Microfluidics-Based Fabrication of Cell-Laden Hydrogel Microfibers for Potential Applications in Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussion
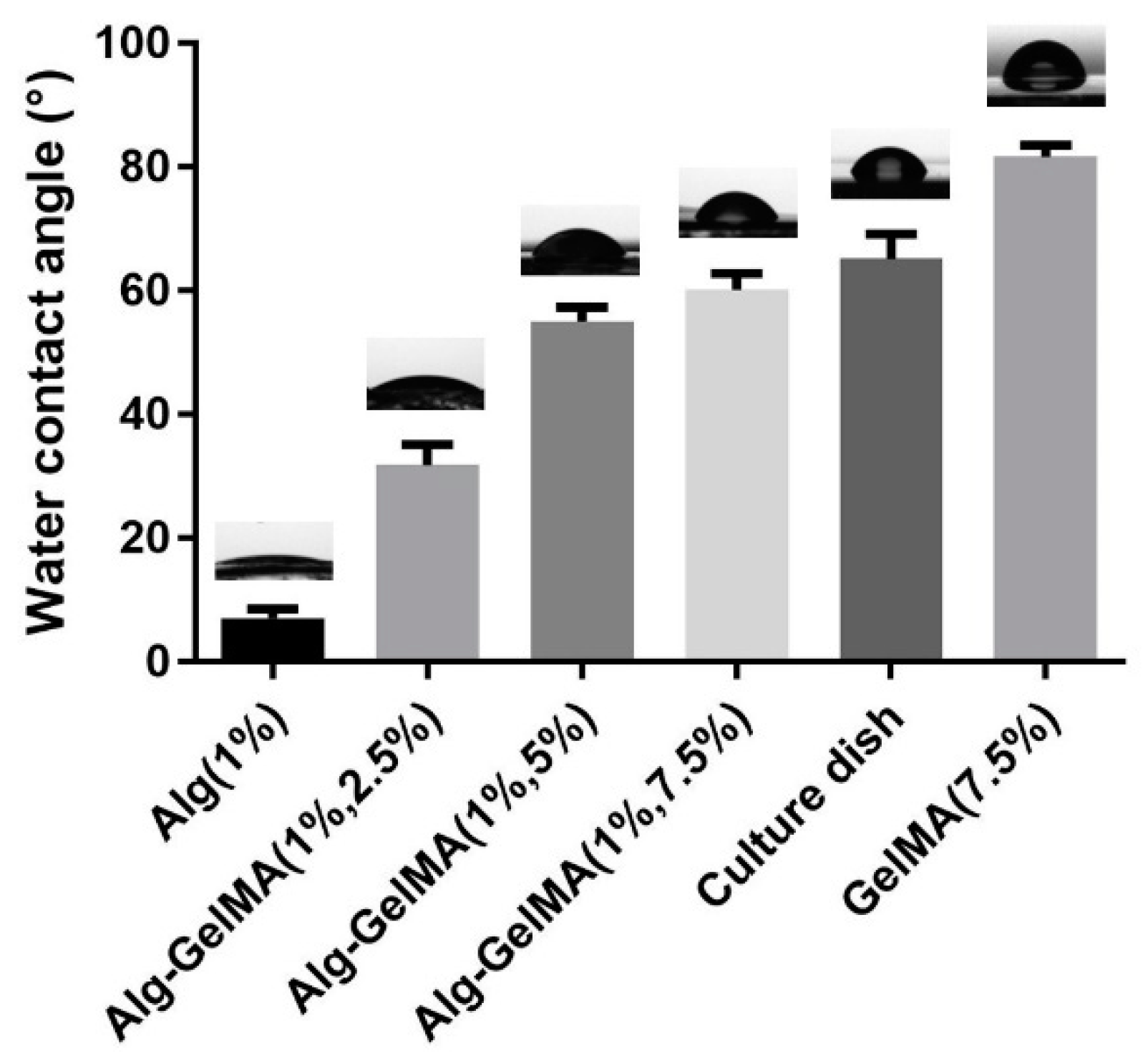
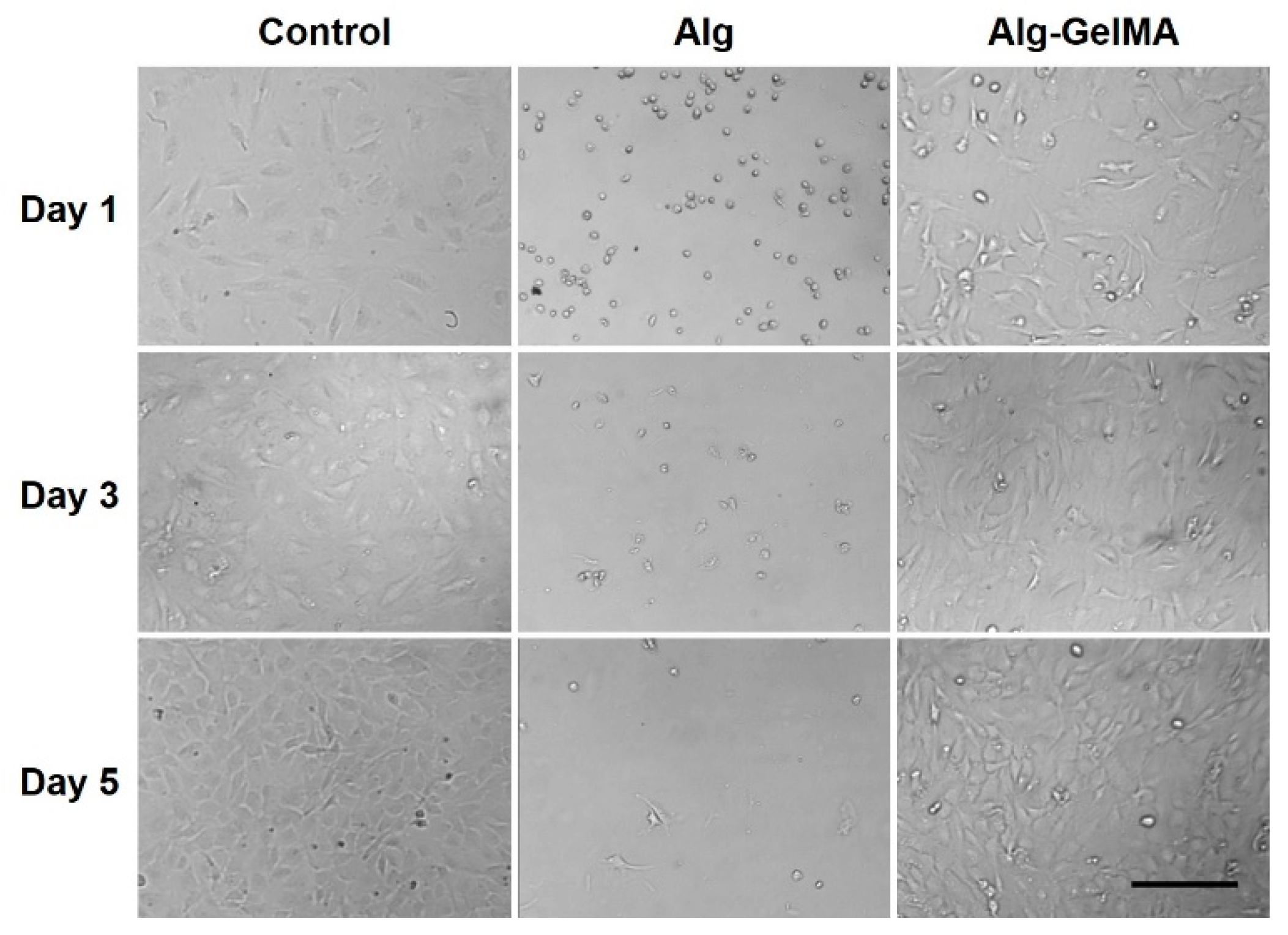
2.1. Fabrication and Characterizations of Alg-GelMA Composite Hydrogel
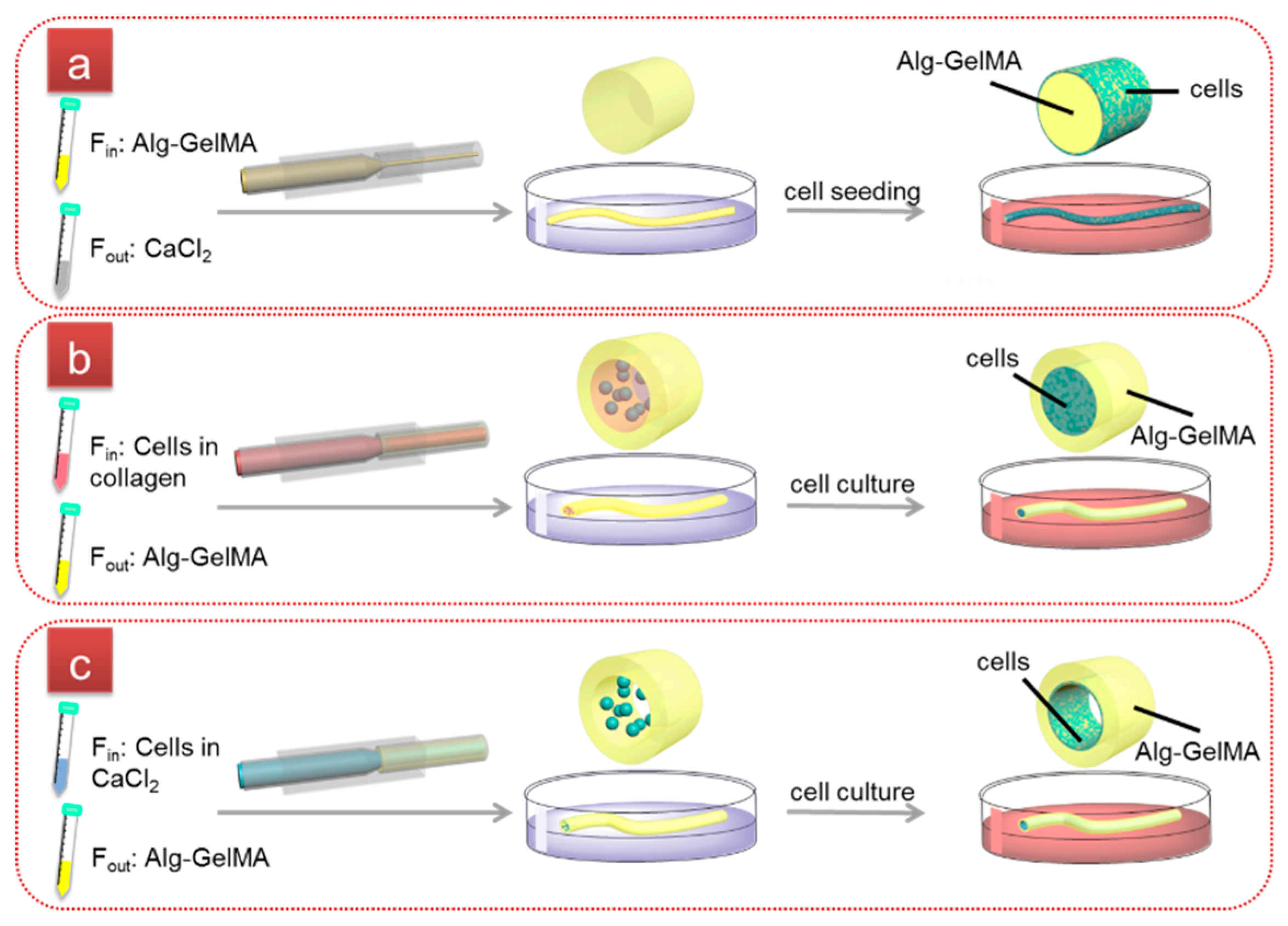
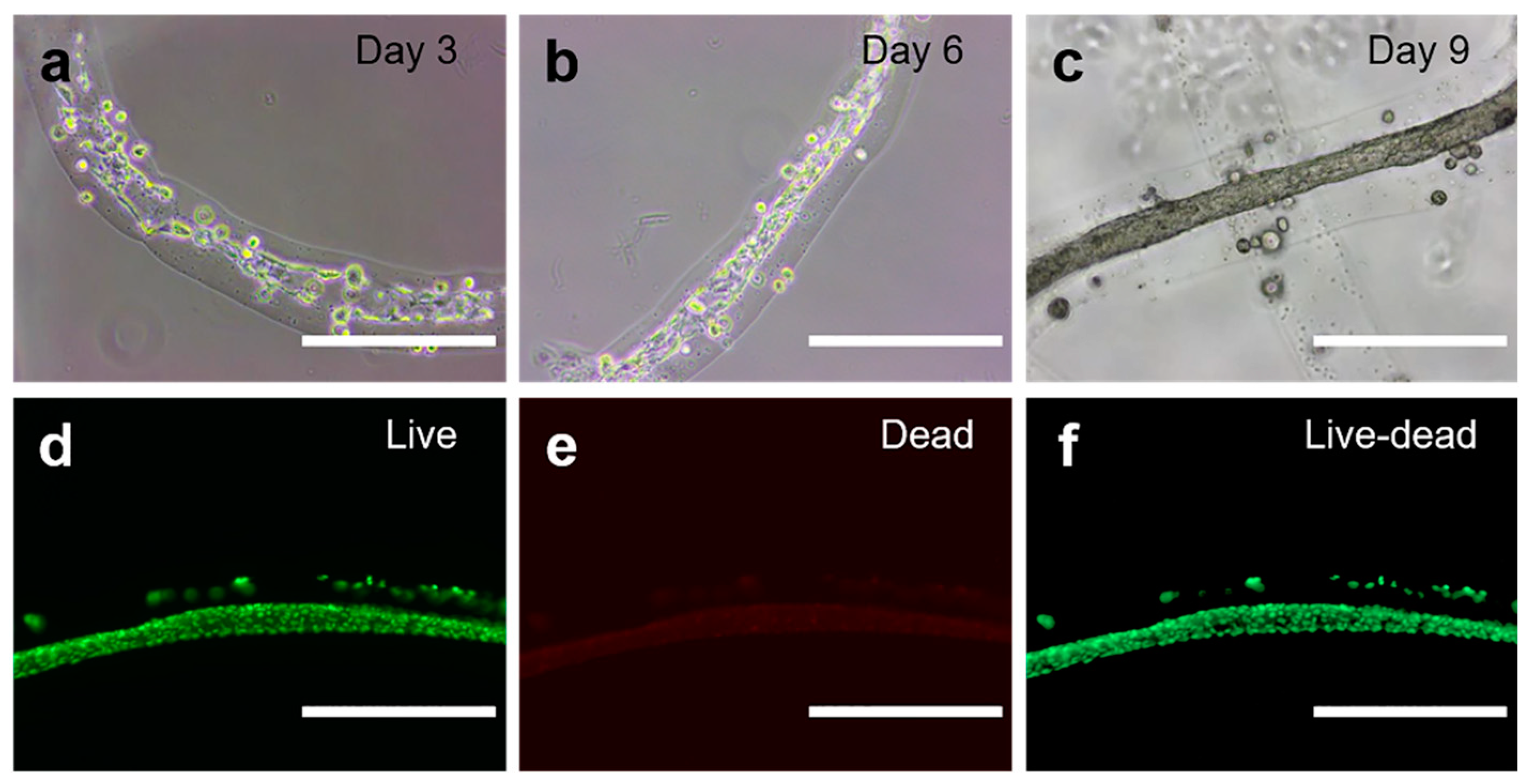
2.2. Fabrication of Cell-laden Single-layer Microfibers
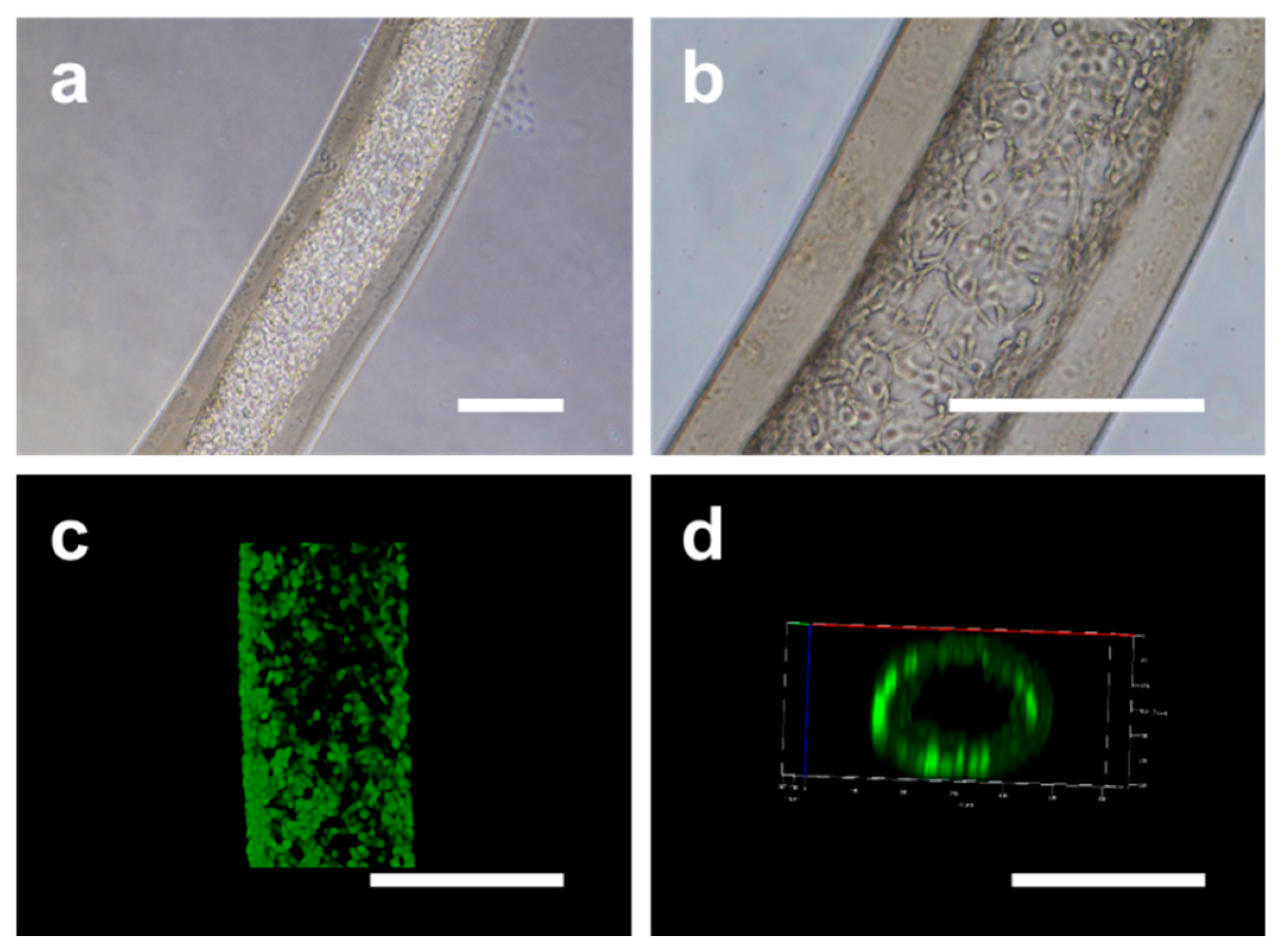
2.3. Fabrication of Cell-laden Double-layer Microfibers
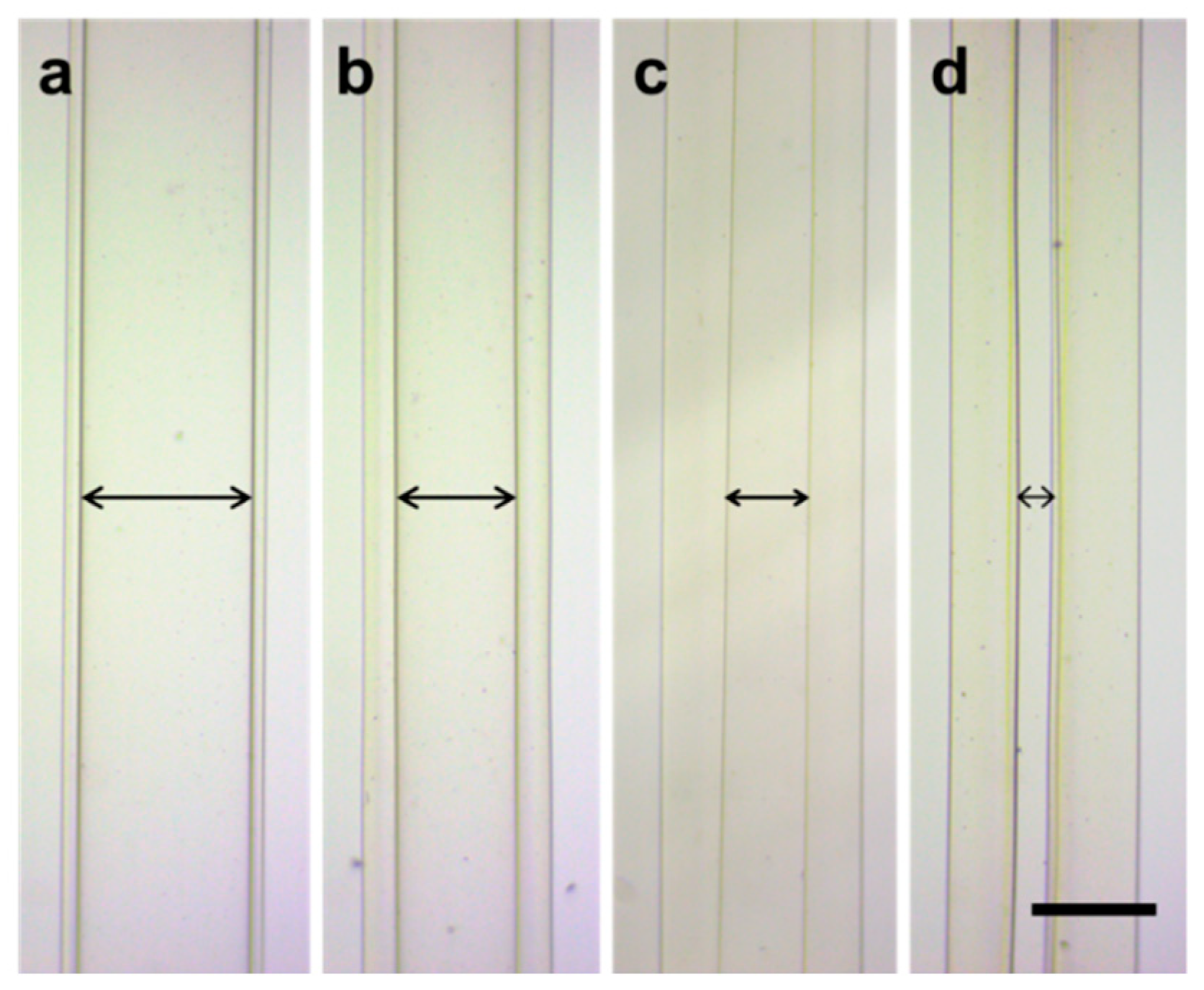
2.4. Fabrication and Characterization of Cell-laden Hollow Microfibers
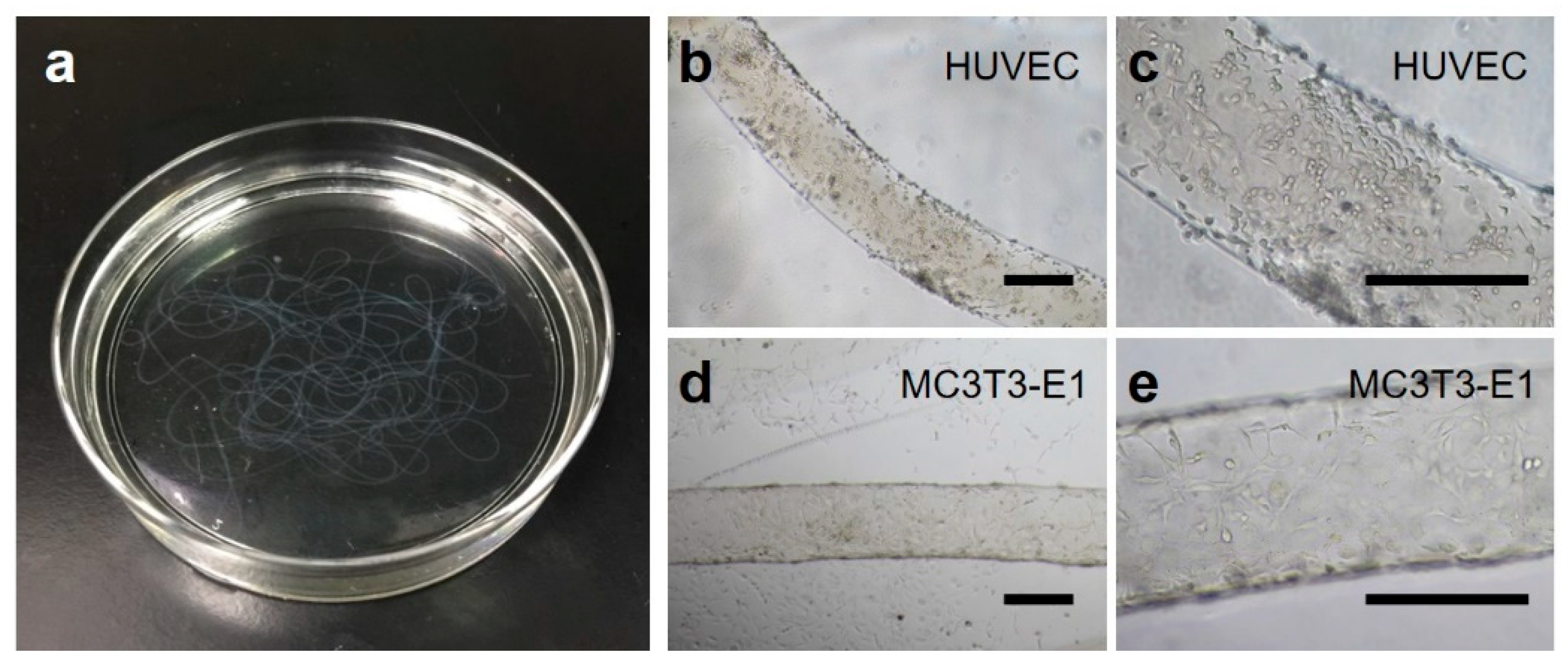
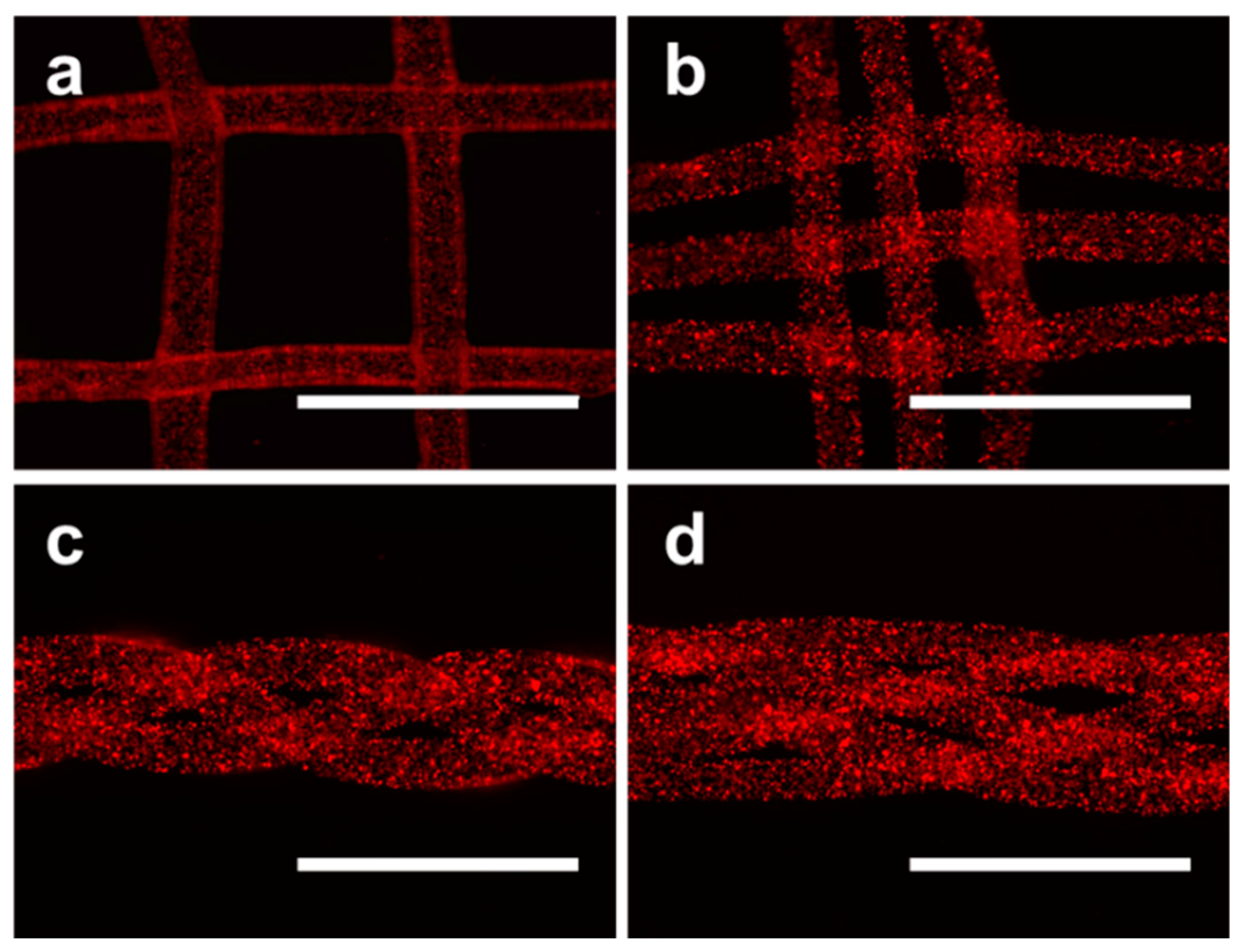
2.5. Higher-order Assembly Using Hydrogel Microfibers
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Hydrogel Membranes
3.3. Mechanical Performance Test of Hydrogel
3.4. Microfluidics
3.5. Morphology Characterization and Perfusion Test of Hollow Microfibers
3.6. Cell Culture
3.7. Fabrication of Cell-laden Microfibers
3.8. Cell Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Del. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol. Biol. 2011, 695, 17–39. [Google Scholar] [PubMed]
- Wang, H.; Sun, W.; Fu, D.; Shen, Y.; Chen, Y.Y.; Wang, L.L. Update on biomaterials for prevention of epidural adhesion after lumbar laminectomy. J. Orthop. Translat. 2018, 13, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: a literature review. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, T.; Chen, G.P. Biodegradable Polymer Scaffold for Tissue Engineering. Key Eng. Mater. 2005, 49, 288–289. [Google Scholar]
- Li, L.; Long, J.; Li, L.; Cao, H.; Tang, T.; Xi, X.; Qin, L.; Lai, Y.; Wang, X. Quantitative determination of residual 1,4-dioxane in three-dimensional printed bone scaffold. J. Orthop. Translat. 2018, 13, 58–67. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Wang, Y.; Zhang, P.; Liu, W. Fibrous scaffolds for tissue engineering. Inflamm. Regen. 2014, 34, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Chae, S.K.; Kang, E.; Khademhosseini, A.; Lee, S.H. Micro/Nanometer-scale fiber with highly ordered structures by mimicking the spinning process of silkworm. Adv. Mater. 2013, 25, 3071–3078. [Google Scholar] [CrossRef]
- Tamayol, A.; Akbari, M.; Annabi, N.; Paul, A.; Khademhosseini, A.; Juncker, D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 2013, 31, 669–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito-Pereira, R.; Correia, D.M.; Ribeiro, C.; Francesko, A.; Etxebarria, I.; Pérez-Álvarez, L.; Vilas, J.L.; Martins, P.; Lanceros-Mendez, S. Silk fibroin-magnetic hybrid composite electrospun fibers for tissue engineering applications. Composites Part B 2018, 141, 70–75. [Google Scholar] [CrossRef]
- Chung, B.G.; Lee, K.H.; Khademhosseini, A.; Lee, S.H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab. Chip 2012, 12, 45–59. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef]
- Wei, D.; Sun, J.; Bolderson, J.; Zhong, M.; Dalby, M.J.; Cusack, M.; Yin, H.; Fan, H.; Zhang, X. Continuous fabrication and assembly of spatial cell-laden fibers for a tissue-like construct via a photolithographic-based microfluidic chip. ACS Appl. Mater. Interfaces 2017, 9, 14606–14617. [Google Scholar] [CrossRef]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.H. Microfluidic spinning of micro- and nano-scale fibers for tissue engineering. Lab. Chip 2014, 14, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Choi, Y.Y.; Chae, S.-K.; Moon, J.-H.; Chang, J.-Y.; Lee, S.-H. Microfluidic spinning of flat alginate fibers with grooves for cell-aligning scaffolds. Adv. Mater. 2012, 24, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- He, X.-H.; Wang, W.; Deng, K.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Microfluidic fabrication of chitosan microfibers with controllable internals from tubular to peapod-like structures. RSC Adv. 2015, 5, 928–936. [Google Scholar] [CrossRef]
- Pedde, R.D.; Mirani, B.; Navaei, A.; Styan, T.; Wong, S.; Mehrali, M.; Thakur, A.; Mohtaram, N.K.; Bayati, A.; Dolatshahi-Pirouz, A.; et al. Emerging biofabrication strategies for engineering complex tissue constructs. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, Y.; Fu, F.; Wang, J.; Shang, L.; Gu, Z.; Zhao, Y. Controlled fabrication of bioactive microfibers for creating tissue constructs using microfluidic techniques. ACS Appl. Mater. Interfaces 2016, 8, 1080–1086. [Google Scholar] [CrossRef]
- Jansen, E.J.; Sladek, R.E.; Bahar, H.; Yaffe, A.; Gijbels, M.J.; Kuijer, R.; Bulstra, S.K.; Guldemond, N.A.; Binderman, I.; Koole, L.H. Hydrophobicity as a design criterion for polymer scaffolds in bone tissue engineering. Biomaterials 2005, 26, 4423–4431. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Del. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, T.; Lin, Y.; Cai, X. Vascularization in graniofacial bone tissue engineering. J. Dent. Res. 2018, 97, 969–976. [Google Scholar] [CrossRef]
- Daniele, M.A.; Boyd, D.A.; Adams, A.A.; Ligler, F.S. Microfluidic strategies for design and assembly of microfibers and nanofibers with tissue engineering and regenerative medicine applications. Adv. Healthc. Mater. 2015, 4, 11–28. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Jia, L.; Han, F.; Wang, J.; Yu, L.; Yu, Y.; Turnbull, G.; Guo, M.; Shu, W.; Li, B. Microfluidics-Based Fabrication of Cell-Laden Hydrogel Microfibers for Potential Applications in Tissue Engineering. Molecules 2019, 24, 1633. https://doi.org/10.3390/molecules24081633
Wang G, Jia L, Han F, Wang J, Yu L, Yu Y, Turnbull G, Guo M, Shu W, Li B. Microfluidics-Based Fabrication of Cell-Laden Hydrogel Microfibers for Potential Applications in Tissue Engineering. Molecules. 2019; 24(8):1633. https://doi.org/10.3390/molecules24081633
Chicago/Turabian StyleWang, Gen, Luanluan Jia, Fengxuan Han, Jiayuan Wang, Li Yu, Yingkang Yu, Gareth Turnbull, Mingyu Guo, Wenmiao Shu, and Bin Li. 2019. "Microfluidics-Based Fabrication of Cell-Laden Hydrogel Microfibers for Potential Applications in Tissue Engineering" Molecules 24, no. 8: 1633. https://doi.org/10.3390/molecules24081633
APA StyleWang, G., Jia, L., Han, F., Wang, J., Yu, L., Yu, Y., Turnbull, G., Guo, M., Shu, W., & Li, B. (2019). Microfluidics-Based Fabrication of Cell-Laden Hydrogel Microfibers for Potential Applications in Tissue Engineering. Molecules, 24(8), 1633. https://doi.org/10.3390/molecules24081633





