and phytotoxicity of terpenes from Tectona grandis. J. Chem. Ecol. 2010, 36, 396–404. [Google Scholar] [CrossRef]
- Ayeb-Zakhama, A.E.; Sakka-Rouis, L.; Bergaoui, A.; Flamini, G.; Jannet, H.B.; Harzallah-Skhiri, F. Chemical composition and allelopathic potential of essential oils from Tipuana tipu (Benth.) Kuntze cultivated in Tunisia. Chem. Biodivers. 2016, 13, 309–318. [Google Scholar] [CrossRef]
- Perez-Corona, M.E.; Heras, P.; Vazquez de Aldana, B.R. Allelopathic potential of invasive Ulmus pumila on understory plant species. Allelopathy J. 2013, 32, 101–112. [Google Scholar]
- Elalouia, M.; Ghazghazia, H.; Ennajaha, A.; Manaab, S.; Guezmirb, W.; Karrayb, N.B.; Laamouria, A. Phenolic profile, antioxidant capacity of five Ziziphus spina-christi (L.) Willd provenances and their allelopathic effects on Trigonella foenum-graecum L. and Lens culinaris L. seeds. Nat. Prod. Res. 2017, 31, 1209–1213. [Google Scholar] [CrossRef]
- Singh, H.P.; Kohli, R.K.; Batish, D.R.; Kaushal, P.S. Allelopathy of gymnospermous trees. J. For. Res. 1999, 4, 245–254. [Google Scholar] [CrossRef]
- Lee, I.K.; Monsi, M. Ecological studies on Pinus densiflora forest. I. Effects of plant substances on the floristic composition of the undergrowth. Bat. Mag. 1963, 76, 400–413. [Google Scholar] [CrossRef]
- Braine, J.W.; Curcio, G.R.; Wachowicz, C.M.; Hansel, F.A. Allelopathic effects of Araucaria angustifolia needle extracts in the growth of Lactuca sativa seeds. J. For. Res. 2012, 17, 440–445. [Google Scholar] [CrossRef]
- Huang, Z.; Liao, L.; Wang, S.; Cao, G. Allelopathy of phenolics from decomposing stump-roots in replant Chinese fir woodland. J. Chem. Ecol. 2000, 26, 2211–2219. [Google Scholar] [CrossRef]
- Chen, L.-C.; Wang, S.-L. Allelopathic behaviour of Chinese fir from plantations of different ages. Forestry 2013, 86, 225–230. [Google Scholar] [CrossRef][Green Version]
- Lin, S.; Cao, G.; Du, L.; Wang, A. Effect of Allelochemicals of Chinese-fir root extracted by supercritical CO2 extraction on Chinese fir. J. Forestry Res. 2003, 14, 122–126. [Google Scholar] [CrossRef]
- Kong, C.H.; Chen, L.C.; Xu, X.H.; Wang, P.; Wang, S.L. Allelochemicals and activities in a replanted Chinese fir (Cunninghamia lanceolata (Lamb.) Hook) tree ecosystem. J. Agric. Food Chem. 2008, 56, 11734–11739. [Google Scholar] [CrossRef]
- Liu, B.; Daryanto, S.; Wang, L.; Li, Y.; Liu, Q.; Zhao, C.; Wang, Z. Excessive accumulation of Chinese fir litter inhibits its own seedling emergence and early growth—A greenhouse perspective. Forests 2017, 8, 341. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Takeshita, S.; Kimura, F.; Ohno, O.; Suenaga, K. A novel substance with allelopathic activity in Ginkgo biloba. J. Plant Physiol. 2013, 170, 1595–1599. [Google Scholar] [CrossRef]
- Young, G.P.; Bush, J.K. Assessment of the allelopathic potential of Juniperus ashei on germination and growth of Bouteloua curtipendula. J. Chem. Ecol. 2009, 35, 74–80. [Google Scholar] [CrossRef]
- Yang, L. Effect of water extracts of larch on growth of Manchurian walnut seedlings. J. Forestry Research 2005, 16, 285–288. [Google Scholar] [CrossRef]
- Ruan, X.; Li, Z.-H.; Wang, Q.; Pan, C.-D.; Jiang, D.-A.; Wang, G.G. Autotoxicity and allelopathy of 3,4-dihydroxyacetophenone isolated from Picea schrenkiana needles. Molecules 2011, 16, 8874–8893. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Pan, C.-D.; Liu, R.; Li, Z.-H.; Li, S.-L.; Jiang, D.-A.; Zhang, J.-C.; Wang, G.; Zhao, Y.-X.; Wang, Q. Effects of climate warming on plant autotoxicity in forest evolution: A case simulation analysis for Picea schrenkiana regeneration. Ecol. Evol. 2016, 6, 5854–5866. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Fushimi, Y.; Tanaka, Y.; Teruya, T.; Suenaga, K. Allelopathy of red pine: Isolation and identification of an allelopathic substance in red pine needles. Plant Growth Regul. 2011, 65, 299–304. [Google Scholar] [CrossRef]
- Kato-Noguchia, H.; Kimura, F.; Ohnob, O.; Suenaga, K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Nektarios, P.A.; Economou, G.; Avgoulas, C. Allelopathic effects of Pinus halepensis needles on trufgrasses and biosensor plants. HortScience 2005, 40, 246–250. [Google Scholar] [CrossRef]
- Monnier, Y.; Vila, B.; Montes, N.; Bousquet-Melou, A.; Prevosto, B.; Fernandez, C. Fertilization and allelopathy modify Pinus halepensis saplings crown acclimation to shade. Trees 2011, 25, 497–507. [Google Scholar] [CrossRef]
- Fernandez, C.; Lelong, B.; Vila, B.; Mévy, J.-P.; Robles, C.; Greff, S.; Dupouyet, S.; Bousquet-Mélou, A. Potential allelopathic effect of Pinus halepensis in the secondary succession: An experimental approach. Chemoecology 2006, 16, 97–105. [Google Scholar] [CrossRef]
- Fernandez, C.; Monnier, Y.; Ormeno, E.; Baldy, V.; Greff, S.; Pasqualini, V.; Mévy, J.-P.; Bousquet-Mélou, A. Variations in allelochemical composition of leachates of different organs and maturity stages of Pinus halepensis. J. Chem. Ecol. 2009, 35, 970–979. [Google Scholar] [CrossRef]
- Fernandez, C.; Santonja, M.; Gros, R.; Monnier, Y.; Chomel, M.; Baldy, V.; Bousquet-Mélou, A. Allelochemicals of Pinus halepensis as drivers of biodiversity in Mediterranean open mosaic habitats during the colonization stage of secondary succession. J. Chem. Ecol. 2013, 39, 298–311. [Google Scholar] [CrossRef]
- Fernandez, C.; Voiriot, S.; Mévy, J.-P.; Vila, B.; Ormeno, E.; Dupouyet, S.; Bousquet-Mélou, A. Regeneration failure of Pinus halepensis Mill.: The role of autotoxicity and some abiotic environmental parameters. For. Ecol. Manag. 2008, 255, 2928–2936. [Google Scholar] [CrossRef]
- Amri, I.; Samia Gargouri, S.; Hamrouni, L.; Hanana, M.; Fezzani, T.; Jamoussi, B. Chemical composition, phytotoxic and antifungal activities of Pinus pinea essential oil. J. Pest Sci. 2012, 85, 199–207. [Google Scholar] [CrossRef]
- Sharma, N.K.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Allelopathic effect of Pinus roxburghii on an understorey plant, Bidens pilosa. Ann. Plant Sci. 2016. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.X.; Gao, Y.; Zhao, Y.J. Study on allelopathy of three species of Pinus in North China. Applied Ecol. Environ. Res. 2018, 16, 6409–6417. [Google Scholar] [CrossRef]
- Devaney, J.L.; Whelan, P.M.; Jansen, M.A.K. Conspecific negative density dependence in a long-lived conifer, yew Taxus baccata L. Eur. J. For. Res. 2018, 137, 69–78. [Google Scholar] [CrossRef]
- Lobiuc, A.; Cuibari, R.; Frunzete, M.; Naela, C.; Burducea, M.; Mirela, A.; Zamfirache, M. The effects of Taxus baccata L. aqueous extracts on germination, seedling growth and physiological parameters of test species. J. Horticult. For. Biotechnol. 2016, 20, 118–125. [Google Scholar]
- Keeling, C.I.; Lewis, A.R.; Kolotelo, D.; Russell, J.H.; Kermode, A.R. Resin vesicles in conifer seeds: Morphology and allelopathic effects. Canadian J. For. Res. 2018, 48, 1515–1525. [Google Scholar] [CrossRef]
- Seal, A.N.; Pratley, J.E.; Haig, T.J.; An, M.; Wua, H. Plants with phytotoxic potential: Wollemi pine (Wollemia nobilis). Agric. Ecosyst. Environ. 2010, 135, 52–57. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, J.S.; Sezirahiga, J.; Kwon, J.; Jeong, M.; Lee, D.; Choi, J.-H.; Jang, D.S. A new canthinone-type alkaloid isolated from Ailanthus altissima Swingle. Molecules 2016, 21, 642. [Google Scholar] [CrossRef]
- Ni, J.-C.; Shi, J.-T.; Tan, Q.-W.; Chen, Q.-J. Phenylpropionamides, piperidine, and phenolic derivatives from the fruit of Ailanthus altissima. Molecules 2017, 22, 2107. [Google Scholar] [CrossRef]
- Curcino Vieira, I.J.; Braz-Filho, R. Quassinoids: Structural diversity, biological activityand synthetic studies. Stud. Nat. Prod. Chem. 2006, 33, 433–492. [Google Scholar] [CrossRef]
- Morre, D.J.; Grieco, P.A.; Morre, D.M. Mode of action of the anticancer quassinoids—Inhibition of the plasma membrane NADH oxidase. Life Sci. 1998, 63, 595–604. [Google Scholar] [CrossRef]
- Heisey, R.M. Identification of an allelopathic compound from Ailanthus altissima (Simaroubaceae) and characterization of its herbicidal activity. Am. J. Bot. 1996, 83, 192–200. [Google Scholar] [CrossRef]
- Lin, L.-J.; Peiser, G.; Ying, B.-P.; Mathias, K.; Karasina, F.; Wang, Z.; Itatani, J.; Green, L.; Hwang, Y.-S. Identification of plant growth inhibitory principles in Ailanthus altissima Castela tortuosa. J. Agri. Food Chem. 1995, 43, 1706–1711. [Google Scholar] [CrossRef]
- Gao, W.; Ge, S.; Sun, J. Ailanthone exerts anticancer effect by up-regulating miR-148a expression in MDA-MB-231 breast cancer cells and inhibiting proliferation, migration and invasion. Biomed. Pharmacother. 2019, 109, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.L.; Xiong, J.; Wu, S.B.; Zhu, J.J.; Hong, J.L.; Zhao, Y.; Hu, J.F. Tetracyclic triterpenoids and terpenylated coumarins from the bark of Ailanthus altissima (“Tree of Heaven”). Phytochemistry 2013, 86, 159–167. [Google Scholar] [CrossRef]
- Heisey, R.M. Allelopathic and herbicidal effects of extracts from tree-of-heaven (Ailanthus altissima). Am. J. Bot. 1990, 77, 662–670. [Google Scholar] [CrossRef]
- Heisey, R.M.; Heisey, T.K. Herbicidal effects under field conditions of Ailanthus altissima bark extract, which contains ailanthone. Plant Soil 2003, 256, 85–99. [Google Scholar] [CrossRef]
- Ayeb-Zakhama, A.E.; Salem, S.B.; Sakka-Rouis, L.; Flamini, G.; Jannet, H.B.; Harzallah-Skhiri, F. Chemical Composition and phytotoxic effects of essential oils obtained from Ailanthus altissima (Mill.) Swingle cultivated in Tunisia. Chem. Biodivers. 2014, 11, 1216–1227. [Google Scholar] [CrossRef]
- Vogel, A., Jr.; Reinschauer, C. Reinschauer. Uever einen neuen organischen Körper in den Fruchtschalen der Juglans regia. Neues Repertorium Für die Pharmacie 1856, 5, 106–110. [Google Scholar]
- Berntsen, A.; Semper, A. Ueber die constitution des juglons und seine synthese aus naphtalin. Berichte der Deutschen Chemischen Gesellschaft 1887, 20, 934–941. [Google Scholar] [CrossRef]
- Davis, E.F. The toxic principle of Juglans nigra as identified with synthetic juglone, and itstoxic effects on tomato and alfalfa plants. Am. J. Biol. 1928, 15, 620. [Google Scholar]
- Hook, I.; Mills, C.; Sheridan, H. Bioactive naphthoquinones from higher plants. Studies Nat. Prod. Chem. 2014, 41, 119–160. [Google Scholar] [CrossRef]
- Babula, P.; Vaverkova, V.; Poborilova, Z.; Ballova, L.; Masarik, M.; Provaznik, I. Phytotoxic action of naphthoquinone juglone demonstrated on lettuce seedling roots. Plant Physiol Biochem. 2014, 84, 78–86. [Google Scholar] [CrossRef]
- Hejl, A.M.; Koster, K.L. Juglone disrupts root plasma membrane HC-ATPase activity and impairs water uptake, root respiration, and growth in soybean (Glycine max) and corn (Zea mays). J. Chem. Ecol. 2004, 30, 453–471. [Google Scholar] [CrossRef]
- Catanzaro, E.; Greco, G.; Potenza, L.; Calcabrini, C.; Fimognari, C. Natural products to fight cancer: A focus on Juglans regia. Toxins 2018, 10, 469. [Google Scholar] [CrossRef]
- Coder, K.D. Black Walnut Allelopathy: Tree Chemical Warfare; University of Georgia: Athens, GA, USA, 2017. [Google Scholar]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef]
- Thakur, A.; Cahalan, C. Geographical variation of Juglans regia L. in juglone content: Rapid analysis using micro plate reader. Curr. Sci. 2011, 100, 1483–1485. [Google Scholar]
- Solar, A.; Colaric, M.; Usenik, V.; Stampar, F. Seasonal variations of selected flavonoids, phenolic acids and quinones in annual shoots of common walnut (Juglans regia L.). Plant Sci. 2006, 170, 453–461. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Seasonal variation of the main individual phenolics and juglone in walnut (Juglans regia) leaves. Pharm. Biol. 2014, 52, 575–580. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Funk, D.T.; Case, P.J.; Rietveld, W.J.; Phares, R.E. Effects of juglone on the growth of coniferous seedlings. Forest Science 1979, 25, 452–454. [Google Scholar] [CrossRef]
- Kessler, C.T. Effect of juglone on freshwater algal growth. J. Chem. Ecol. 1989, 152, 127–2134. [Google Scholar] [CrossRef]
- Jose, S.; Gillespie, A.R. Allelopathy in black walnut (Juglans nigra L.) alley cropping. II. Effects of juglone on hydroponically grown corn (Zea mays L.) and soybean (Glycine max L. Merr.) growth and physiology. Plant Soil 1998, 203, 199–206. [Google Scholar] [CrossRef]
- Terzi, I.; Kocaçalişkan, I.; Benlioğlu, O.; Solak, K. Effects of juglone on growth of cucumber seedlings with respect to physiological and anatomical parameters. Acta Physiol. Plant 2003, 25, 353. [Google Scholar] [CrossRef]
- Bohm, P.A.F.; Bohm, F.; Ferrarese, M.L.L.; Salvador, V.H.; Soares, A.R.; Ferrarese, O. Effects of juglone on soybean root growth and induction of lignification. Allelopathy J. 2010, 25, 465–474. [Google Scholar]
- Klotz, L.O.; Hou, X.; Jacob, C. 1,4-Naphthoquinones: From oxidative damage to cellular and inter-cellular signaling. Molecules 2014, 19, 14902–14918. [Google Scholar] [CrossRef]
- Sytykiewicz, H. Expression patterns of glutathione transferase gene (GstI) in maize seedlings under juglone-induced oxidative stress. Int. J. Mol. Sci. 2011, 12, 7982–7995. [Google Scholar] [CrossRef]
- Chi, W.-C.; Fu, S.-F.; Huang, T.-L.; Chen, Y.-A.; Chen, C.-C.; Huang, H.-J. Identification of transcriptome profiles and signaling pathways for the allelochemical juglone in rice roots. Plant. Mol. Biol. 2011, 7, 591–607. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chi, W.-C.; Trinh, N.N.; Cheng, K.-T.; Chen, Y.-A.; Lin, T.C.; Lin, Y.-C.; Huang, L.-Y.; Huang, H.-J.; Chiang, T.-Y. Alleviation of allelochemical juglone-induced phytotoxicity in tobacco plants by proline. J. Plant Interact. 2015, 10, 167–172. [Google Scholar] [CrossRef]
- Kurtyka, R.; Pokora, W.; Tukaj, Z.; Karcz, W. Effects of juglone and lawsone on oxidative stress in maize coleoptile cells treated with IAA. AoB Plants 2016, 8. [Google Scholar] [CrossRef]
- Sytykiewicz, H.; Kozak, A.; Leszczyński, B.; Sempruch, C.; Łukasik, I.; Sprawka, I.; Kmieć, K.; Kurowska, M.; Kopczyńska, A.; Czerniewicz, P. Transcriptional profiling of catalase genes in juglone-treated seeds of maize (Zea mays L.) and wheat (Triticum aestivum L.). Acta Biol. Hung. 2018, 69, 449–463. [Google Scholar] [CrossRef]
- Hou, X.; Huang, J.; Tang, J.; Wang, N.; Zhang, L.; Gu, L.; Sun, Y.; Yang, Z.; Huang, Y. Allelopathic inhibition of juglone (5-hydroxy-1,4-naphthoquinone) on the growth and physiological performance in Microcystis aeruginosa. J. Environ. Manage. 2019, 232, 382–386. [Google Scholar] [CrossRef]
- Topal, S.; Kocacaliskan, I.; Arslan, O.; Tel, A.Z. Herbicidal effects of juglone as an allelochemical. Phyton Ann. Rei Botan. 2007, 46, 259–269. [Google Scholar]
- Schmidt, S.K. Degradation of juglone by soil bacteria. J. Chem. Ecol. 1988, 14, 1561–1571. [Google Scholar] [CrossRef]
- Von Kiparski, G.R.; Lee, L.S.; Gillespie, A.R. Occurrence and fate of the phytotoxin juglone in alley soils under black walnut trees. J. Environ. Qual. 2007, 36, 709–717. [Google Scholar] [CrossRef]
- Strugstad, M.P.; Despotovski, S. A summary of extraction, synthesis, properties, and potential uses of juglone: A literature review. J. Ecosyst. Manag. 2012, 13, 1–16. [Google Scholar]
- Yang, L.; Wang, P.; Kong, C. Effect of larch (Larix gmelini Rupr.) root exudates on Manchurian walnut (Juglans mandshurica Maxim.) growth and soil juglone in a mixed-species plantation. Plant Soil 2010, 329, 249–258. [Google Scholar] [CrossRef]
- Salahuddin; Rewald, B.; Razaq, M.; Lixue, Y.; Li, J.; Khan, F.; Jie, Z. Root order-based traits of Manchurian walnut & larch and their plasticity under interspecific competition. Sci. Rep. 2018, 8, 9815. [Google Scholar] [CrossRef]
- Nguyen, B.C.Q.; Tawata, S. The chemistry and biological activities of mimosine: A review. Phytother. Res. 2016, 30, 1230–1242. [Google Scholar] [CrossRef]
- Bickel, A.F. On the structure of leucaenine (leucaenol) from Leucaena glauca Bentham. I. J. Am. Chem. Soc. 1947, 69, 1801–1803. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tawata, S.; Khanh, T.D. Herbicidal activity of mimosine and its derivatives. In Herbicides—Advances in Research; Price, A.J.K., Jessica, A., Eds.; InTech: Rijeka, Croatia, 2013; pp. 299–312. [Google Scholar]
- Xuan, T.D.; Elzaawely, A.A.; Deba, F.; Fukuta, M.; Tawata, S. Mimosine in Leucaena as a potent bio-herbicide. Agron. Sustain. Dev. 2006, 26, 89–97. [Google Scholar] [CrossRef]
- Ogita, S.; Kato, M.; Watanabe, S.; Ashihara, H. The Co-occurrence of two pyridine alkaloids, mimosine and trigonelline, in Leucaena leucocephala. Z. Naturforsch 2014, 69, 124–132. [Google Scholar] [CrossRef]
- Chou, C.-H.; Kuo, Y.-L. Allelopathic research of subtropical vegetation in Taiwan. III. Allelopathic exclusion of understory by Leucaena leucocephala (Lam.) de Wit. J. Chem. Ecol. 1986, 12, 1431–1448. [Google Scholar] [CrossRef]
- Lalitha, K.; Vargheese, C.M.; Balasubramanian, N. Spectrophotometric determination of mimosine and 3-hydroxy-4 (1H)-pyridone—the toxic principles of Leucaena leucocephala. Anal. Biochem. 1993, 213, 57–62. [Google Scholar] [CrossRef]
- Gilbert, D.; Neilson, A.; Miyazawa, H.; Depamphilis, M.L.; Burhans, W.C. Mimosine arrests DNA synthesis at replication forks by inhibiting deoxyribonucleotide metabolism. J. Biol. Chem. 1995, 270, 95957–99606. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, J.T. EIF3 p 170, a mediator of mimosine effect on protein synthesis and cell cycle progression. Mol. Biol. Cell 2003, 14, 3942–3951. [Google Scholar] [CrossRef]
- Negi, V.S.; Bingham, J.-P.; Li, Q.X.; Borthakur, D. A carbon-nitrogen lyase from Leucaena leucocephala catalyzes the first step of mimosine degradation. Plant Physiol. 2014, 164, 922–934. [Google Scholar] [CrossRef]
- Prasad, M.N.V.; Subhashini, P. Mimosine inhibited seed germination, seedling growth, and enzymes of Oryza sativa L. J. Chem. Ecol. 1994, 20, 1689–1696. [Google Scholar] [CrossRef]
- Williams, R.D.; Hoagland, R.E. Phytotoxicity of mimosine and albizziine on seed germination and seedling growth of crops and weeds. Allelopathy J. 2007, 19, 423–430. [Google Scholar]
- Andrade, A.B.; Ferrarese, A.F.; Teixeira, A.F.; Ferrarese-Filho, O. Mimosine-inhibited soybean (Glycine max) root growth, lignification and related enzymes. Allelopathy J. 2008, 21, 133–143. [Google Scholar]
- Rodrigues-Corrêa, K.C.D.S.; Honda, M.D.H.; Borthakur, D.; Fett-Neto, A.G. Mimosine accumulation in Leucaena leucocephala in response to stress signaling molecules and acute UV exposure. Plant Physiol. Biochem. 2019, 135, 432–440. [Google Scholar] [CrossRef]
- Yeung, P.K.K.; Wong, F.T.W.; Wong, J.T.W. Mimosine, the allelochemical from the leguminous tree Leucaena leucocephala, selectively enhances cell proliferation in dinoflagellates. Appl. Environ. Microbiol. 2002, 68, 5160–5163. [Google Scholar] [CrossRef]
- Einhellig, F.A. Mode of Allelochemical Action of Phenolic Compounds. In Allelopathy: Chemistry and Mode of Action of Allelochemicals; Macías, F.A., Galindo, J.C.G., Molinillo, J.M.G., Cutler, H.G., Eds.; CRC Press: Boca Raton, FL, USA, 2004; Chapter 11; pp. 217–238. [Google Scholar]
- Souza-Alonso, P.; González, L.; Cavaleiro, C. Ambient has become strained. Identification of Acacia dealbata Link volatiles interfering with germination and early growth of native species. J. Chem. Ecol. 2014, 40, 1051–1061. [Google Scholar] [CrossRef]
- Ayeb-Zakhama, A.; Sakka-Rouis, L.; Bergaoui, A.; Flamini, G.; Jannet, H.B.; Harzallah-Skhiri, F. Chemical composition and allelopathic potential of essential oils obtained from Acacia cyanophylla Lindl. cultivated in Tunisia. Chem. Biodivers. 2015, 12, 615–626. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; González, L.; López-Nogueira, A.; Cavaleiro, C.; Pedrol, N. Volatile organic compounds of Acacia longifolia and their effects on germination and early growth of species from invaded habitats. Chem. Ecol. 2018, 34, 126–145. [Google Scholar] [CrossRef]
- Leicach, S.R.; Grass, M.A.Y.; Chludil, H.D.; Garau, A.M.; Guarnaschelli, A.B.; Fernandez, P.C. Chemical defenses in Eucalyptus species: A sustainable strategy based on antique knowledge to diminish agrochemical dependency. In New Advances and Contributions to Forestry Research; Oteng-Amoako, A.A., Ed.; Intech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Zhang, J.; An, M.; Wu, H.; Stanton, R.; Lemerle, D. Chemistry and bioactivity of eucalyptus essential oils. Allelopathy J. 2010, 25, 313–330. [Google Scholar]
- Barbosa, L.C.A.; Filomeno, C.A.; Teixeira, R.R. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Yu, S.; Wang, Y.; Fang, B.; Cai, C.; Liu, S. Identification and allelopathic effects of 1,8-cineole from Eucalyptus urophylla on lettuce. Allelopathy J. 2010, 26, 255–264. [Google Scholar]
- Kaur, S.; Singh, H.P.; Rani, D.; Batish, D.R.; Kohli, R.K. Chemical characterization and allelopathic potential of volatile oil of Eucalyptus tereticornis against Amaranthus viridis. J. Plant Interact. 2011, 6, 297–302. [Google Scholar] [CrossRef][Green Version]
- Wu, H.; Zhang, J.; Stanton, R.; An, M.; Liu, D.L.; Lemerle, D. Allelopathic effects of Eucalyptus dundasii on germinationand growth of ryegrass and barley grass. Allelopathy J. 2011, 28, 87–94. [Google Scholar]
- Ghnaya, A.D.; Hanana, M.; Amri, I.; Balti, H.; Gargouri, S.; Jamoussi, B.; Hamrouni, L. Chemical composition of Eucalyptus erythrocorys essential oils and evaluation of their herbicidal and antifungal activities. J. Pest. Sci. 2013. [Google Scholar] [CrossRef]
- Jaime, M.D.I.; Ferrer, M.A.B. Post-emergent herbicidal activity of Eucalyptus globulus Labill. essential oil. Nereis 2018, 10, 25–36. [Google Scholar]
- Setia, N.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Phytotoxicity of volatile oil from Eucalyptus citriodora against some weedy species. J. Environ. Biol. 2007, 28, 63–66. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Setia, N.; Kohli, R.K. Herbicidal activity of volatile oils from Eucalyptus citriodora against Parthenium hysterophorus. Ann. Appl. Biol. 2005, 146, 89–94. [Google Scholar] [CrossRef]
- Benchaa, S.; Hazzit, M.; Abdelkrim, H. Allelopathic effect of Eucalyptus citriodora essential oil and its potential use as bioherbicide. Chem. Biodivers. 2018, 15, e1800202. [Google Scholar] [CrossRef]
- Batish, D.R.; Setia, N.; Singh, H.P.; Kohli, R.K. Phytotoxicity of lemon-scented eucalypt oil and its potential use as a bioherbicide. Crop Protect. 2004, 23, 1209–1214. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Ramezani, H.; Kohli, R.K. Comparative phytotoxicity of four monoterpenes against Cassia occidentalis. Ann. Appl. Biol. 2002, 141, 111–116. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Nidhi, S.; Kohli, R.K.; Shalinder, K.; Yadav, S.S. Alternative control of littleseed canary grass using eucalypt oil. Agron. Sustain. Dev. 2007, 27, 171–177. [Google Scholar] [CrossRef]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 2000, 26, 303–313. [Google Scholar] [CrossRef]
- Zhang, J.; An, M.; Wu, H.; Liu, D.L.; Stanton, R. Chemical composition of essential oils of four Eucalyptus species and their phytotoxicity on silver leaf nightshade (Solanum elaeagnifolium Cav.) in Australia. Plant Growth Regul. 2012, 68, 231–237. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Shalinder, K.; Komal, A.; Kohli, R.K. α-Pinene inhibits growth and induces oxidative stress in roots. Ann. Botan. 2006, 98, 1261–1269. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Nidhi, S.; Shalinder, K.; Kohli, R.K. Chemical composition and phytotoxicity of volatile essential oil from intact and fallen leaves of Eucalyptus citriodora. Zeitschrift fur Naturforschung 2006, 61, 465–471. [Google Scholar] [CrossRef]
- Singh, I.P.; Batish, D.R.; Kaur, S.; Kohli, R.K.; Arora, K. Phytotoxicity of the volatile monoterpene citronellal against some weeds. Zeitschrift fur Naturforschung 2006, 61, 334–340. [Google Scholar] [CrossRef]
- Areco, V.A.; Figueroa, S.; Cosa, M.T.; Dambolena, J.S.; Zygadlo, J.A.; Zunino, M.P. Effect of pinene isomers on germination and growth of maize. Biochem. Syst. Ecol. 2014, 55, 27–33. [Google Scholar] [CrossRef]
- Del Moral, R.; Muller, C.H. The allelopathic effects of Eucalyptus camaldulensis. Am. Midland Nat. 1970, 83, 254–282. [Google Scholar] [CrossRef]
- Souto, X.C.; Gonzalez, L.; Reigosa, M.J. Comparative-analysis of allelopathic effects produced by 4 forestry species during decomposition process in their soils in Galicia (NW Spain). J. Chem. Ecol. 1994, 20, 3005–3015. [Google Scholar] [CrossRef]
- Sasikumar, K.; Vijayalakshmi, C.; Parthiban, K.T. Allelopathic effects of Eucalyptus on blackgram (Phaseolus mungo L.). Allelopathy J. 2002, 9, 205–214. [Google Scholar]
- Grichi, A.; Nasr, Z.; Khouja, M.L. Identification and phytotoxicity of phenolic compounds in Eucalyptus camaldulensis. Allelopathy J. 2018, 44, 75–88. [Google Scholar] [CrossRef]
- Gershenzon, J. Metabolic costs of terpenoid accumulation in higher plants. J. Chem. Ecol. 1994, 20, 1281–1328. [Google Scholar] [CrossRef]
- Goodger, J.Q.D.; Heskes, A.M.; Woodrow, I.E. Contrasting ontogenetic trajectories for phenolic and terpenoid defences in Eucalyptus froggattii. Ann. Botan. 2013, 112, 651–659. [Google Scholar] [CrossRef]
- Lu, F.; Zheng, L.; Chen, Y.; Li, D.; Zeng, R.; Li, H. Soil microorganisms alleviate the allelopathic effect of Eucalyptus grandis × E. urophylla leachates on Brassica chinensis. J. For. Res. 2017, 28, 1203–1207. [Google Scholar] [CrossRef]
- Zhang, J.; An, M.; Wu, H.; Liu, D.L.; Stanton, R. Phytotoxic activity and chemical composition of aqueous volatile fractions from Eucalyptus species. PLoS ONE 2014, 9, e93189. [Google Scholar] [CrossRef]
- Ieri, F.; Cecchi, L.; Giannini, E.; Clemente, C.; Romani, A. GC-MS and HS-SPME-GC_GC-TOFMS determination of the volatile composition of essential oils and hydrosols (by-products) from four Eucalyptus species cultivated in Tuscany. Molecules 2019, 24, 226. [Google Scholar] [CrossRef]
- He, H.; Song, Q.; Wang, Y.; Yu, S. Phytotoxic effects of volatile organic compounds in soil water taken from a Eucalyptus urophylla plantation. Plant Soil 2014, 377, 203–215. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, D.J.; Zhang, J.; Ji, T.W.; Tang, Z.Q.; Zhao, Y.Y. Allelopathic effects of volatile compounds from Eucalyptus grandis on Vigna radiata, Raphanus sativus and Lactuca sativa. Allelopathy J. 2015, 36, 273–282. [Google Scholar]
- Becerra, P.I.; Catford, J.A.; Inderjit; McLeod, M.L.; Andonian, K.; Erik, T.; Aschehoug, E.T.; Montesinos, D.; Callaway, R.M. Inhibitory effects of Eucalyptus globulus on understorey plant growth and species richness are greater in non-native regions. Global Ecol. Biogeogr 2017, 27, 68–76. [Google Scholar] [CrossRef]
- Frick, K.M.; Kamphuis, L.G.; Siddique, K.H.M.; Singh, K.B.; Foley, R.C. Quinolizidine Alkaloid Biosynthesis in Lupins and Prospects for Grain Quality Improvement. Front. Plant Sci. 2017, 8, 87. [Google Scholar] [CrossRef]
- Lehle, F.R.; Frans, R.; McClelland, M. Allelopathic potential of hope white lupine (Lupinus albus) herbage and herbage extracts. Weed Sci. 1983, 31, 513–519. [Google Scholar] [CrossRef]
- Wink, M. Inhibition of seed germination by quinolizidine alkaloids: Aspects of allelopathy in Lupinus albus L. Planta 1983, 158, 365–368. [Google Scholar] [CrossRef]
- Turner, B.L. A new species of Lupinus (Fabaceae) from Oaxaca, Mexico: A shrub or tree mostly three to height meters high. Phytologia 1995, 79, 102–107. [Google Scholar]
- Kato-Noguchi, H.; Fushimi, Y.; Shigemori, H. An allelopathic substance in red pine needles (Pinus densiflora). J. Plant Physiol. 2009, 166, 442–446. [Google Scholar] [CrossRef]
- Anwar, T.; Ilyas, N.; Qureshi, R.; Munazir, M.; Rahim, B.Z.; Qureshi, H.; Kousar, R.; Maqsood, M.; Abbas, Q.; Bhatti, M.I.; et al. Allelopathic potential of Pinus roxburghii needles against selected weeds of wheat crop. Appl. Ecol. Environ. Res. 2019, 17, 1717–1739. [Google Scholar] [CrossRef]
- Gallet, C. Allelopathic potential in bilberry-spruce forests: Influence of phenolic compounds on Spruce seedlings. J. Chem. Ecol. 1994, 20, 211–218. [Google Scholar] [CrossRef]
- Yang, L.; Ruan, X.; Jiang, D.; Zhang, J.; Pan, C.; Wang, Q. Physiological effects of autotoxicity due to DHAP stress on Picea schrenkiana regeneration. PLoS ONE 2017, 12, e0177047. [Google Scholar] [CrossRef]
- Bi, J.; Blanco, J.A.; Seely, B.; Kimmins, J.P.; Ding, Y.; Welham, C. Yield decline in Chinese-fir plantations: A simulation investigation with implications for model complexity. Can. J. For. Res. 2007, 37, 1615–1630. [Google Scholar] [CrossRef]
- Xia, Z.-C.; Kong, C.-H.; Chen, L.-C.; Wang, P.; Wang, S.-L. A broadleaf species enhances an autotoxic conifers growth through belowground chemical interactions. Ecology 2016, 97, 2283–2292. [Google Scholar] [CrossRef]
- Blum, U. Allelopathic interactions involving phenolic acids. J. Nematol. 1996, 28, 259–267. [Google Scholar]
- Daglish, C. The isolation and identification of a hydrojuglone glycoside occurring in the walnut. Biochemical J. 1950, 47, 452–457. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Rhodes, D. Biosynthesis and molecular actions of specialized 1,4-naphthoquinone natural products produced by horticultural plants. Horticult. Res. 2016, 3, 16046. [Google Scholar] [CrossRef]
- Müller, W.; Leistner, E. 1,4-Naphthoquinone, an intermediate in juglone (5-hydroxy-1,4-naphthoquinone) biosynthesis. Phytochemistry 1976, 15, 407–410. [Google Scholar] [CrossRef]
- McCoy, R.M.; Utturkar, S.M.; Joseph, W.; Crook, J.W.; Thimmapuram, J.; Widhalm, J.R. The origin and biosynthesis of the naphthalenoid moiety of juglone in black walnut. Horticult. Res. 2018, 5, 67. [Google Scholar] [CrossRef]
- Vestena, S.; Fett-Neto, A.G.; Duarte, R.C.; Ferreira, A.G. Regulation of mimosine accumulation in Leucaena leucocephala seedlings. Plant Sci. 2001, 161, 597–604. [Google Scholar] [CrossRef]
- Yafuso, J.T.; Negi, V.S.; Bingham, J.-P.; Borthakur, D. An O-acetylserine (thiol) lyase from Leucaena leucocephala is a cysteine synthase but not a mimosine synthase. Appl. Biochem. Biotechnol. 2014, 173, 1157–1168. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Iwasaki, H.; Oogai, S.; Fukuta, M.; Parveen, S.; Hossain, M.A.; Anai, T.; Oku, H. Molecular characterization of cytosolic cysteine synthase in Mimosa pudica. J. Plant Res. 2018, 131, 319–329. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Iwasaki, H.; Parveen, S.; Oogai, S.; Fukuta, M.; Hossain, M.A.; Anai, T.; Oku, H. Cytosolic cysteine synthase switch cysteine and mimosine production in Leucaena leucocephala. Appl. Biochem. Biotechnol. 2018, 186, 613–632. [Google Scholar] [CrossRef]
- Tangendjaja, B.; Lowry, J.B.; Wills, R.B.H. Isolation of a mimosine degrading enzyme from Leucaena leaf. J. Sci. Food Agric. 1986, 37, 523–526. [Google Scholar] [CrossRef]
- Xuan, T.D.; Minh, T.N.; Khan, T.D. Isolation and biological activities of 3-hydroxy-4(1H)-pyridone. J. Plant Interact. 2016, 11, 94–100. [Google Scholar] [CrossRef]
- Xu, Y.; Tao, Z.; Jin, Y.; Chen, S.; Zhou, Z.; Gong, A.G.W.; Yuan, Y.; Dong, T.T.X.; Tsim, K.W.K. Jasmonate-elicited stress induces metabolic change in the leaves of Leucaena leucocephala. Molecules 2018, 232, 188. [Google Scholar] [CrossRef]
- Cheng, A.; Lou, Y.; Mao, Y.; Lu, S.; Wang, L.; Chen, X. Plant terpenoids: Biosynthesis and ecological functions. J. Int. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Andrew, R.L.; Kesze, A.; Foley, W.J. Intensive sampling identifies previously unknown chemotypes, population divergence and biosynthetic connections among terpenoids in Eucalyptus tricarpa. Phytochemistry 2013, 94, 148–158. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Hofberger, J.A.; Ramirez, A.M.; Bergh, E.; Zhu, X.; Bouwmeester, H.J.; Schuurink, R.C.; Schranz, M.E. Large-scale evolutionary analysis of genes and supergene clusters from terpenoid modular pathways provides insights into metabolic diversification in flowering plants. PLoS ONE 2015, 10, e0128808. [Google Scholar] [CrossRef]
- Külheim, C.; Padovan, A.; Hefer, C.; Krause, S.T.; Köllner, T.G.; Myburg, A.A.; Degenhardt, J.; Foley, W.J. The eucalyptus terpene synthase gene family. BMC Genom. 2015, 16, 450. [Google Scholar] [CrossRef]
- Guo, L.; Qiu, J.; Li, L.-F.; Lu, B.; Olsen, K.; Fan, L. Genomic clues for crop–weed interactions and evolution. Trends Plant Sci. 2018, 23, 1102–1115. [Google Scholar] [CrossRef]
- Takos, A.M.; Rook, F. Why biosynthetic genes for chemical defense compounds cluster. Trends Plant Sci. 2012, 17, 383–388. [Google Scholar] [CrossRef]
- Guo, Q.; Lu, N.; Luo, Z.; Sun, Y.; Jin, S.; Wang, S.; Guo, Z.; Li, F.; Chen, S.; Zhang, W.; et al. An assessment of the environmental impacts of transgenic triploid Populus tomentosa in field condition. Forests 2018, 9, 482. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Zhong, Y.; Wu, Y.; Li, Z.; Xu, L.-A.; Xu, M. Transcriptome analysis and identification of genes related to terpenoid biosynthesis in Cinnamomum camphora. BMC Genom. 2018, 19, 550. [Google Scholar] [CrossRef]
- Mao, J.; He, Z.; Hao, J.; Liu, T.; Chen, J.; Huang, S. Identification, expression, and phylogenetic analyses of terpenoid biosynthesis-related genes in secondary xylem of loblolly pine (Pinus taeda L.) based on transcriptome analyses. Peer J. 2019, 7, e6124. [Google Scholar] [CrossRef]
- Guo, X.; Cui, M.; Deng, M.; Liu, X.; Huang, X.; Zhang, X.; Luo, L. Molecular differentiation of five Cinnamomum camphora chemotypes using desorption atmospheric pressure chemical ionization mass spectrometry of raw leaves. Sci. Rep. 2017, 7, 46579. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Swanton, C.J.; Nkoa, R.; Blackshaw, R.E. Experimental methods for crop-weed competition studies. Weed Sci. 2015, 63, 2–11. [Google Scholar] [CrossRef]
- FAO. 2019. Available online: http://www.fao.org/faostat/en/#home (accessed on 11 March 2019).
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef]
- Saha, D.; Marble, S.C.; Pearson, B.J. Allelopathic effects of common landscape and nursery mulch materials on weed control. Front. Plant Sci. 2018, 9, 733. [Google Scholar] [CrossRef]
- De Feo, V.; Mancini, E.; Voto, E.; Curini, M.; Digilio, M. Bioassayoriented isolation of an insecticide from Ailanthus altissima. J. Plant Interact. 2009, 4, 119–123. [Google Scholar] [CrossRef]
- Farooq, M.; Bajwa, A.A.; Cheema, S.A.; Cheema, Z.A. Application of allelopathy in crop production. Int. J. Agric. Biol. 2013, 15, 1367–1378. [Google Scholar]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H.M. The role of allelopathy in agricultural pest management. Pest. Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef]
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.M.; Putievsky, E.; Lerner, H.R. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Brophy, J.J.; Davies, N.W.; Southwell, I.A.; Stiff, I.A.; Williams, L.R. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J. Agric. Food Chem. 1989, 37, 1330–1335. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Spencer, G.F. Synthesis and herbicidal activity of modified monoterpenes structurally similar to cinmethylin. Weed Science 1996, 44, 7–11. [Google Scholar]
- Jones, R.G. Cinmethylin—A new herbicide developed for use in rice. In Pest Management in Rice; Grayson, B.T., Green, M.B., Copping, L.G., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 349–357. [Google Scholar]
- Owens, D.K.; Nanayakkara, N.P.; Dayan, F.E. In planta mechanism of action of leptospermone: Impact of its physico-chemical properties on uptake, translocation, and metabolism. J. Chem. Ecol. 2013, 39, 262–270. [Google Scholar] [CrossRef]
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest. Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef]
- Duke, S.O.; Cantrell, C.L.; Meepagala, K.M.; Wedge, D.E.; Tabanca, N.; Kevin, K.; Schrader, K.R. Natural toxins for use in pest management. Toxins 2010, 2, 1943–1962. [Google Scholar] [CrossRef]
- Ooka, J.K.; Owens, D.K. Allelopathy in tropical and subtropical species. Phytochem Rev. 2018, 17, 1225–1237. [Google Scholar] [CrossRef]
- Macias, F.A.; Galindo, J.C.G.; Molinillo, J.M.G.; Castellano, D.; Velasco, R.F.; Chinchilla, D. Developing new herbicide models from allelochemicals. Pestic. Sci. 1999, 55, 633–675. [Google Scholar] [CrossRef]
- Barton, A.F.M.; Dell, B.; Knight, A.R. Herbicidal activity of cineole derivatives. J. Agric. Food Chem. 2010, 58, 10147–10155. [Google Scholar] [CrossRef]
- Barton, A.F.M.; Clarke, B.R.; Dell, B.; Knight, A.R. Post-emergent herbicidal activity of cineole derivatives. J. Pest. Sci. 2014, 87, 531–541. [Google Scholar] [CrossRef]
- Durán, A.G.; Chinchilla, N.; Molinillo, J.M.; Macías, F.A. Influence of lipophilicity in O-acyl and O-alkyl derivatives of juglone and lawsone: A structure-activity relationship study in the search for natural herbicide models. Pest Manag. Sci. 2018, 74, 682–694. [Google Scholar] [CrossRef]
- Kato, T.; Sizumura, Y.; Fukishima, M.; Honda, T.; Nakanishi, T.; Noguchi, T. Antitumor activity of novel ailanthone derivatives in vitro and in vivo. Anticancer Res. 1988, 8, 573–579. [Google Scholar] [PubMed]
- Chen, S.; Zheng, T.; Ye, C.; Huannixi, W.; Yakefu, Z.; Meng, Y.; Peng, X.; Tian, Z.; Wang, J.; Ma, Y.; et al. Algicidal properties of extracts from Cinnamomum camphora fresh leaves and their main compounds. Ecotoxicol. Environ. Saf. 2018, 163, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Puig, C.G.; Gonçalves, R.F.; Valentão, P.; Andrade, P.B.; Reigosa, M.J.; Pedrol, N. The consistency between phytotoxic effects and the dynamics of allelochemicals release from Eucalyptus globulus leaves used as bioherbicide green manure. J. Chem. Ecol. 2018, 44, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Quddus, M.S.; Bellairs, S.M.; Wurm, P.A.S. Acacia holosericea (Fabaceae) litter has allelopathic and physical effects on mission grass (Cenchrus pedicellatus and C. polystachios) (Poaceae) seedling establishment. Aust. J. Bot. 2014, 62, 189–195. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.B.; Melo, J.W.S.; Guedes, N.M.P.; Gontijo, L.M.; Guedes, R.N.C.; Gondim, M.G.C., Jr. Bioinsecticide-predator interactions: Azadirachtin behavioral and reproductive impairment of the coconut mite predator Neoseiulus baraki. PLoS ONE 2015, 10, e0118343. [Google Scholar] [CrossRef]
- McKenzie, N.; Helson, B.; Thompson, D.; Otis, C.; McFarlane, J.; Buscarini, T.; Meating, J. Azadirachtin: An effective systemic insecticide for control of Agrilus planipennis (Coleoptera: Buprestidae). J. Econ. Entomol. 2010, 103, 708–717. [Google Scholar] [CrossRef]
- Grimalt, S.; Thompson, D.; Chartrand, D.; McFarlane, J.; Helson, B.; Lyons, B.; Meating, J.; Scarr, T. Foliar residue dynamics of azadirachtins following direct stem injection into white and green ash trees for control of emerald ash borer. Pest Manage. Sci. 2011, 67, 1277–1284. [Google Scholar] [CrossRef]
- Kreutzweiser, D.; Thompson, D.; Grimalt, S.; Chartrand, D.; Good, K.; Scarr, T. Environmental safety to decomposer invertebrates of azadirachtin (neem) as a systemic insecticide in trees to control emerald ash borer. Ecotoxicol. Environ. Saf. 2011, 74, 1734–1741. [Google Scholar] [CrossRef]
- Koul, O.; Wahab, S. Neem: Today and in the New Millennium; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Sindhu, A.; Kumar, S.; Sindhu, G.; Ali, H.; Abdulla, M.K. Effect of neem (Azadirachta indica A. Juss) leachates on germination and seedling growth of weeds. Allelopathy. J. 2005, 16, 329–334. [Google Scholar]
- Cloyd, R.A.; Cycholl, N.L. Phytotoxicity of selected insecticides on greenhouse-grown herbs. Hortscience 2002, 37, 671–672. [Google Scholar] [CrossRef]
- Kulkarni, N.S.; Sawant, I.S.; Sawant, S.D.; Adsule, P.G. Bio-efficacy of neem formulations (azadirachtin 1% and 5%) on important insect pests of grapes and their effect on shelf life. Acta Hortic. 2008, 785, 305–312. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Lee, M.Y. Essential oils as repellents against arthropods. Biomed. Res. Int. 2018, 6860271. [Google Scholar] [CrossRef]
- Maia, M.F.; Moore, S.J. Plant-based insect repellents: A review of their efficacy, development and testing. Malaria J. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Geng, Z.; Zhang, W.; Liang, J.; Wang, C.; Deng, Z.; Du, S. The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dai, G. Acaricidal activity of compounds from Cinnamomum camphora (L.) Presl against the carmine spider mite, Tetranychus cinnabarinus. Pest Manag. Sci. 2015, 71, 1561–1571. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Song, L.; Cao, X.; Yao, X.; Tang, F.; Yue, Y.D. GC×GC-TOFMS analysis of essential oils composition from leaves, twigs and seeds of Cinnamomum camphora L. Presl and their insecticidal and repellent activities. Molecules 2016, 21, 423. [Google Scholar] [CrossRef]
- Tsao, R.; Romanchuk, F.; Peterson, C.J.; Coats, J.R. Plant growth regulatory effect and insecticidal activity of the extracts of the tree of heaven (Ailanthus altissima). BMC Ecol. 2002, 2. [Google Scholar] [CrossRef]
- Ishaaya, I.; Hirashima, A.; Yablonski, S.; Tawata, S.; Eto, M. Mimosine, a nonprotein amino acid, inhibits growth and enzyme systems in Tribolium castaneum. Pesticide Biochem. Physiol. 1991, 39, 35–42. [Google Scholar] [CrossRef]
- Nguyen, B.C.Q.; Chompoo, J.; Tawata, S. Insecticidal and nematicidal activities of novel mimosine derivatives. Molecules 2015, 20, 16741–16756. [Google Scholar] [CrossRef]
- Thiboldeaux, R.L.; Lindroth, R.L.; Tracy, J.W. Differential toxicity of juglone (5-hydroxy-1,4-naphthoquinone) and related naphthoquinones to saturniid moths. J. Chem. Ecol. 1994, 20, 1631–1641. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Song, Z.; Fang, G. Insecticidal activities and active components of the alcohol extract from green peel of Juglans mandshurica. J. Forestry Res. 2007, 18, 62–64. [Google Scholar] [CrossRef]
- Piskorski, R.; Ineichen, S.; Dorn, S. Ability of the oriental fruit moth Grapholita molesta (Lepidoptera: Tortricidae) to detoxify juglone, the main secondary metabolite of the non-host plant walnut. J. Chem. Ecol. 2011, 37, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Smith, S.L. Effects of the chitin synthetase inhibitor plumbagin and its 2-demethyl derivative juglone on insect ecdysone 20-monooxygenase activity. Experientia 1988, 44, 990–991. [Google Scholar] [CrossRef]
- Magiri, E.N.; Konji, V.N.; Makawiti, D.W.; Midiwo, J. Effect of plant quinones on insect flight muscle mitochondria. Int. J. Trop. Insect. Sci. 1995, 16, 183. [Google Scholar] [CrossRef]
- Lv, S.T.; Du, W.X.; Bai, S.M.; Chen, G. Insecticidal effect of juglone and its disturbance analysis in metabolic profiles of Aphis gossypii glover using 1H NMR-based metabonomics approach. Phytoparasitica 2018, 46, 521–531. [Google Scholar] [CrossRef]
- Hu, W.; Du, W.; Bai, S.; Lv, S.; Chen, G. Phenoloxidase, an effective bioactivity target for botanical insecticide screening from green walnut husks. Nat. Prod. Res. 2018, 32, 2848–2851. [Google Scholar] [CrossRef]
- Ribeiro, K.A.L.; Carvalho, C.M.; Molina, M.T.; Lima, E.P.; Montero, E.L.; Reys, J.R.M.; Oliveira, M.B.F.; Pinto, A.V.; Santana, A.E.G.; Goulart, M.O.F. Activities of naphthoquinones against Aedes aegypti (Linnaeus, 1762) (Diptera:Culicidae), vector of dengue and Biomphalaria glabrata (Say, 1818), intermediate host of Schistosoma mansoni. Acta Trop. 2009, 111, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Huang, C.G.; Chen, W.J.; Kuo, Y.H.; Chang, S.T. Larvicidal activity of tectoquinone isolated from red heartwood-type Cryptomeria japonica against two mosquito species. Bioresour. Technol. 2008, 99, 3617–3622. [Google Scholar] [CrossRef] [PubMed]
- Rudman, P.; da Costa, E.W.B.; Gay, F.J.; Wetherly, A.H. Relationship of tectoquinone to durability in Tectona grandis. Nature 1958, 181, 721–722. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Khanh, T.D.; Chung, I.M.; Tawata, S.; Xuan, T.D. Allelopathy for weed management in sustainable agriculture. Health 2007, 1, 2. [Google Scholar] [CrossRef]
- Isman, M.B.; Machial, C.M. Pesticides based on plant essential oils: From traditional practice to commercialization. In Naturally Occurring Bioactive Compounds; Rai, M., Carpinella, M.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 3, pp. 29–44. [Google Scholar] [CrossRef]
- Secretariat to the Convention on Biological Diversity. The Cartagena Protocol on Biosafety; Secretariat to the Convention on Biological Diversity: Montreal, QC, USA, 2000. [Google Scholar]
- Khan, M.S.; Khan, M.A.; Ahmad, D. Assessing utilization and environmental risks of important genes in plant abiotic stress tolerance. Front. Plant Sci. 2016, 7, 792. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Yu, X.; Shimazaki, T.; Kawaoka, A.; Ebinuma, H.; Kazuo, N. Watanabe Allelopathy assessments for the environmental biosafety of the salt-tolerant transgenic Eucalyptus camaldulensis, genotypes codA 12-5B, codA 12-5C, and codA 20C. J. Wood Sci. 2009, 55, 149–153. [Google Scholar] [CrossRef]
- Yu, X.; Kikuchi, A.; Matsunaga, E.; Shimada, T.; Watanabe, K.N. Environmental biosafety assessment on transgenic Eucalyptus globulus harboring the choline oxidase (codA) gene in semi-confined condition. Plant Biotechnol. 2013, 30, 73–76. [Google Scholar] [CrossRef]
- Yu, X.; Kikuchi, A.; Matsunaga, E.; Kawaoka, A.; Ebinuma, H.; Watanabe, K.N. A field trial to assess the environmental biosafety of codA-transgenic Eucalyptus camaldulensis cultivation. Plant Biotechnol. 2013, 30, 357–363. [Google Scholar] [CrossRef]
- Gilani, S.A.; Kikuchi, A.; Yu, X.; Ahmad, M.Z.; Sugano, M.; Fujii, Y.; Kazuo, N. Watanabe difference between non-transgenic and salt tolerant transgenic Eucalyptus camaldulensis for diversity and allelopathic effects of essential oils. Pak. J. Bot. 2017, 49, 345–351. [Google Scholar]
- Ohara, K.; Matsunaga, E.; Nanto, K.; Yamamoto, K.; Sasaki, K.; Ebinuma, H.; Yazaki, K. Monoterpene engineering in a woody plant Eucalyptus camaldulensis using a limonene synthase cDNA. Plant Biotech. J. 2010, 8, 28–37. [Google Scholar] [CrossRef]
- Lucas, A.M.; Pasquali, G.; Astarita, L.V.; Cassel, E. Comparison of genetically engineered (GE) and non-GE Eucalyptus trees using secondary metabolites obtained by steam distillation. J. Essential Oil Res. 2017, 29, 22–31. [Google Scholar] [CrossRef]
- USDA APHIS. Petition for Determination of Non-regulated Status for Freeze Tolerant Hybrid Eucalyptus Lines. 2011. Available online: http://www.aphis.usda.gov/brs/aphisdocs/11_01901p.pdf (accessed on 12 September 2018).
- Rastogi, S.; Dwivedi, U.N. Down-regulation of lignin biosynthesis in transgenic Leucaena leucocephala harboring O-methyltransferase gene. Biotechnol. Prog. 2006, 22, 609–616. [Google Scholar] [CrossRef]
- Jube, S.; Borthakur, D. Transgenic Leucaena leucocephala expressing the Rhizobium gene pydA encoding a meta-cleavage dioxygenase shows reduced mimosine content. Plant Physiol. Biochem. 2010, 48, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Ohmiya, Y.; Kurita, M.; Tsubomura, M.; Kondo, T.; Park, Y.W.; Baba, K.; Hayashi, T. Biosafety assessment of transgenic poplars overexpressing xyloglucanase (AaXEG2) prior to field trials. J. Wood Sci. 2008, 54, 408–413. [Google Scholar] [CrossRef]
- Newhouse, A.E.; Oakes, A.D.; Pilkey, H.C.; Roden, H.E.; Horton, T.R.; Powell, W.A. Transgenic American chestnuts do not inhibit germination of native seeds or colonization of mycorrhizal fungi. Front. Plant Sci. 2018, 9, 1046. [Google Scholar] [CrossRef]
- Kovalitskaya, Y.A.; Dayanova, L.K.; Azarova, A.B.; Shestibratov, K.A. RNA Interference-mediated down-regulation of 4-coumarate: Coenzyme A ligase in Populus tremula alters lignification and plant growth. Int. J. Environ. Sci. Educ. 2016, 11, 12259–12271. [Google Scholar]
- Kovalitskaya, Y.A.; Kovalenko, N.P.; Shestibratov, K.A. Populus tremula plants with reduced expression of the 4-coumarate-CoA ligase demonstrated defects of the rhizogenesis. Int. J. Engineer. Technol. 2018, 7, 1139–1144. [Google Scholar]
- Laitinen, M.; Julkunen-Tiitto, R.; Tahvanainen, J.; Heinonen, J.; Rousi, M. Variation in birch (Betula pendula) shoot secondary chemistry due to genotype, environment, and ontogeny. J. Chem. Ecol. 2005, 31, 697–717. [Google Scholar] [CrossRef]
- Peltonen, P.A.; Vapaavuori, E.; Julkunen-Tiitto, R. Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Global Change Biol. 2005, 11, 1305–1324. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Schestibratov, K.A.; Shadrina, T.E.; Bulatova, I.V.; Abramochkin, D.G.; Miroshnikov, A.I. Cotransformation of aspen and birch with three T-DNA regions from two different replicons in one Agrobacterium tumefaciens strain. Rus. J. Genet. 2010, 46, 1282–1289. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Korobova, A.V.; Shendel, G.V.; Kudoyarova, G.R.; Schestibratov, K.A. Effect of glutamine synthetase gene overexpression in birch (Betula pubescens) plants on auxin content and rooting in vitro. Dokl. Biochem. Biophys. 2018, 480, 143–145. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Kovalenko, N.P.; Shestibratov, K.A. Influence of nitrogen availability on growth of two transgenic birch species carrying the pine GS1a gene. Plants 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Galhano, R.; Wiemann, P.; Bueno, E.; Tiernan, M.; Wu, W.; Chung, I.-M.; Gershenzon, J.; Tudzynski, B.; Sesma, A.; et al. Genetic evidence for natural product-mediated plant–plant allelopathy in rice (Oryza sativa). New Phytol. 2012, 193, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.X.; Zhuang, Y.E.; Xu, T.C.; Li, Y.Z.; Li, Y.; Lin, W.X. Changes in rice allelopathy and rhizosphere microflora by inhibiting rice phenylalanine ammonia-lyase gene expression. J. Chem. Ecol. 2013, 39, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Stanišić, M.; Ćosić, T.; Savić, J.; Krstić-Milošević, D.; Mišić, D.; Smigocki, A.; Ninković, S.; Banjac, N. Hairy root culture as a valuable tool for allelopathic studies in apple. Tree Physiol. 2019. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
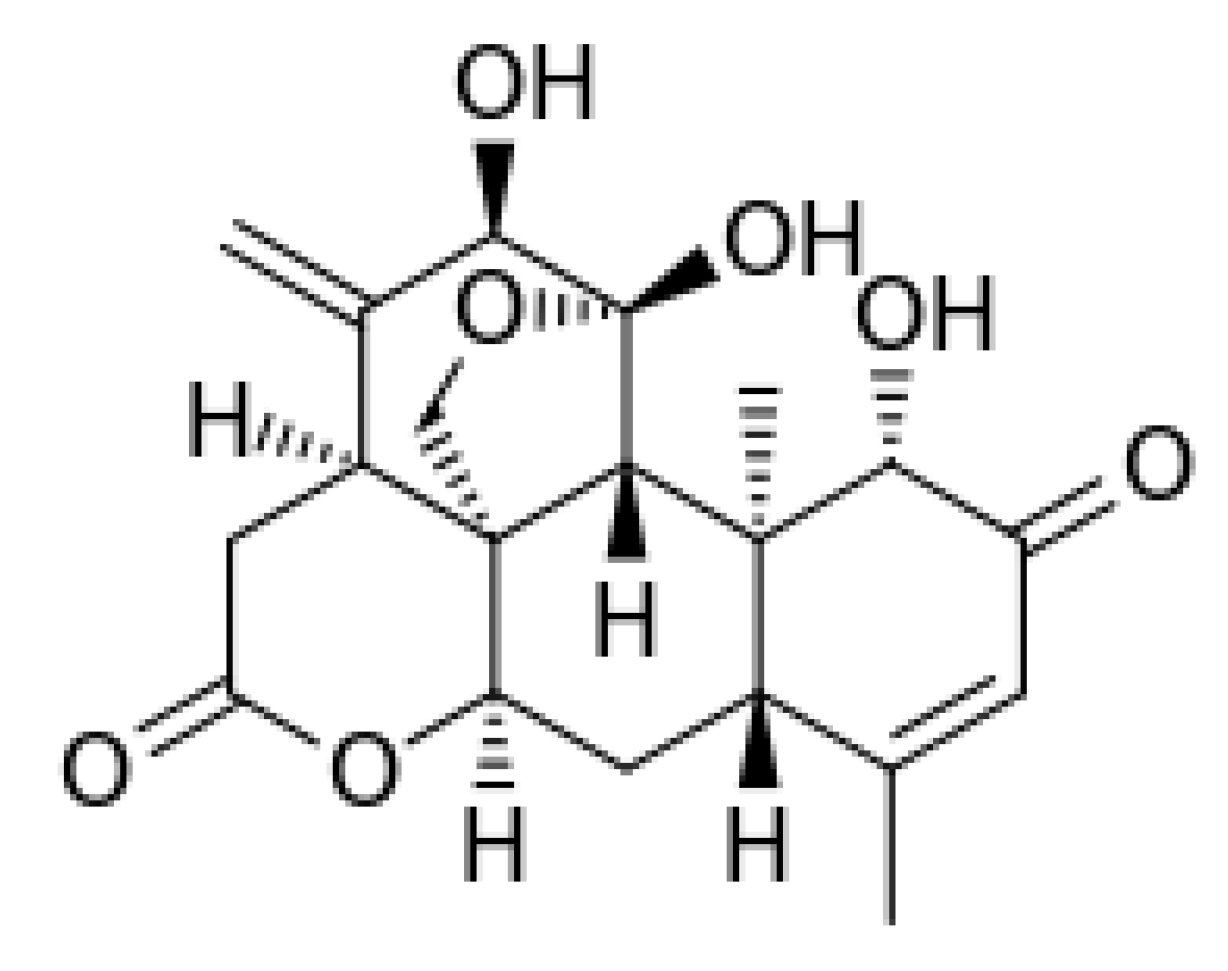
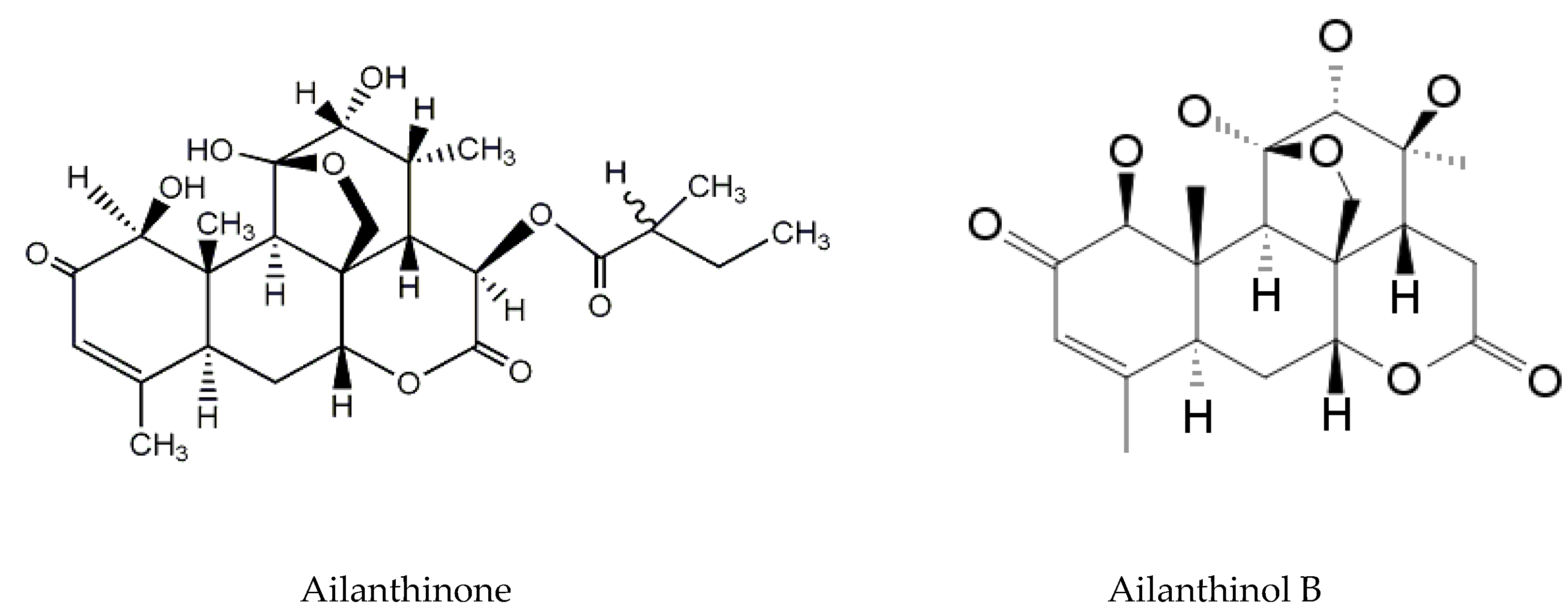
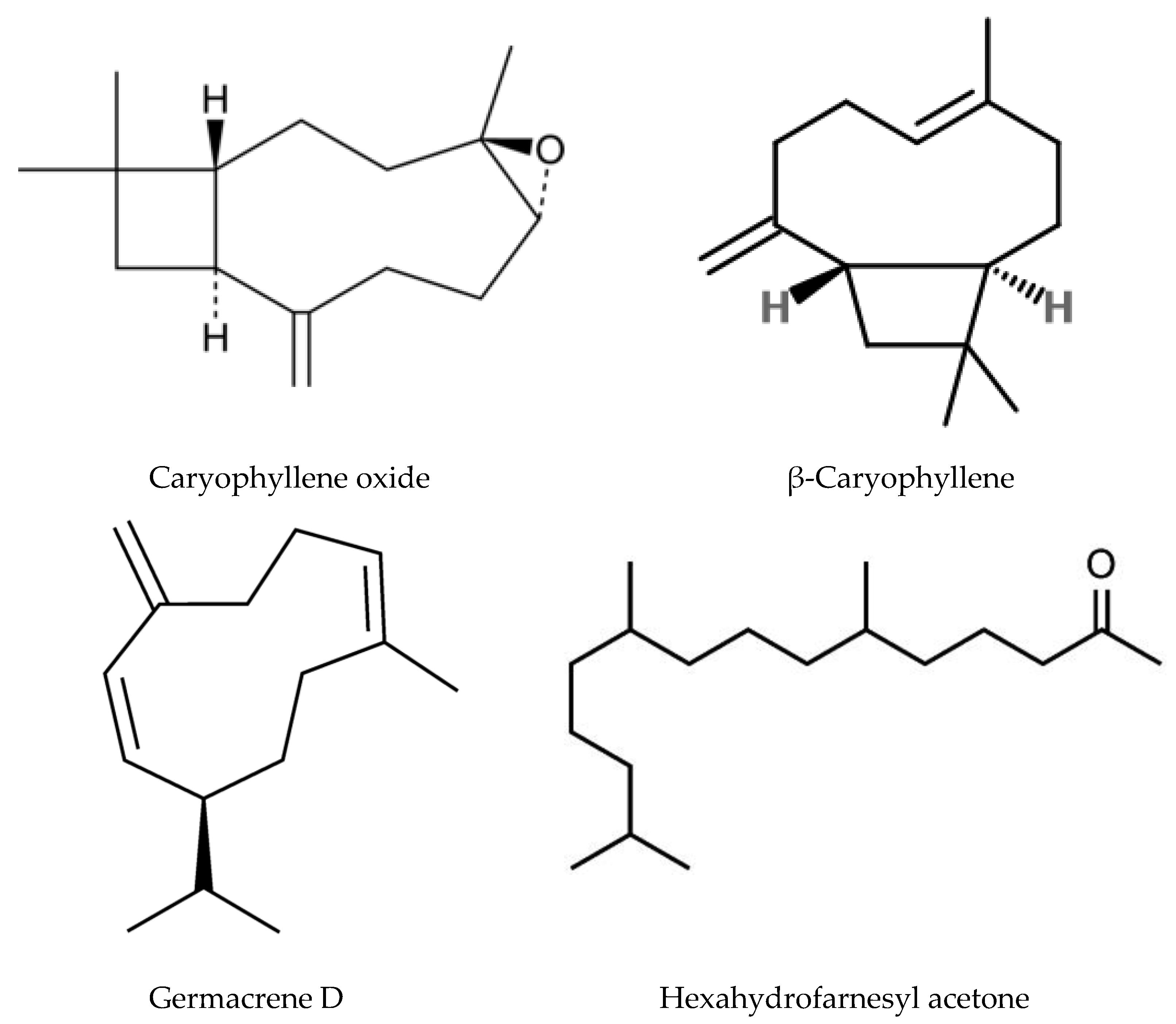
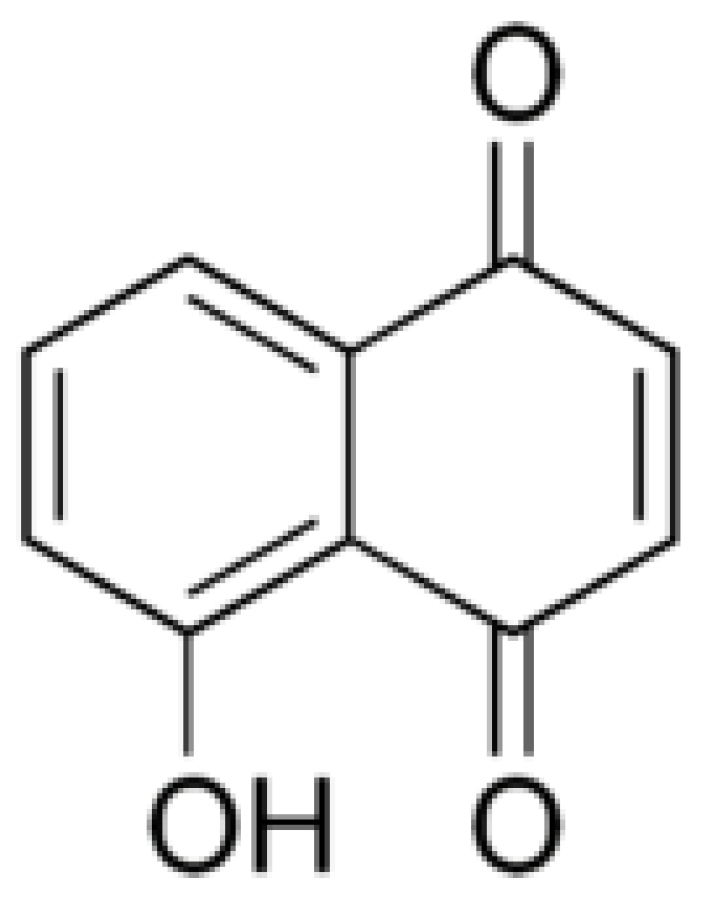
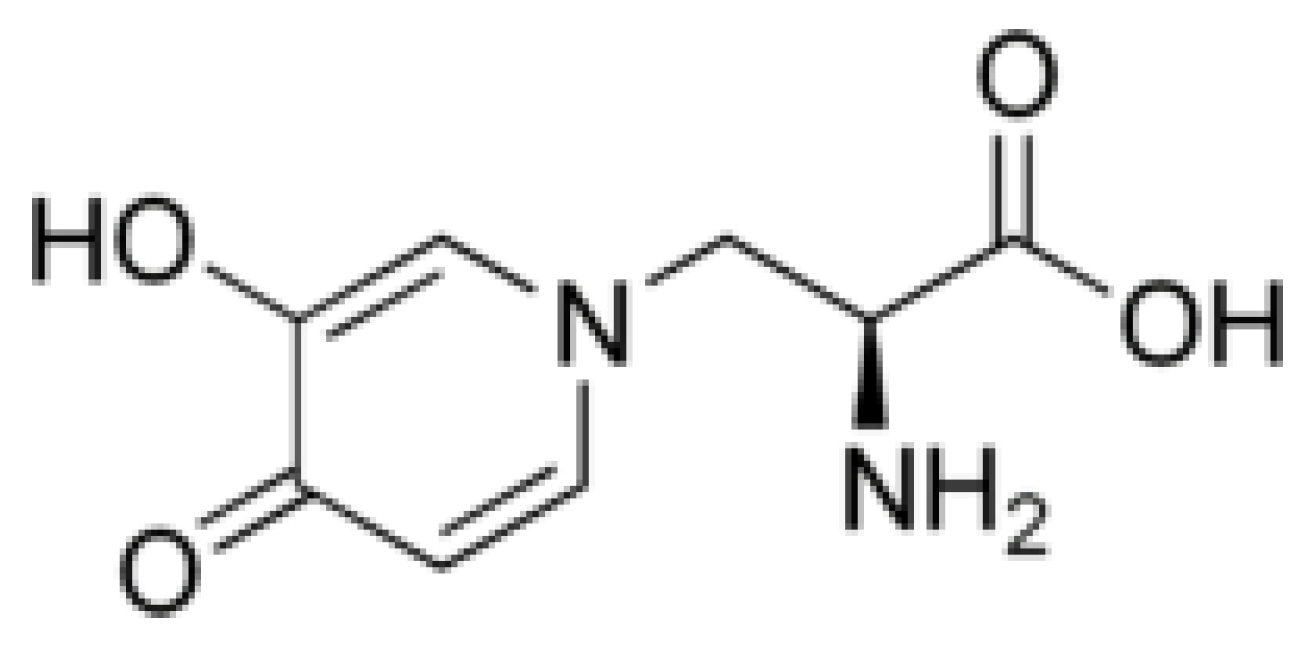
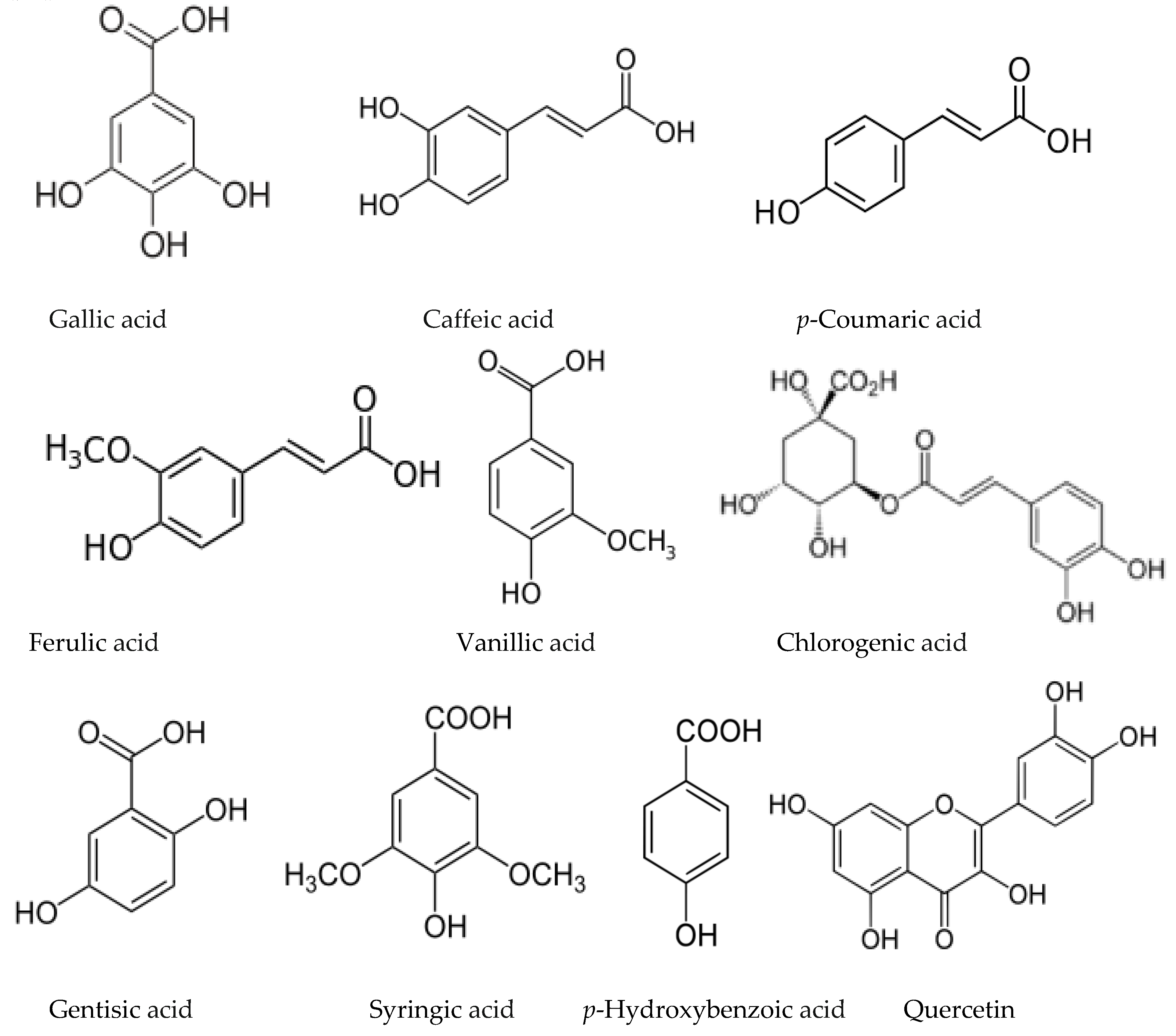
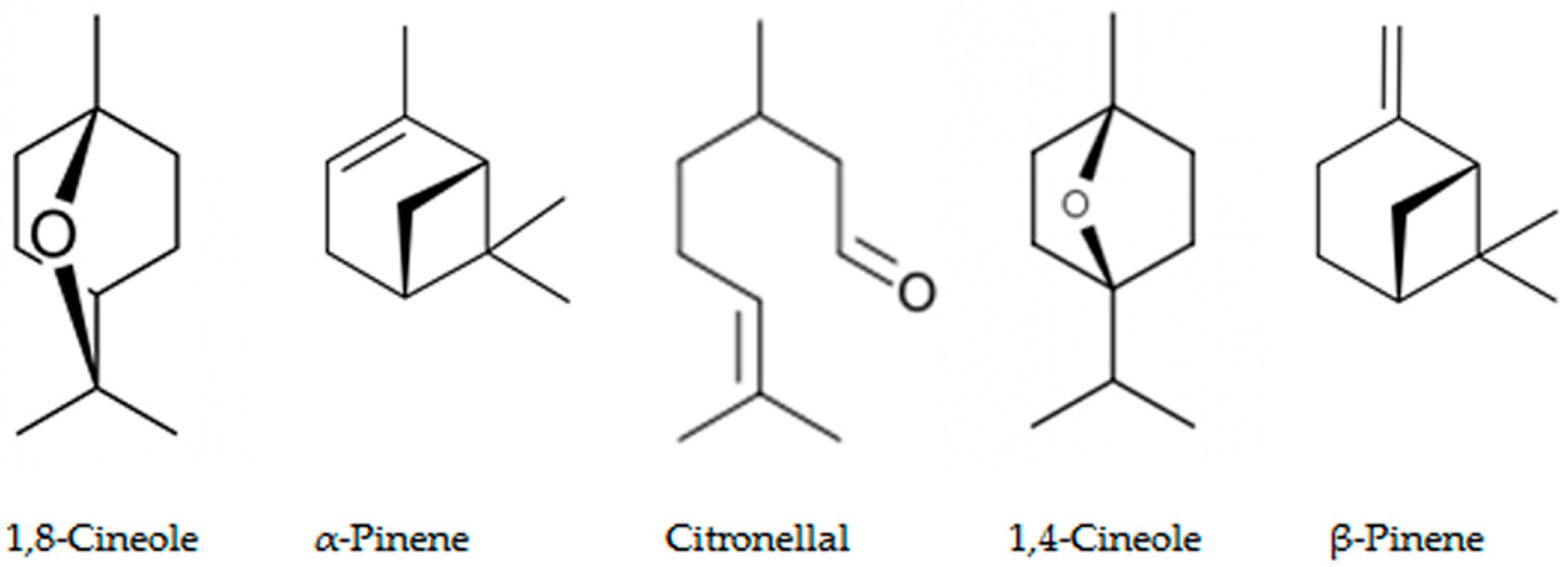
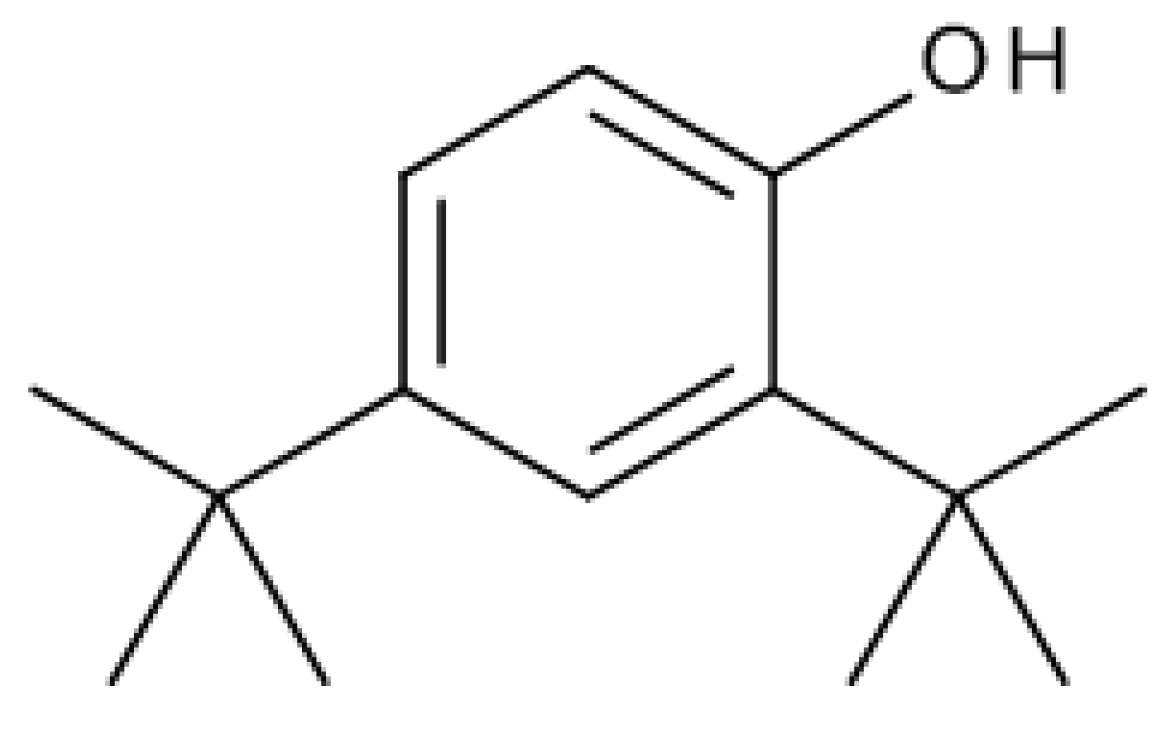
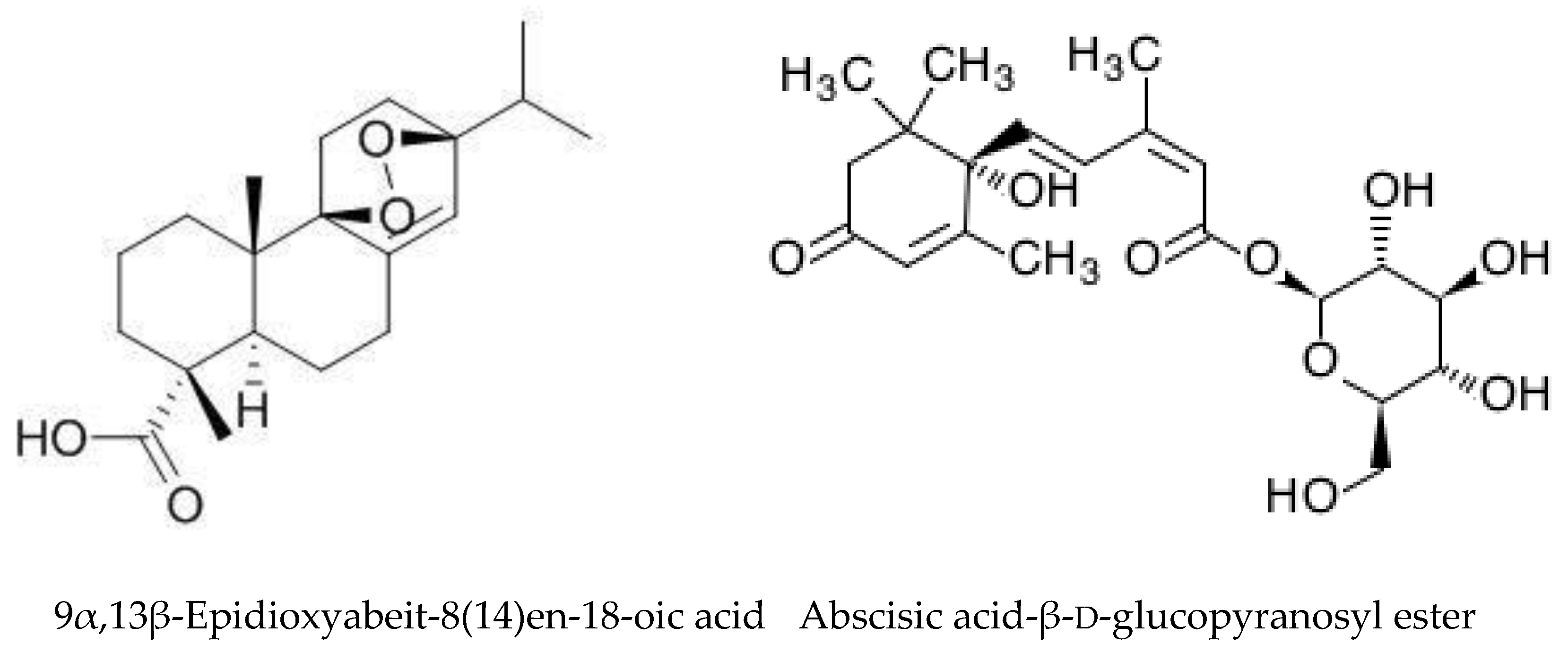

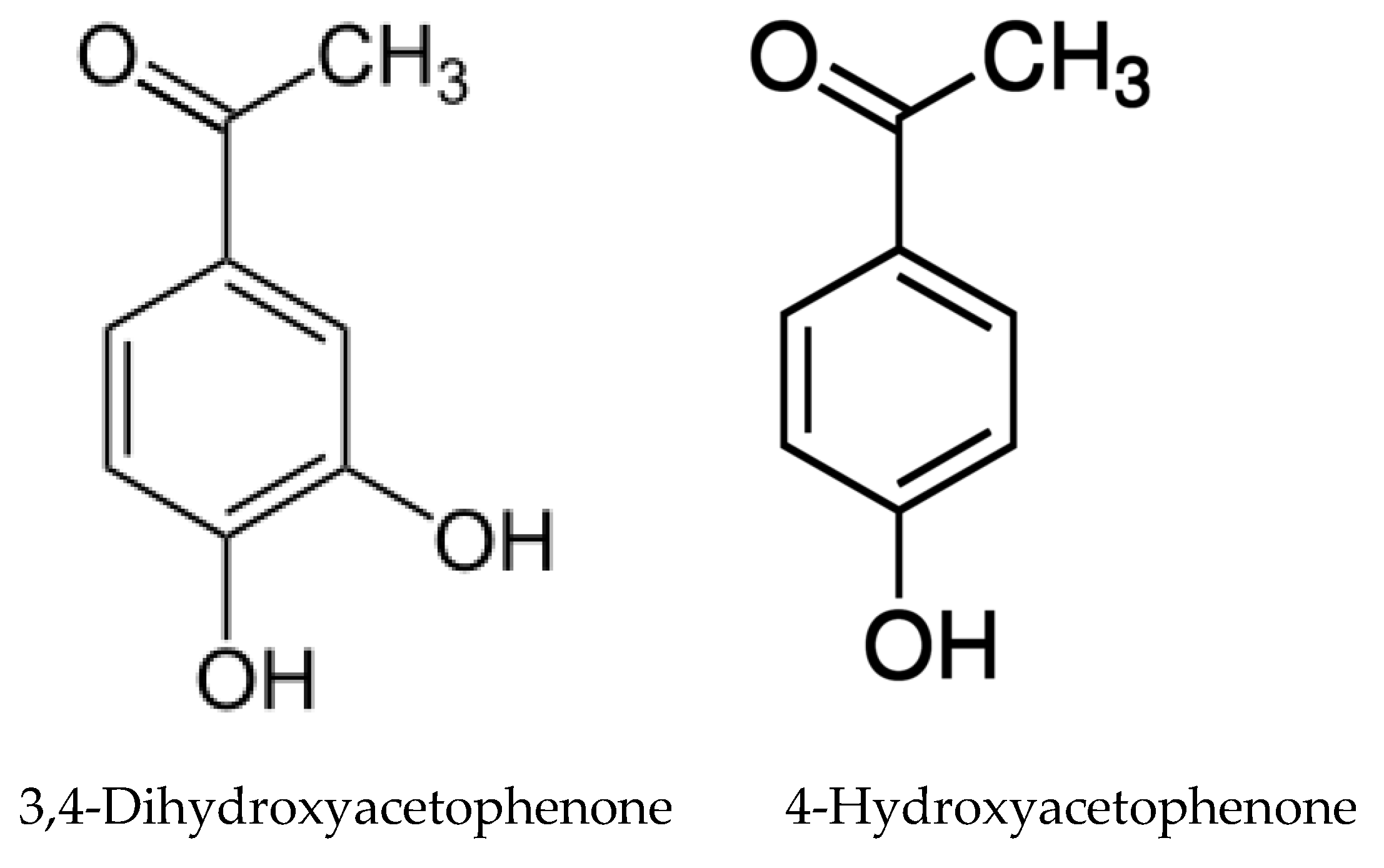
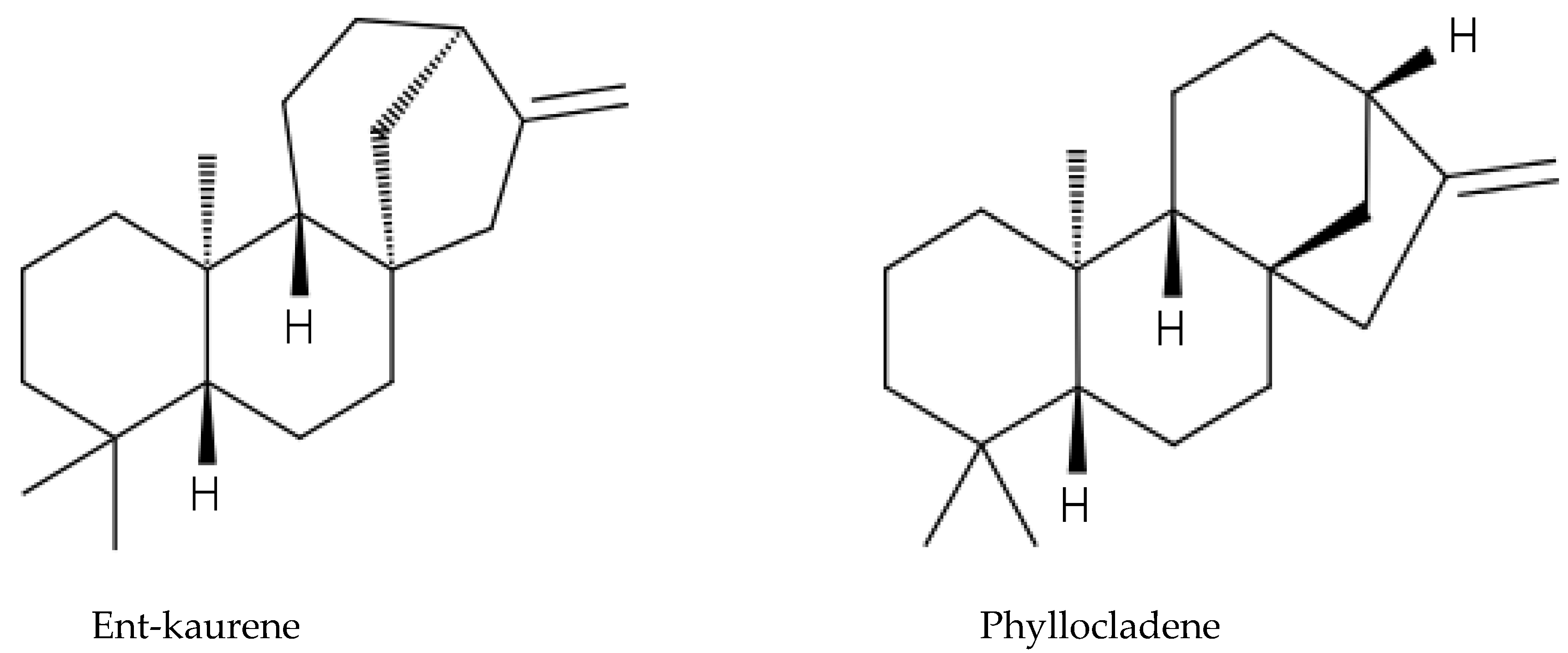
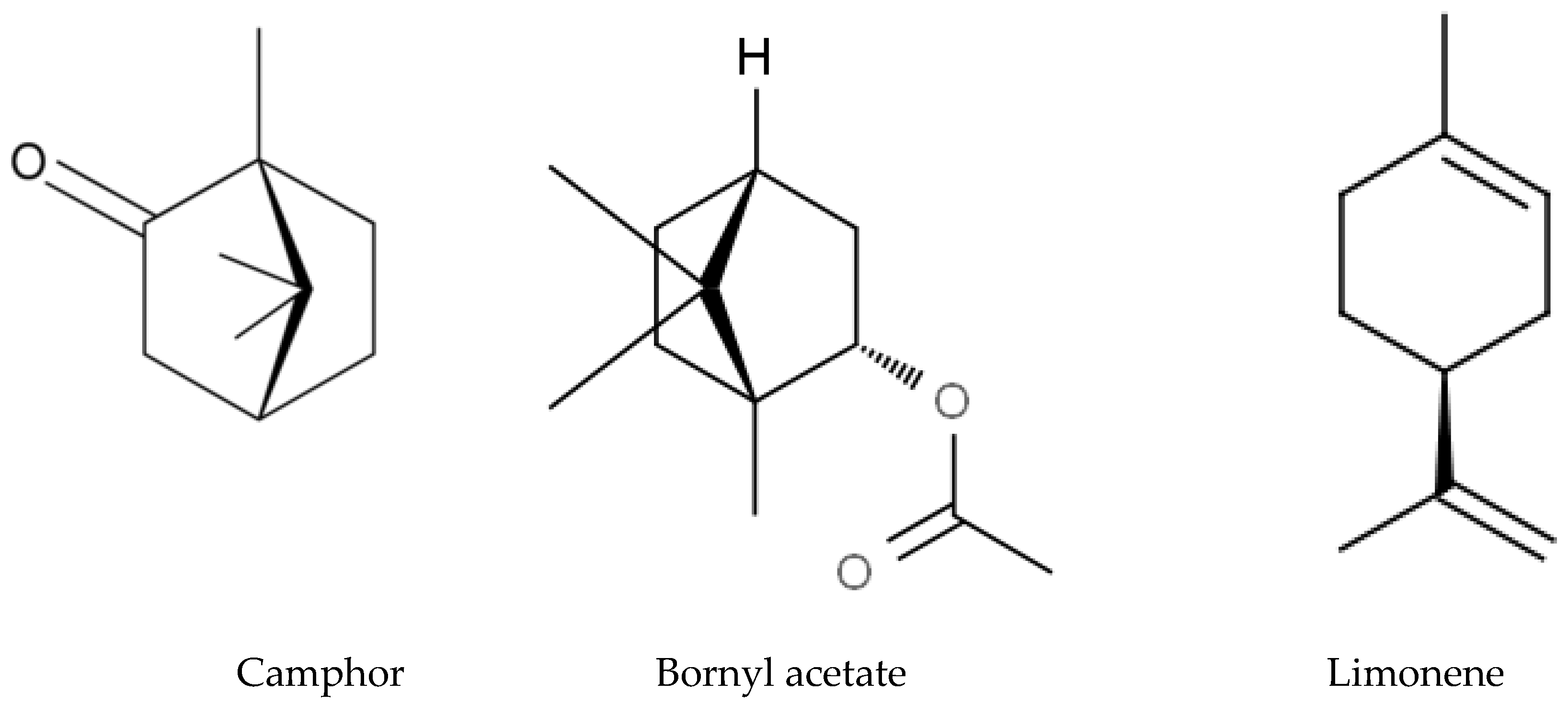
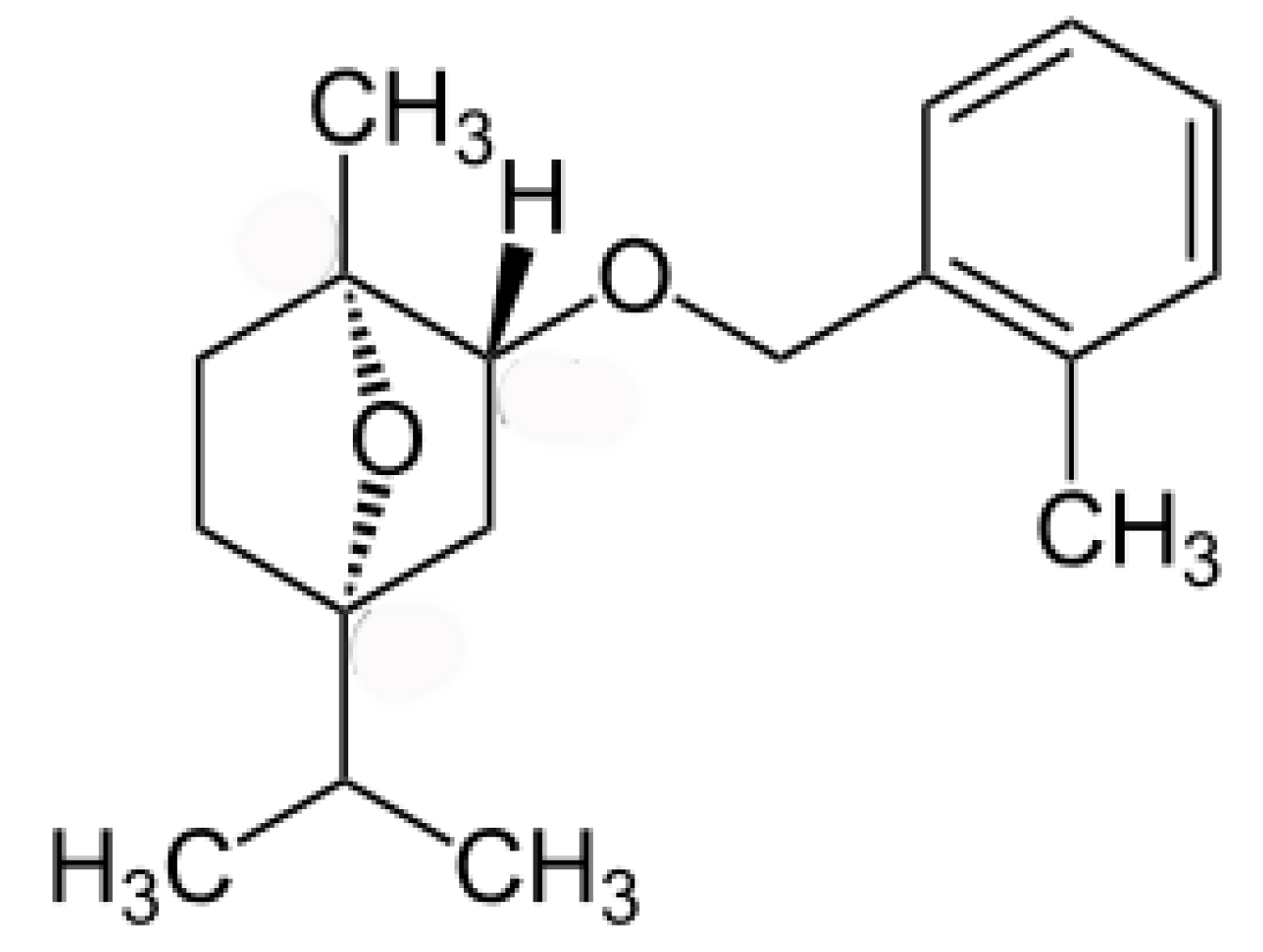
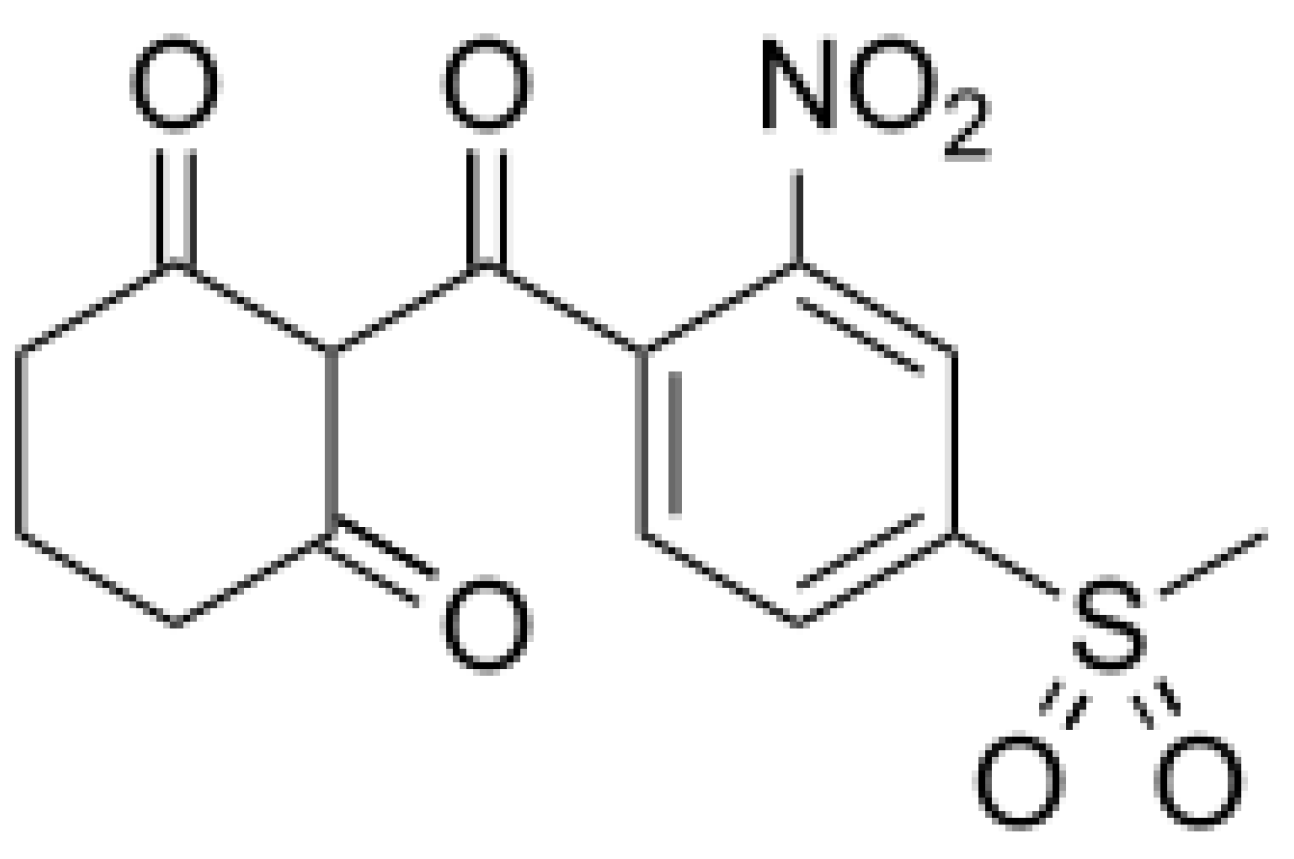
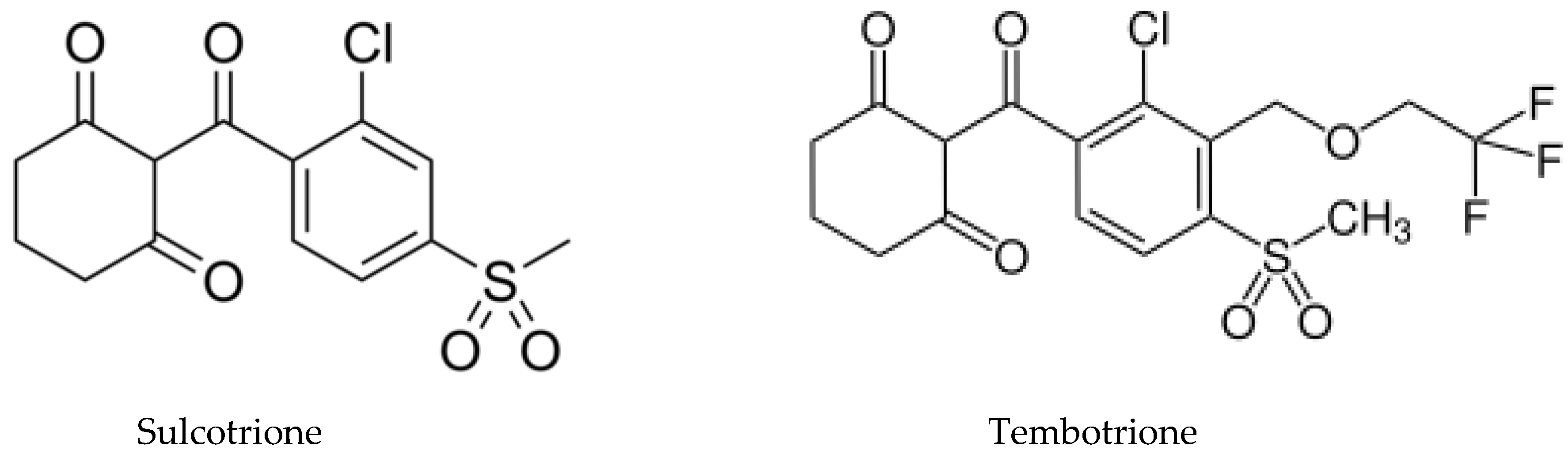
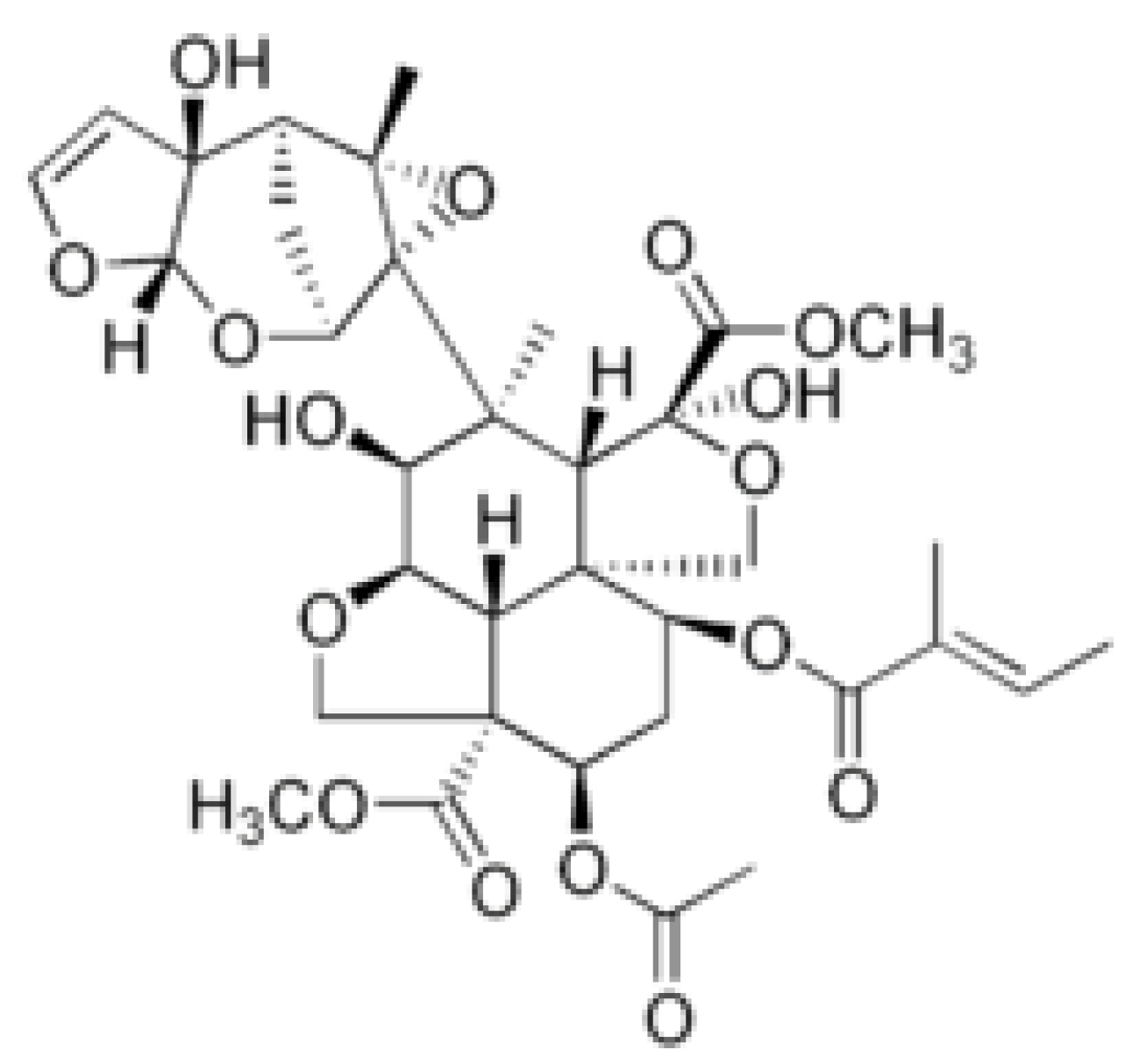
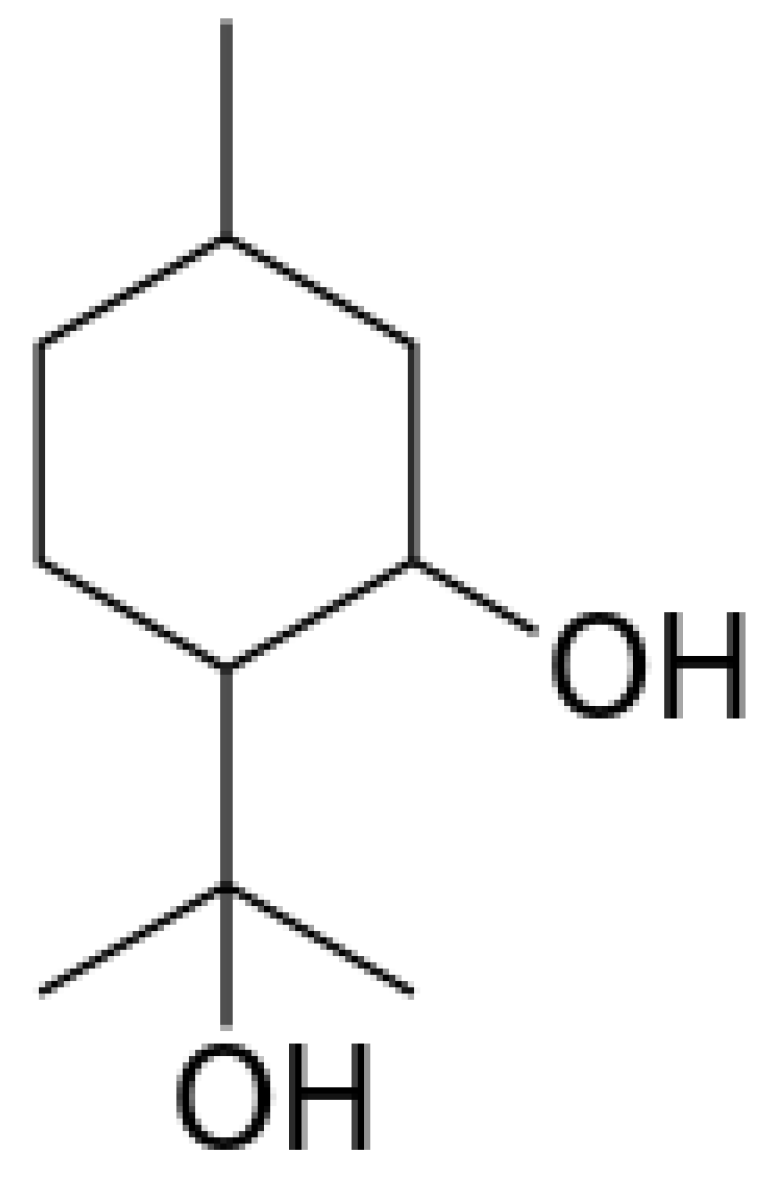
| Allelopathic Species | Test Species a | Analysis Type | Compounds | Reference |
|---|---|---|---|---|
| Albizia adianthifolia, Buddleja saligna, Combretum kraussii, Halleria lucida, Rapanea melanophloeos, Vachellia sieberiana, | Lactuca sativa | laboratory bioassay | leaf extracts | [91] |
| Albizia lebbeck | crops (5) | laboratory bioassay | leaf extracts | [92] |
| Alstonia scholaris | Lactuca sativa, crop (1), weed (1) | laboratory bioassays, pot culture | leaf and litter extracts, leaf and litter powder, allelochemicals | [93] |
| A. scholaris | weed (1) | laboratory bioassays | leaf and bark extracts | [94] |
| Ailanthus altissima, Ulmus pumilla, Robinia pseudoacacia, Populus alba | grasses (6) | laboratory bioassays | leaf litter extracts | [95] |
| A. altissima, Robinia pseudoacacia, Fraxinus angustifolia, Populus alba | R. pseudoacacia, P. alba, grasses (11) | laboratory bioassays | leaf litter extracts | [96] |
| Amorpha fruticosa, Hedysarum mongolicum, Sabina vulgaris, Hippophae rhamnoides | shrub (1) | laboratory bioassays | leaf extracts | [97] |
| Azadirachta indica | weeds (4), crops (3), grass (1) | laboratory bioassay | leaf extracts, allelochemicals | [98] |
| A. indica | crops (6), weeds (3) | laboratory bioassay | bark and leaf extracts | [99] |
| Cabralea canjerana, Carapa guianensis, Cedrela odorata, Hortia oreadica, Spiranthera odoratissima, Toona ciliate, Zanthoxylum petiolare | Lactuca sativa, crops (3) | laboratory bioassays | allelochemicals | [100] |
| Castanea dentata | Lactuca saliva, trees (6) | laboratory bioassay | leaf extracts | [101] |
| Castanea dentata, C. mollissima | Lactuca sativa, crops (5) | laboratory bioassays | leaf extracts | [102] |
| Casuarina equisetifolia | crops (1), weed (1) | laboratory bioassay | fog-drip leachates; needle, litter, cones and soil extracts | [103] |
| C. equisetifolia | trees (3) | laboratory bioassays | root, litter and soil extracts | [104] |
| C. equisetifolia | C. equisetifolia | laboratory bioassays | allelochemicals | [105] |
| Cinnamomum camphora | crop (1) | pot culture | leaf powder, allelochemical | [106] |
| C. camphora, C. glaucescens, C. tamala | Lactuca sativa, grass (1) | laboratory bioassays | leaf, root and fruit essential oils | [107] |
| C. septentrionale | tree (1) | pot culture | leaf litter | [108] |
| Cinnamomum septentrionale | crop (1) | pot culture | leaf litter | [109] |
| Diospyros kaki | Lactuca sativa, crops (5) | laboratory bioassays | leaf extracts | [110] |
| Lonicera maackii | Lonicera maackii, grasses (5) | laboratory bioassay | leaf extract | [111] |
| L. maackii | Arabidopsis thaliana | laboratory bioassay | leaf extract | [112] |
| Lupinus jaimehintoniana | Lactuca sativa | laboratory bioassays | leaf, seed, shoot and phloem extracts | [113] |
| Morus alba | crops (1), weeds (1) | laboratory bioassay, pot culture | leaf extract | [114] |
| Nerium oleander | weeds (1) | laboratory bioassay, pot culture | leaf extract | [115] |
| Nyssa yunnanensis | N. yunnanensis | laboratory bioassays, greenhouse, field | extracts, soil, litter | [116] |
| Paulownia tomentosa, P. elongata × P. fortunei | crop (1), grasses (2) | laboratory bioassays | leaf extracts | [117] |
| Populus deltoides | crops (7) | laboratory bioassay, pot culture | leaves leaf | [118] |
| Quercus coccifera | crops (5) | laboratory bioassays | leaf and soil extracts | [119] |
| Q. leucotrichophora | Quercus leucotrichophora | laboratory bioassay, pot culture | bark, leaf, bark and leaf litter extracts | [120] |
| Rhamnus cathartica | herbs (4) | greenhouse, field | leaves and fruits | [121] |
| R. cathartica | herbs (3), trees (2) | pot culture | leaves and roots | [122] |
| R. cathartica | crops (1) | laboratory bioassay | fruits, leaf, root and bark extracts | [123] |
| Rhododendron formosanum | Lactuca sativa, crops (2), grasses (2), weed (1) | laboratory bioassays | flower, leaf and litter extracts | [124] |
| R. formosanum | Lactuca sativa | laboratory bioassays | allelochemical | [125] |
| Robinia pseudo-acacia | Lactuca sativa, crops (2), weeds (2), herbs (2) | laboratory bioassay | leaf extracts | [126] |
| Tectona grandis | crops (1), weeds (2) | laboratory bioassay | leaf extracts | [127] |
| T. grandis | Lactuca sativa, crops (4) | laboratory bioassay | allelochemicals | [128] |
| T. grandis, Aleurites fordii, Gliricidia sepium, Maytenus buxifolia | Lactuca sativa, crops (4) | laboratory bioassay | allelochemicals | [129] |
| Tipuana tipu | Lactuca sativa | laboratory bioassays | root, stem, leaf, flower and pod essential oils | [130] |
| Ulmus pumila | grasses (3) | laboratory bioassay, pot culture | leaf litter extracts | [131] |
| Ziziphus spinachristi | crops (4) | laboratory bioassay | leaf extracts | [132] |
| Allelopathic Species | Test Species a | Analysis Type | Compounds | Reference |
|---|---|---|---|---|
| Araucaria angustifolia | Lactuca sativa | laboratory bioassay | needle extracts | [135] |
| Cunninghamia lancealata | Cunninghamia lancealata | laboratory bioassay, pot culture | stump-roots extracts, stump-roots | [136] |
| C. lancealata | Cunninghamia lancealata | laboratory bioassay | leaf and root extracts, rhizosphere soil | [137] |
| C. lancealata | Cunninghamia lancealata | laboratory bioassay | root extracts | [138] |
| C. lancealata | Cunninghamia lancealata | laboratory bioassay | allelochemicals | [139] |
| C. lancealata | Cunninghamia lancealata | pot culture | leaf and plastic litter | [140] |
| Ginkgo biloba | Lactuca sativa, crop (1), grass (1), weed (1) | laboratory bioassay | leaf extracts, allelochemical | [141] |
| Juniperus ashei | grass (1) | sandwich method, field | leaf and litter leachate | [142] |
| Latix gmelini | tree (1) | pot culture | root, bark, branch and leaf extracts | [143] |
| Picea schrenkiana | Picea schrenkiana, Latuca sativa, crops (5) | laboratory bioassay | allelochemicals | [144] |
| P. schrenkiana | Picea schrenkiana | laboratory bioassay | needle extracts, allelochemicals | [145] |
| Pinus densiflora | crops (1), weeds (1) | laboratory bioassay | allelochemicals | [146] |
| P. densiflora | Latuca sativa, crops (2), weeds (3) | laboratory bioassay | allelochemicals | [147] |
| Pinus halepensis | Lemna minor, weeds (3) | laboratory bioassay, pot culture | needles, needle extracts | [148] |
| P. halepensis | Pinus halepensis | field | needle leachates | [149] |
| P. halepensis | Lactuca sativa, herb (1) | laboratory bioassay | root and needle extracts | [150] |
| P. halepensis | Pinus halepensis | laboratory bioassay | needle and roots extracts, litter | [151] |
| P. halepensis | Pinus halepensis, Lactuca sativa, herb (1) | laboratory bioassay | needle and roots extracts | [152] |
| P. halepensis | grasses (12) | laboratory bioassay | needle extracts, soil rhizosphere | [153] |
| P. pinea | weeds (3) | laboratory bioassay | essential oils | [154] |
| P. roxburghii | herb (1) | laboratory bioassay, pot culture | needles and bark extracts, needle litter | [155] |
| P. thunbergii, P. tabuliformis, P. koraiensis | Pinus thunbergii, Pinus tabuliformis, Pinus koraiensis | field | needle leachates | [156] |
| Taxus baccata | Taxus baccata | field | needls | [157] |
| T. baccata | crops (2) | laboratory bioassay | aril, leaf and bark extracts | [158] |
| Thuja plicata, T. occidentalis, Abies amabilis, A. balsamea, A. grandis, A. lasiocarpa, Tsuga canadensis, T. mertensiana, T. heterophylla | Arabidopsis thaliana | laboratory bioassay | extracts of resin vesicles from seeds | [159] |
| Wollemia nobilis | crops (1), grass (1) | laboratory bioassay | leaf extract | [160] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedev, V.G.; Krutovsky, K.V.; Shestibratov, K.A. …Fell Upas Sits, the Hydra-Tree of Death †, or the Phytotoxicity of Trees. Molecules 2019, 24, 1636. https://doi.org/10.3390/molecules24081636
Lebedev VG, Krutovsky KV, Shestibratov KA. …Fell Upas Sits, the Hydra-Tree of Death †, or the Phytotoxicity of Trees. Molecules. 2019; 24(8):1636. https://doi.org/10.3390/molecules24081636
Chicago/Turabian StyleLebedev, Vadim G., Konstantin V. Krutovsky, and Konstantin A. Shestibratov. 2019. "…Fell Upas Sits, the Hydra-Tree of Death †, or the Phytotoxicity of Trees" Molecules 24, no. 8: 1636. https://doi.org/10.3390/molecules24081636
APA StyleLebedev, V. G., Krutovsky, K. V., & Shestibratov, K. A. (2019). …Fell Upas Sits, the Hydra-Tree of Death †, or the Phytotoxicity of Trees. Molecules, 24(8), 1636. https://doi.org/10.3390/molecules24081636







