New UHPLC-QqQ-MS/MS Method for the Rapid and Sensitive Analysis of Ascorbic and Dehydroascorbic Acids in Plant Foods
Abstract
1. Introduction
2. Results and Discussion
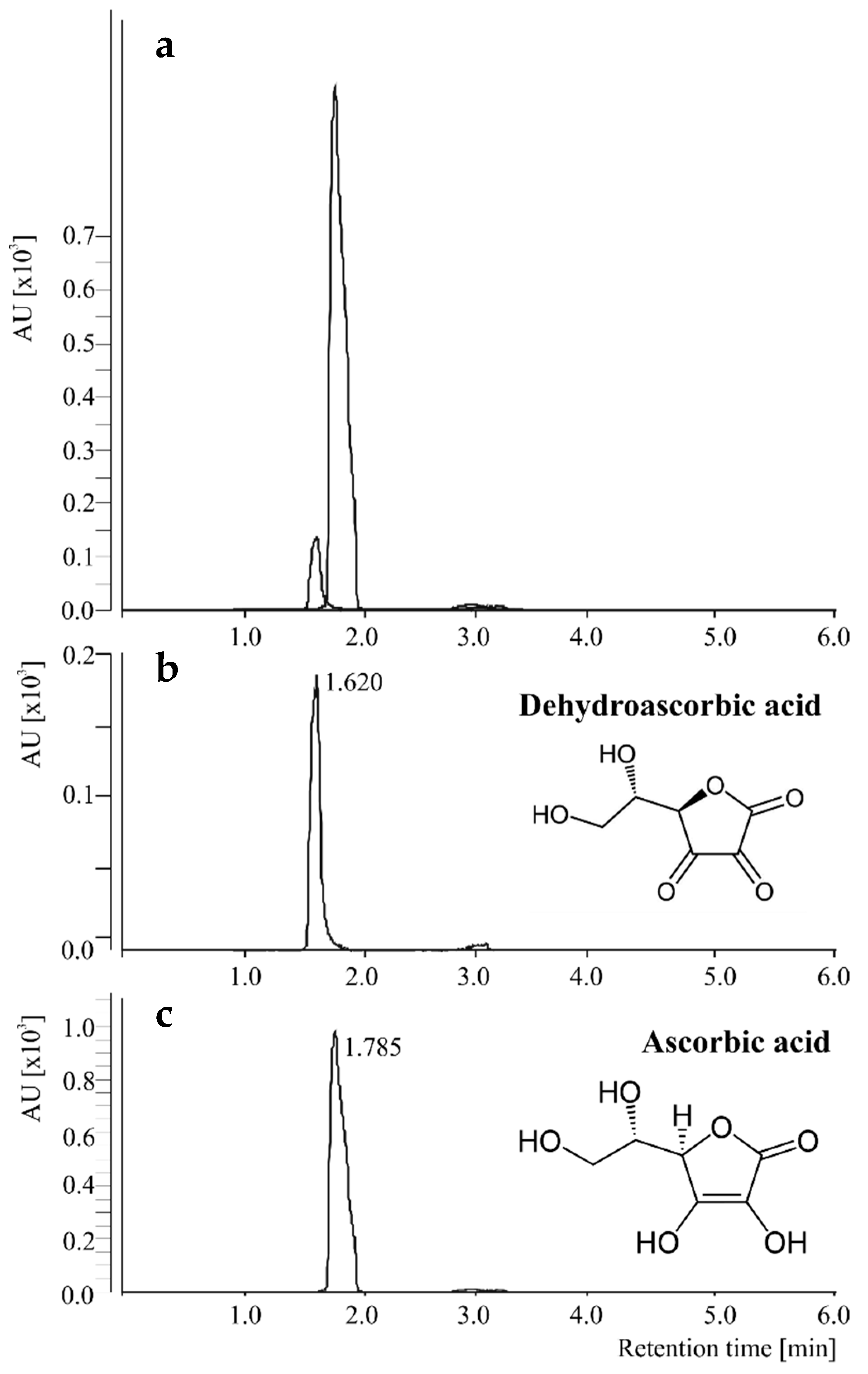
2.1. Qualitative and Quantitative MRM Transitions
2.2. Calibration Curves and Sensitivity
2.3. Precision and Accuracy
2.4. Sample Preparation, Matrix Effect, Sensitivity and Recovery
3. Materials and Methods
3.1. Chemicals
3.2. Sample Preparation
3.3. UHPLC-ESI-QqQ-MS/MS Conditions
3.4. Method Validation Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dasgupta, A.; Klein, K. Chapter 15-Antioxidant Vitamins and Minerals, in Antioxidants. In Food, Vitamins and Supplements; Elsevier: San Diego, CA, USA, 2014; pp. 277–294. [Google Scholar]
- Johnston, C.S. Vitamin C. In Present Knowledge in Nutrition, 10th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 248–260. [Google Scholar]
- Gonzalez-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: a review of evidence. Front Biosci. (Landmark Ed) 2013, 18, 1017–1209. [Google Scholar]
- Varvara, M.; Bozzo, G.; Celano, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The Use of Ascorbic Acid as a Food Additive: Technical-Legal Issues. IJFS 2016, 5, 4313. [Google Scholar] [CrossRef]
- Beyer, R.E. The role of ascorbate in antioxidant protection of biomembranes: Interaction with vitamin E and coenzyme Q. J. Bioenerg. Biomembr. 1994, 26, 349–358. [Google Scholar] [CrossRef]
- Sagun, K.C.; Cárcamo, J.M.; Golde, D.W. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005, 19, 1657–1667. [Google Scholar] [CrossRef]
- Ogiri, Y.; Sun, F.; Hayami, S.; Fujimura, A.; Yamamoto, K.; Yaita, M.; Kojo, S. Very Low Vitamin C Activity of Orally Administered l-Dehydroascorbic Acid. J. Agric. Food. Chem. 2002, 50, 227–229. [Google Scholar] [CrossRef]
- Mazurek, A.; Włodarczyk-Stasiak, M. A comparison of vitamin C content determination by chromatographic and spectrophotometric methods according to standard PN-A-040. Current Issues Pharm. Med. Sci. 2013, 26, 443–447. [Google Scholar] [CrossRef]
- Mazurek, A.; Jamroz, J. Precision of dehydroascorbic acid quantitation with the use of the subtraction method--validation of HPLC-DAD method for determination of total vitamin C in food. Food Chem. 2015, 173, 543–550. [Google Scholar] [CrossRef]
- Nováková, L.; Solich, P.; Solichová, D. HPLC methods for simultaneous determination of ascorbic and dehydroascorbic acids. Trends Anal. Chem. 2008, 27, 942–958. [Google Scholar] [CrossRef]
- Van de Velde, F.; Pirovani, M.E.; Camara, M.S.; Guemes, D.R.; Bernardi, C.M.H. Optimization and Validation of a UV–HPLC Method for Vitamin C Determination in Strawberries (Fragaria ananassa Duch.) Using Experimental Designs. Food Anal. Methods 2012, 5, 1097–1104. [Google Scholar] [CrossRef]
- Kall, M.A.; Andersen, C. Improved method for simultaneous determination of ascorbic acid and dehydroascorbic acid, isoascorbic acid and dehydroisoascorbic acid in food and biological samples. J. Chromatogr. B Biomed. Sci. Appl. 1999, 730, 101–111. [Google Scholar] [CrossRef]
- Simões, S.I.D.; Eleutério, C.V.; Cruz, M.E.; Corvo, M.L.; Martins, M.B. Biochemical changes in arthritic rats: Dehydroascorbic and ascorbic acid levels. Eur. J. Pharm. Sci. 2003, 18, 185–189. [Google Scholar] [CrossRef]
- Fenoll, J.; Martínez, A.; Hellín, P.; Flores, P. Simultaneous determination of ascorbic and dehydroascorbic acids in vegetables and fruits by liquid chromatography with tandem-mass spectrometry. Food Chem. 2011, 127, 340–344. [Google Scholar] [CrossRef]
- Gutiérrez-Quequezana, L.; Vuorinen, A.L.; Kallio, H.; Yang, B. Improved analysis of anthocyanins and vitamin C in blue-purple potato cultivars. Food Chem. 2018, 242, 217–224. [Google Scholar] [CrossRef]
- Spinola, V.; Mendes, B.; Camara, J.S.; Castilho, P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef]
- Klimczak, I.; Gliszczyńska-Świgło, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef]
- Harmonised Tripartite Guideline. Guidelines on Validation of Analytical Procedures, Text and Methodology Q2 (R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human use (ICH). November 2005. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 10 January 2019).
- Bioanalytical Method Validation. Guidance for Industry. U.S. Department of Health and Human Services, Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Biopharmaceutics. May 2018. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf (accessed on 10 January 2019).
- Caban, M.; Migowska, N.; Stepnowski, P.; Kwiatkowski, M.; Kumirska, J. Matrix effects and recovery calculations in analyses of pharmaceuticals based on the determination of β-blockers and β-agonists in environmental samples. J. Chromatogr. A 2012, 1258, 117–127. [Google Scholar] [CrossRef]
- Koh, E.; Wimalasiri, K.M.S.; Chassy, A.W.; Mitchell, A.E. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Composit. Anal. 2009, 22, 637–643. [Google Scholar] [CrossRef]
- Shigenaga, T.; Yamauchi, N.; Funamoto, Y.; Shigyo, M. Effects of heat treatment on an ascorbate–glutathione cycle in stored broccoli (Brassica oleracea L.) florets. Postharvest Biol. Technol. 2005, 38, 152–159. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; García-Viguera, C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur. Food Res. Technol. 2002, 215, 310–316. [Google Scholar] [CrossRef]
- Leong, S.Y.; Oey, I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food chem. 2012, 133, 1577–1587. [Google Scholar] [CrossRef]
- Munyaka, A.W.; Makule, E.E.; Oey, I.; Lowy, A.V.; Hendrickx, M. Thermal Stability of l-Ascorbic Acid and Ascorbic Acid Oxidase in Broccoli (Brassica oleracea var. italica). J. Food Sci. 2010, 75, C336–C340. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Compounds are commercially available. |
| Compound | Retention Time (min) | Quantification Transition 1 (m/z) | Qualifier Transition (m/z) | Collision Energy (eV) | Fragmentor (V) |
|---|---|---|---|---|---|
| Ascorbic acid | 1.785 | 175 > 115 | 175 > 71 | 5 | 90 |
| Dehydroascorbic acid | 1.620 | 173 > 143 | 173 > 113 | 5 | 60 |
| Parameters | AA | DHAA |
|---|---|---|
| Range of concentrations for standard curves (ng mL−1) | 10.00–500.00 | 50.00–500.00 |
| Calibration curve | y = 67817 x + 766.99 | y = 3031.2 x + 440.71 |
| Coefficient of regression (R2) | 0.9952 | 0.9936 |
| LOD (ng mL−1) | 6.25 | 12.50 |
| LOQ (ng mL−1) | 10.00 | 50.00 |
| Analyte | Intra-Day Accuracy/Precision (CV) | Inter-Day Accuracy/Precision (CV) | ||||
|---|---|---|---|---|---|---|
| 80 ng mL−1 | 200 ng mL−1 | 400 ng mL−1 | 80 ng mL−1 | 200 ng mL−1 | 400 ng mL−1 | |
| Ascorbic acid | 86.4/10.6 | 104.2/9.2 | 98.7/3.1 | 87.6/12.2 | 99.2/8.0 | 90.6/8.3 |
| Dehydroascorbic acid | 90.7/13.7 | 114.8/8.6 | 95.8/2.5 | 86.7/10.6 | 113.5/6.3 | 98.7/9.9 |
| Food Matrix | Nominal Added Concentration (ng mL−1) | AA | DHAA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measured | SD | RSD (%) | ME (%) | RE (%) | Measured | SD | RSD (%) | ME (%) | RE (%) | ||
| Orange Juice | 400 | 621.42 | 72.88 | 13.7 | 118.6 | 101.6 | 464.80 | 6.79 | 1.5 | 118.3 | 116.8 |
| 200 | 397.30 | 41.36 | 13.3 | 99.9 | 104.8 | 225.13 | 17.18 | 7.6 | 98.0 | 112.6 | |
| 80 | 267.64 | 21.98 | 10.4 | 107.0 | 91.4 | 77.75 | 8.84 | 11.4 | 111.0 | 97.2 | |
| 0 | 194.53 | 14.35 | 7.4 | - | - | - | - | - | - | - | |
| Lyophilized Orange Juice | 400 | 574.19 | 28.66 | 3.9 | 103.7 | 90.0 | 455.67 | 30.89 | 6.8 | 115.4 | 113.9 |
| 200 | 430.11 | 33.35 | 8.7 | 95.2 | 108.0 | 180.39 | 4.90 | 2.7 | 85.0 | 90.2 | |
| 80 | 299.60 | 25.26 | 12.4 | 120.0 | 106.8 | 71.02 | 9.57 | 13.5 | 100.8 | 88.8 | |
| 0 | 214.15 | 14.64 | 6.8 | - | - | - | - | - | - | - | |
| Lyophilized Broccoli Florets | 400 | 403.42 | 42.42 | 10.5 | 117.8 | 100.9 | 3455.24 | 306.67 | 2.7 | 120.1 | 118.6 |
| 200 | 174.58 | 16.28 | 9.3 | 86.0 | 87.3 | 3216.86 | 307.63 | 5.7 | 102.7 | 117.9 | |
| 80 | 74.23 | 5.11 | 6.9 | 86.0 | 92.8 | 3060.30 | 302.44 | 10.4 | 113.12 | 99.1 | |
| 0 | - | - | - | - | - | 2981.05 | 294.24 | 9.9 | - | - | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baenas, N.; Salar, F.J.; Domínguez-Perles, R.; García-Viguera, C. New UHPLC-QqQ-MS/MS Method for the Rapid and Sensitive Analysis of Ascorbic and Dehydroascorbic Acids in Plant Foods. Molecules 2019, 24, 1632. https://doi.org/10.3390/molecules24081632
Baenas N, Salar FJ, Domínguez-Perles R, García-Viguera C. New UHPLC-QqQ-MS/MS Method for the Rapid and Sensitive Analysis of Ascorbic and Dehydroascorbic Acids in Plant Foods. Molecules. 2019; 24(8):1632. https://doi.org/10.3390/molecules24081632
Chicago/Turabian StyleBaenas, Nieves, Francisco J. Salar, Raúl Domínguez-Perles, and Cristina García-Viguera. 2019. "New UHPLC-QqQ-MS/MS Method for the Rapid and Sensitive Analysis of Ascorbic and Dehydroascorbic Acids in Plant Foods" Molecules 24, no. 8: 1632. https://doi.org/10.3390/molecules24081632
APA StyleBaenas, N., Salar, F. J., Domínguez-Perles, R., & García-Viguera, C. (2019). New UHPLC-QqQ-MS/MS Method for the Rapid and Sensitive Analysis of Ascorbic and Dehydroascorbic Acids in Plant Foods. Molecules, 24(8), 1632. https://doi.org/10.3390/molecules24081632






