Optimization of Ultrasound-Assisted Extraction of Some Bioactive Compounds from Tobacco Waste
Abstract
:1. Introduction
2. Results and Discussion
2.1. Detected Bioactive Compounds in Tobacco Leaf and Waste Extracts
2.2. Antioxidant Activity of Tobacco Leaf and Waste Extracts
2.3. Statistical Analysis
2.4. Optimization of Extraction Conditions
3. Materials and Methods
3.1. Ultrasound-Assisted Extraction (UAE) of Phenols
3.2. Ultrasound-Assisted Extraction of Solanesol
3.3. HPLC Analysis
3.4. Antioxidant Activity of Obtained Extracts
3.5. Statistical Analysis
3.6. Optimization of the Extraction Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tayoub, G.; Sulaiman, H.; Alorfi, M. Determination of nicotine levels in the leaves of some Nicotiana tabacum varieties cultivated in Syria. Herba Pol. 2015, 61, 23–30. [Google Scholar] [CrossRef]
- Ruiz–Rodriguez, A.; Bronze, M.R.; da Ponte, M.N. Supercritical fluid extraction of tobacco leaves: A preliminary study on the extraction of solanesol. J. Supercrit. Fluids 2008, 45, 171–176. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Xu, C.P.; Xiao, Y.; Mao, D.B. Antioxidant activities of polysaccharide fractions isolated from burley tobacco flowers. Croat. J. Food Sci. Technol. 2013, 5, 46–52. [Google Scholar]
- Vinatorua, M.; Mason, T.J.; Calinescua, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC 2017, 97, 159–178. [Google Scholar] [CrossRef]
- González de Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido; Ferreiro-González, X.; Amores-Arrocha, A.; Palma, M.; Barbero, G.F.; Jiménez-Cantizano, A. Alternative Ultrasound-Assisted Method for the Extraction of the Bioactive Compounds Present in Myrtle (Myrtus communis L.). Molecules 2019, 24, 882. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Xu, X.; Lee, F.S.C.; Wang, X. Rapid determination of total solanesol in tobacco leaf by ulstrasound–assisted extraction with RP–HPLC and ESI–TOF/MS. J. Pharm. Biomed. Anal. 2007, 43, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Q.J.; Li, X.; Luo, Y.; Liu, H. Extraction and HPLC Characterization of Chlorogenic Acid from Tobacco Residuals. Sep. Sci. Technol. 2007, 42, 3481–3492. [Google Scholar] [CrossRef] [Green Version]
- Docheva, M.; Dagnon, S.; Statkova–Abeghe, S. Flavonoid content and radical scavenging potential of extracts prepared from tobacco cultivars and waste. Nat. Prod. Res. 2014, 28, 1328–1334. [Google Scholar] [CrossRef]
- Karabegović, I.T.; Veljković, V.B.; Lazić, M.L. Ultrasound–assisted extraction of total phenols and flavonoids from dry tobacco (Nicotiana tabacum) leaves. Nat. Prod. Commun. 2011, 6, 1855–1856. [Google Scholar] [CrossRef]
- Docheva, M.; Dagnon, S.; Statkova, S.; Dimanov, D. Isolation of bioflavonoids from tobacco. Trakia J. Sci. 2012, 10, 79–83. [Google Scholar]
- Gu, X.; Cai, J.; Zhu, X.; Su, Q. Dynamic ultrasound–assisted extraction of polyphenols in tobacco. J. Sep. Sci. 2005, 28, 2477–2481. [Google Scholar] [CrossRef]
- Mazvimba, M.T.; Ying, Y.; Zhi–Qin, C.; Zhang, Y. Optimization and orthogonal design of an ultrasonic–assisted aques extraction process for extracting chlorogenic acid from dry tobacco leaves. Chin. J. Nat. Med. 2012, 10, 311–320. [Google Scholar] [CrossRef]
- Xie, F.; Yu, A.; Hou, D.; Liu, H.; Ding, L.; Zhang, S. Rapid and Sensitive Analysis of Eight Polyphenols in Tobacco by Rapid Resolution Liquid Chromatography. Am. J. Analyt. Chem. 2011, 2, 929–933. [Google Scholar] [CrossRef]
- Zhao, C.J.; Zu, Y.G.; Li, C.Y.; Tian, C.Y. Distribution of solanesol in Nicotiana tabacum. J. Forest Res. 2007, 18, 69–72. [Google Scholar] [CrossRef]
- Liu, Y.; Yong, G.; Xu, Y.; Zhu, D.; Tong, H.; Liu, S. Simultaneous Determination of Free and Esterified Fatty Alcohols, Phytosterols and Solanesol in Tobacco Leaves by GC. Chromatographia 2010, 71, 727–732. [Google Scholar] [CrossRef]
- Popova, V.; Ivanova, T.; Nikolova, V.; Stoyanova, A.; Docheva, M.; Hristeva, T.; Damyanova, S.; Nikolov, N. Biologically Active and Volatile Compounds in Leaves and Extracts of Nicotiana alata Link & Otto from Bulgaria. J. Pharm. Sci. Res. 2017, 9, 2045–2051. [Google Scholar]
- Sun, Y.; Li, W.; Wang, J.; Bi, J.; Su, S. Determination of Rutin in Cigarette Tobacco, Filters, Mainstream Smoke and Burned Ash of Different Branded Cigarettes by High Performance Liquid Chromatography. Molecules 2012, 17, 3751–3760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cvetanovska, A.; Krstic, M.; Cvetanovska, L. Content of total phenolic compounds and antioxidant potential of oriental tobacco varieties (Nicotiana tabacum L.). Eur. J. Pharm. Med. 2017, 4, 2223–2228. [Google Scholar]
- Machado, P.A.; Fu, H.; Kratochvil, R.; Yuan, Y.; Hahm, T.S.; Sabliov, C.M.; Wei, Y.; Lo, M. Recovery of solanesol from tobacco as a value–added byproduct for alternative applications. Bioresour. Techn. 2010, 101, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.; Gochev, V.; Girova, T.; Iliev, I.; Ivanova, T.; Stoyanova, A. Extraction Products from Tobacco–Aroma and Bioactive Compounds and Activities. Curr. Bioact. Compd. 2015, 11, 31–37. [Google Scholar] [CrossRef]
- Wang, J.; Lu, D.; Zhao, H.; Ling, X.; Jiang, B.; Ouyang, P. Application of response surface methodology optimization for the production of caffeic acid from tobacco waste. Afr. J. Biotechnol. 2009, 8, 1416–1424. [Google Scholar]
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta Rev. Biomembr. 2004, 1660, 171–199. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, Y.; Zhang, G.; Yin, Z. Improved extraction of solanesol from tobacco waste by enzymatic cell wall breaking. Chin. J. Biotechnol. 2013, 29, 1706–1710. [Google Scholar]
- Yan, N.; Liu, Y.; Gong, D.; Du, Y.; Zhang, H.; Zhang, Z. Solanesol: A review of its resources, derivatives, bioactivities, medicinal applications, and biosynthesis. Phytochem. Rev. 2015, 14, 403–417. [Google Scholar] [CrossRef]
- Hu, J.Y.; Liang, Y.; Xie, Y.; Huang, Z.F.; Zhong, H.Z. Study on solanesol content in every section of tobacco by high performance liquid chromatography. Chin. J. Anal. Lab. 2007, 26, 106–108. [Google Scholar]
- Chen, Y.K.; Li, X.S.; Yang, G.Y.; Chen, Z.Y.; Hu, Q.F.; Miao, M.M. Phenolic compounds from Nicotiana tabacum and their biological activities. J. Asian. Nat. Prod. Res. 2012, 14, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharmaceut. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Snook, M.E. The polyphenols of nicotiana. CORESTA 1994, 1, 17–18. [Google Scholar]
- Da Silva Caetano, A.C.; De Araújo, C.R.; Galvão De Lima, V.L.A.; Sucupira Maciel, M.I.; de Almeida Melo, E. Evaluation of antioxidant activity of agro–industrial waste of acerola (Malpighia emarginata D.C.) fruit extracts. Ciênc. Tecnol. Aliment. 2011, 31, 769–775. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologicallyactive compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Liu, C.Z. Microwave–assisted extraction of solanesol from tobacco leaves. J. Chromatogr. A 2006, 1129, 135–139. [Google Scholar] [CrossRef]
- Cravotto, G.; Omiccioli, G.; Stevanato, L. An Improved Sonochemical Reactor. Ultrason. Sonochem. 2005, 12, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Mariatti, F.; Gunjevic, V.; Secondo, M.; Villa, M.; Parolin, J.; Cavaglià, G. Pilot Scale Cavitational Reactors and Other Enabling Technologies to Design the Industrial Recovery of Polyphenols from Agro-Food By-Products, a Technical and Economical Overview. Foods 2018, 7, 130. [Google Scholar] [CrossRef]
- Alexandru, L.; Cravotto, G.; Giordana, L.; Binello, A.; Chemat, F. Ultrasound-Assisted Extraction of Clove Buds with Batch- and Flow-Reactors: A comparative Study on a pilot scale. Innov. Food Sci. Emerg. Technol. 2013, 20, 167–172. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, W.; Shao, X. Determination of seven polyphenols in water by high performance liquid chromatography combined with preconcentration. Talanta 2008, 77, 679–683. [Google Scholar] [CrossRef]
- Zhao, H.; Cai, K.; Lei, B.; Ren, Z.; Cai, B.; Pan, W. Development of method to determine c6–c3 phenolic acids in tobacco (Nicotiana tabacum) by HPLC. Asian. J. Chem. 2014, 26, 8621–8624. [Google Scholar] [CrossRef]
- Wang, J.; Lu, D.; Zhao, H.; Jiang, B.; Wang, J.; Ling, X.; Chai, H.; Ouyang, P. Discrimination and classification of tobacco wastes by identification and quantification of polyphenols with LC–MS/MS. J. Serb. Chem. Soc. 2010, 75, 875–891. [Google Scholar] [CrossRef]
- Fathiazad, F.; Delazar, A.; Amiri, R.; Sarker, S.D. Extraction of Flavonoids and Quantification of Rutin from waste Tobacco Leaves. Iran. J. Pharm. Res. 2005, 3, 222–227. [Google Scholar] [CrossRef]
- Hu, R.S.; Wang, J.; Li, H.; Ni, H.; Chen, Y.F.; Zhang, Y.W.; Xiang, S.P.; Li, H.H. Simultaneous extraction of nicotine and solanesol from waste tobacco materials by the column chromatographic extraction method and their separation and purification. Sep. Purif. Technol. 2015, 146, 1–7. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Niuc, H.; Wang, J.; Qin, Y. Bioactivity of solanesol extracted from tobacco leaves with carbon dioxide–ethanol fluids. Biochem. Eng. J. 2008, 42, 92–96. [Google Scholar] [CrossRef]
- Zhao, C.J.; Li, C.Y.; Fu, Y.J.; Zu, Y.G. Extraction and determination of solanesol in waste tobacco leaves by ultrasonic and HPLC. Chin. J. Appl. Chem. 2005, 22, 1265–1267. [Google Scholar]
- Atla, S.R.; Raja, B.; Dontamsetti, B.R. A new method of extraction, isolation and determination of solanesol from tobacco waste (Nicotiana tabacum L.) by non–aqueous RP–HPLC. Int. J. Pharm. Pharm. Sci. 2014, 6, 543–546. [Google Scholar]
- Leffinger, J. Carotenoids as Flavor & Fragrance Precursors. Leffingwell Reports 2002, 2, 1–5. [Google Scholar] [CrossRef]
- Banožić, M.; Šubarić, D.; Jokić, S. Tobacco waste in Bosnia and Herzegovina—Problem or high-value material? Glasnik Zaštite Bilja 2018, 41, 64–72. [Google Scholar] [CrossRef]
- Molnar, M.; Jerković, I.; Suknović, D.; Bilić Rajs, B.; Aladić, K.; Šubarić, D.; Jokić, S. Screening of Six Medicinal Plant Extracts Obtained by Two Conventional Methods and Supercritical CO2 Extraction Targeted on Coumarin Content, 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity and Total Phenols Content. Molecules 2017, 22. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
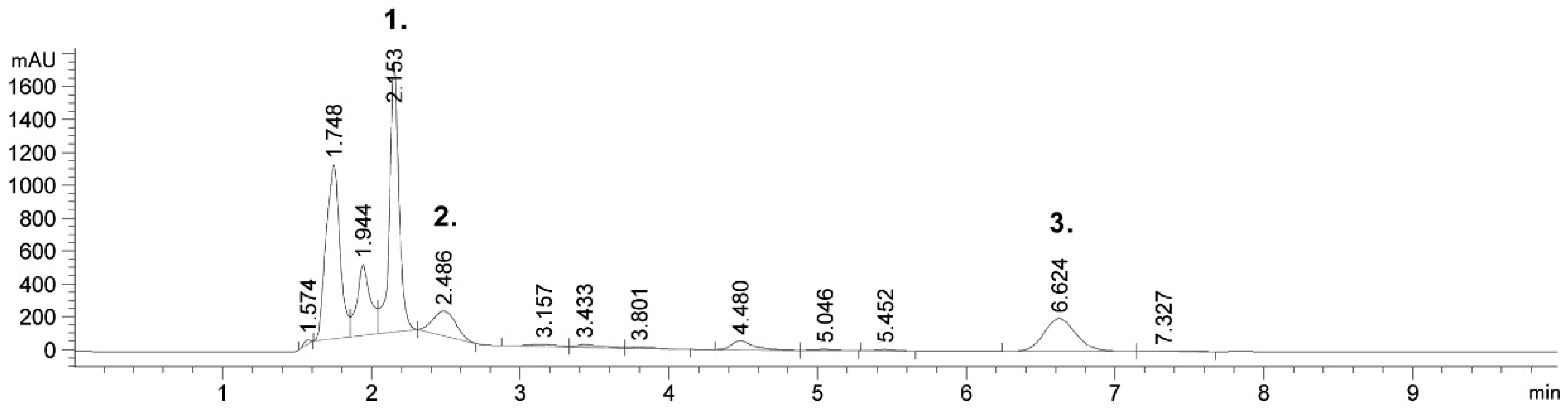
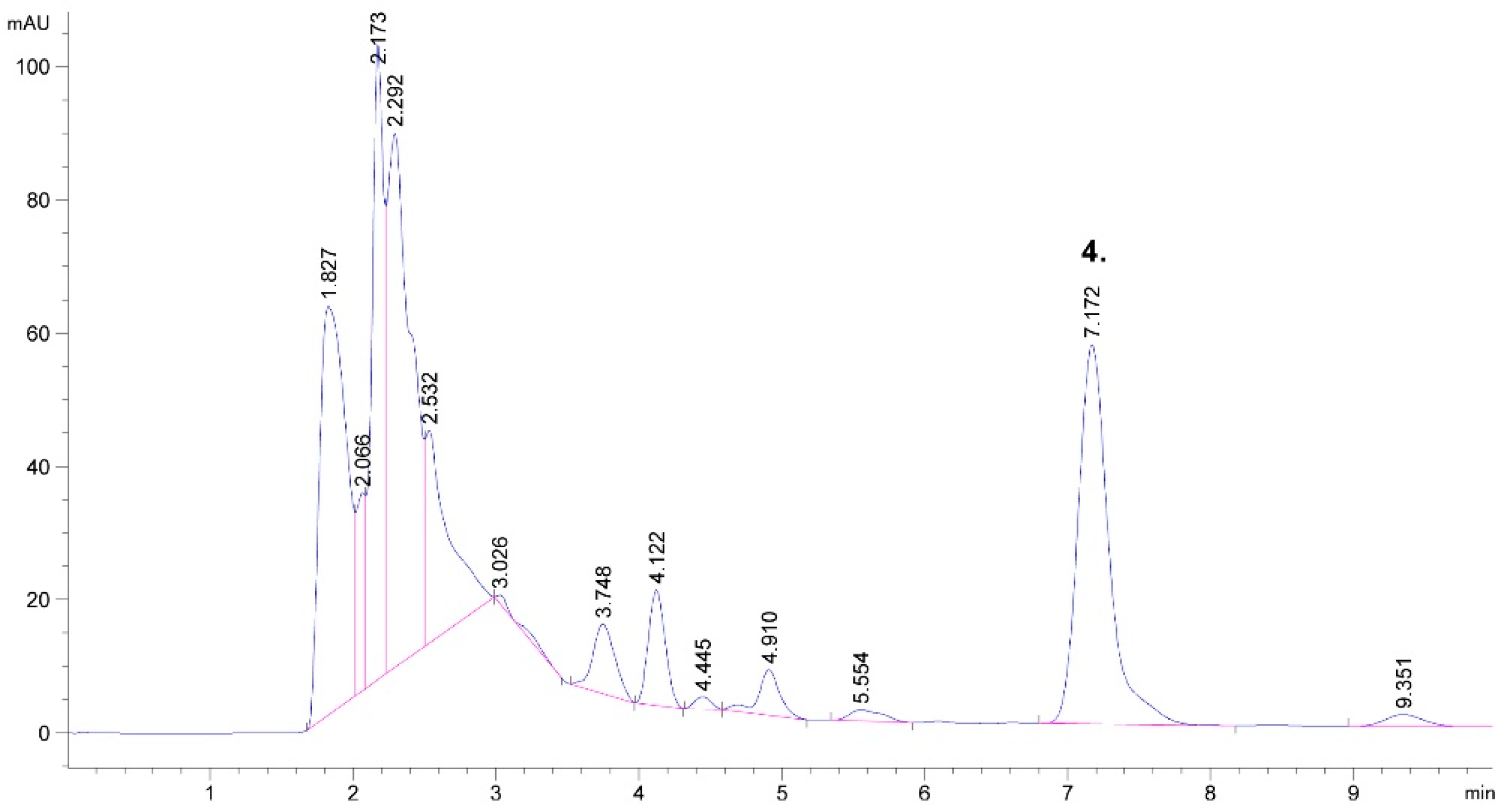
| Plant Part | Process Parameters of UAE | Extracted Compounds | Reference | ||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Solvent | Solid-Solvent Ratio | Frequency/Power | |||
| Leaves and residues | - | 60 | Ethanol | 1:10 | - | Solanesol | [7] |
| Residues | 25 | 15 | Acetone:water (1:2 v/v) | 1:9 | 100 W | Polyphenols | [8] |
| Leaves and waste | 40 | 30 | Ethyl acetate:methanol (1:1 v/v) | 1:30 | - | Phenolic acids Flavonoids | [9] |
| Leaves | 25 and 40 | 20 | Acetone Methanol | 1:10 | 40 Hz | Phenols and flavonoids | [10] |
| Leaves | 40 | 30 | Ethyl acetate:methanol (1:1 v/v) | 1:50 | - | Flavonoids | [11] |
| Leaves | 35 | 30 | Anhydrous methanol with 0.5% ascorbic acid | 1:10 | - | Polyphenols | [12] |
| Leaves | 30–50 | 5–30 | Water + 10–15 drops of ethanol | 20:256 | 24 kHz | Chlorogenic acid | [13] |
| Leaves | - | 30 | Methanol:water (70:30 v/v) | 1:160 | 50 Hz | Polyphenols | [14] |
| Different part of tobacco plant | 45 | 15 | Ethanol:water (85:15 v/v) | 1:15 | 40 Hz | Solanesol | [15] |
| Leaves | - | 30 | Acetone | 1:30 | - | Fatty alcohols Phytosterols Solanesol | [16] |
| Leaves | 70 | 180 | Methanol:water (70:30 v/v) | - | - | Polyphenols | [17] |
| Cigarette tobacco | - | 30 | - | 1:45 | 200 W | Rutin | [18] |
| Lyophilized leaves | - | 15 | Methanol:water (80:20 v/v) | - | Polyphenols | [19] | |
| Fresh leaves | 60 | 240 | Ethanol: hexane (125:75 v/v) | 10:200 | 47 ± 3 kHz | Solanesol | [20] |
| Leaves | 60 | 3 × 20 | Methanol:water (60:40 v/v) | 1:50 | - | Phenolic acids | [21] |
| Waste material | - | 120 | Methanol:water (70:30 v/v) | 150:500 | - | Chlorogenic acids and rutin | [22] |
| RUN | UAE Conditions | Chlorogenic Acid (ug/mL) | Caffeic Acid (ug/mL) | Rutin (ug/mL) | DPPH (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Solvent-Solid ratio (mL/g) | Ethanol-Water ratio (%) | Leaves | Scrap | Dust | Midrib | Leaves | Scrap | Dust | Midrib | Leaves | Scrap | Dust | Midrib | Leaves | Scrap | Dust | Midrib | |
| 1. | 70 | 30 | 30 | 80 | 143.0 | 25.0 | 23.01 | 11.35 | 10.6 | 8.0 | - | - | 119 | 37.8 | 25.31 | 10.38 | 56.28 | 3.40 | 10.38 | 7.61 |
| 2. | 50 | 30 | 30 | 60 | 251.8 | 115.4 | 103.8 | 19.40 | 11.1 | 3.4 | - | - | 126 | 38.7 | 29.67 | 20.76 | 79.69 | 25.1 | 20.76 | 17.51 |
| 3. | 50 | 30 | 30 | 60 | 353.4 | 111.9 | 106.8 | 70.75 | 8.7 | 7.1 | - | - | 89.6 | 38.6 | 28.78 | 22.81 | 80.63 | 25.1 | 22.81 | 14.95 |
| 4. | 70 | 45 | 30 | 60 | 297.0 | 116.3 | 115.1 | 20.69 | 12.8 | 5.7 | - | - | 134 | 36.0 | 32.57 | 26.87 | 91.01 | 29.1 | 26.87 | 10.00 |
| 5. | 30 | 30 | 30 | 40 | 434.3 | 103.6 | 96.95 | 59.93 | 9.9 | 6.2 | - | - | 85.2 | 29.9 | 18.68 | 25.23 | 87.26 | 26.9 | 25.23 | 17.68 |
| 6. | 50 | 15 | 10 | 60 | 2221 | 556.4 | 429 | 141 | 31.5 | - | - | - | 281 | 81.9 | 43.10 | 19.48 | 88.95 | 34.5 | 19.48 | 13.55 |
| 7. | 70 | 30 | 50 | 60 | 74.9 | 15.5 | 15.24 | - | 4.6 | 4.9 | 3.44 | - | 116.1 | 47.7 | 34.68 | 16.09 | 85.01 | 21.4 | 16.09 | 18.36 |
| 8. | 50 | 30 | 30 | 60 | 357.4 | 112.1 | 100.8 | 24.12 | 8.8 | 5.0 | - | - | 99.5 | 35.8 | 29.83 | 21.33 | 76.85 | 25.5 | 21.33 | 18.33 |
| 9. | 30 | 30 | 30 | 80 | 69.7 | 21.7 | 19.77 | 8.28 | 8.7 | - | - | - | 85.2 | 14.9 | 12.77 | 0.71 | 13.94 | 3.36 | 0.71 | 6.14 |
| 10. | 50 | 30 | 30 | 60 | 281.0 | 112.3 | 101.2 | 17.89 | 10.9 | 6.4 | - | - | 121.4 | 43.0 | 23.03 | 23.95 | 75.23 | 24.2 | 23.95 | 15.39 |
| 11. | 50 | 30 | 10 | 40 | 2720.0 | 804.2 | 508.4 | 128.7 | 31.5 | - | - | - | 311.0 | 68.8 | 41.50 | 28.49 | 94.94 | 37.2 | 28.49 | 24.74 |
| 12. | 50 | 30 | 50 | 80 | 44.3 | 26.3 | 8.04 | - | - | 3.3 | 5.20 | 3.32 | 123.5 | 34.4 | 33.78 | 14.85 | 78.83 | 18.3 | 14.85 | 7.41 |
| 13. | 50 | 30 | 50 | 40 | 102.8 | 8.3 | 22.30 | 11.86 | 8.9 | 7.4 | - | 5.70 | 99.6 | 43.6 | 33.40 | 26.54 | 95.02 | 20.8 | 26.54 | 18.43 |
| 14. | 50 | 45 | 50 | 60 | 75.0 | 10.9 | 13.60 | 5.06 | 6.5 | 10.8 | 5.48 | 3.68 | 138.1 | 42.7 | 34.76 | 23.11 | 80.37 | 16.99 | 23.11 | 13.41 |
| 15. | 30 | 15 | 30 | 60 | 193.0 | 121.7 | 105.9 | 41.15 | 6.4 | - | - | - | 91.5 | 33.4 | 19.60 | 23.98 | 94.19 | 16.18 | 23.98 | 9.11 |
| 16. | 50 | 15 | 50 | 60 | 76.3 | 16.2 | 11.84 | - | 4.8 | 3.0 | 2.34 | 3.20 | 119.9 | 42.4 | 30.12 | 19.42 | 78.42 | 28.74 | 19.42 | 11.47 |
| 17. | 50 | 45 | 30 | 80 | 165.0 | 99.7 | 85.40 | 8.78 | 12.1 | 6.9 | - | 7.89 | 171.6 | 43.7 | 32.01 | 18.58 | 93.86 | 14.89 | 18.58 | 17.75 |
| 18. | 50 | 45 | 30 | 40 | 463.8 | 106.6 | 50.56 | 19.73 | 9.1 | 6.4 | - | - | 88.8 | 28.9 | 23.00 | 27.28 | 94.01 | 32.80 | 27.28 | 19.86 |
| 19. | 50 | 45 | 10 | 60 | 2008 | 557.7 | 458.0 | 164.5 | 25.5 | - | - | - | 299.0 | 48.5 | 58.20 | 24.89 | 94.64 | 17.47 | 24.89 | 21.19 |
| 20. | 30 | 30 | 50 | 60 | 54.9 | 17.8 | 13.66 | 3.64 | 3.6 | 3.2 | 3.66 | - | 116.7 | 47.6 | 33.80 | 20.62 | 93.44 | 26.45 | 20.62 | 11.30 |
| 21. | 50 | 15 | 30 | 80 | 77.3 | 22.9 | 23.20 | 8.25 | 11.4 | - | - | - | 77.6 | 22.5 | 25.64 | 11.52 | 26.08 | 10.01 | 11.52 | 5.70 |
| 22. | 70 | 30 | 30 | 40 | 393.2 | 113.9 | 49.43 | 22.90 | 10.7 | 7.8 | - | - | 96.0 | 28.6 | 20.26 | 25.53 | 74.56 | 33.32 | 25.53 | 17.27 |
| 23. | 70 | 30 | 10 | 60 | 1944 | 605.0 | 462.7 | 137.1 | 16.9 | - | - | - | 327.9 | 65.1 | 42.70 | 20.66 | 77.56 | 14.44 | 20.66 | 14.44 |
| 24. | 50 | 30 | 10 | 80 | 1713 | 424.8 | 290.3 | 96.90 | 24.9 | - | - | - | 266.5 | 54.0 | 41.40 | 13.37 | 49.76 | 13.52 | 13.37 | 10.68 |
| 25. | 30 | 45 | 30 | 60 | 313.3 | 124.2 | 103.1 | 67.78 | 8.4 | 6.2 | - | - | 93.7 | 30.1 | 24.92 | 19.05 | 78.16 | 14.33 | 19.05 | 12.83 |
| 26. | 50 | 15 | 30 | 40 | 469.2 | 106.3 | 46.50 | 19.57 | 9.5 | 6.6 | - | - | 82.4 | 24.9 | 14.65 | 24.69 | 74.48 | 19.95 | 24.69 | 16.59 |
| 27. | 50 | 30 | 30 | 60 | 303.1 | 125.8 | 102.5 | 21.88 | 9.6 | 8.4 | - | - | 102.7 | 36.0 | 26.40 | 22.07 | 75.95 | 26.41 | 22.07 | 15.77 |
| 28. | 30 | 30 | 10 | 60 | 2318. | 653.5 | 397.4 | 61.60 | 30.7 | - | - | - | 427.6 | 93.7 | 32.80 | 18.68 | 87.19 | 24.34 | 18.68 | 11.88 |
| 29. | 70 | 15 | 30 | 60 | 228.4 | 116.1 | 105.2 | 44.35 | 11.4 | 7.4 | - | - | 128.5 | 47.8 | 26.27 | 20.89 | 69.99 | 26.93 | 20.89 | 17.44 |
| RUN | UAE Conditions | Solanesol (μg/mL) | ||||
|---|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Leaves | Scraps | Dust | Midrib | |
| 1. | 70 | 45 | 556 | 162.5 | 148 | 61.2 |
| 2. | 55 | 51.2 | 598.9 | 148.2 | 137.7 | 54.2 |
| 3. | 55 | 30 | 485.1 | 148.4 | 128.4 | 53.9 |
| 4. | 55 | 30 | 406.2 | 143.2 | 125.9 | 53.8 |
| 5. | 55 | 30 | 456.1 | 135.0 | 125.8 | 52.0 |
| 6. | 76.2 | 30 | 598.7 | 129.4 | 182.8 | 56.7 |
| 7. | 55 | 8.8 | 416.4 | 114.4 | 106.4 | 57.5 |
| 8. | 33.8 | 30 | 294.9 | 127.3 | 109.4 | 51.7 |
| 9. | 70 | 15 | 457.4 | 134.5 | 81.7 | 55.4 |
| 10. | 40 | 15 | 398.7 | 123.2 | 96.5 | 50.3 |
| 11. | 40 | 45 | 473.7 | 136.3 | 132.7 | 54.3 |
| 12. | 55 | 30 | 539.5 | 133.5 | 122.0 | 62.7 |
| 13. | 55 | 30 | 446.2 | 129.8 | 92.8 | 55.5 |
| Extracted Compound | Leaves Extracts | Waste Extracts | p | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Min | Max | Mean ± SD | Min | Max | ||
| Chlorogenic acid (μg/mL) | 102.05 ± 70.12 | 21.13 | 272.09 | 21.94 ± 17.31 | 1.82 | 80.42 | <0.001 |
| Caffeic acid (μg/mL) | 2.82 ± 0.81 | 0.00 | 4.43 | 2.30 ± 1.14 | 1.04 | 6.66 | 0.048 |
| Rutin (μg/mL) | 37.65 ± 13.18 | 23.52 | 69.06 | 8.74 ± 5.67 | 1.28 | 23.86 | <0.001 |
| Solanesol (ng/μg) | 28.63 ± 17.87 | 29.49 | 59.89 | 8.24 ± 3.65 | 5.03 | 18.28 | <0.001 * |
| DPPH | 77.46 ± 19.41 | 13.94 | 95.02 | 18.86 ± 7.28 | 0.71 | 37.24 | <0.001 * |
| Extracted Compound | Chlorogenic Acid | Caffeic Acid | Rutin | Solanesol | DPPH |
|---|---|---|---|---|---|
| Chlorogenic acid | 1.000 | ||||
| Caffeic acid | −0.282 | 1.000 | |||
| Rutin | 0.088 | −0.045 | 1.000 | ||
| Solanesol | 0.059 | −0.079 | 0.179 | 1.000 | |
| DPPH | 0.537 ** | −0.220 | 0.407 ** | 0.003 | 1.000 |
| Extracted Compound | Leaves Extracts * | Waste Extracts ** | ||
|---|---|---|---|---|
| Solvent-Solid Ratio (mL/g) | Ethanol-Water Ratio (%) | Solvent-Solid Ratio (mL/g) | Ethanol-Water Ratio (%) | |
| Chlorogenic acid (μg/mL) | −0.839 § | −0.409 ¥ | −0.556 § | −0.207 |
| Caffeic acid (μg/mL) | −0.127 | −0.268 | 0.058 | 0.035 |
| Rutin (μg/mL) | 0.685 § | 0.158 | 0.425 § | −0.068 |
| Sample | Solvent Content | Chlorogenic Acid (μg/mL) | Caffeic Acid (μg/mL) | Rutin (μg/mL) | p |
|---|---|---|---|---|---|
| Leaves | 10 mL/g | 215.45 ± 35.00 | 2.68 ± 0.57 | 31.89 ± 5.74 | <0.001 1 <0.001 2 |
| 30 mL/g | 85.45 ± 37.71 | 3.03 ± 0.48 | 31.97 ± 7.48 | ||
| 50 mL/g | 35.68 ± 10.11 | 2.37 ± 1.48 | 59.49 ± 6.23 | ||
| Waste | 10 mL/g | 38.21 ± 21.93 | 0.00 | 5.32 ± 2.02 | <0.001 3 0.001 4 |
| 30 mL/g | 20.69 ± 12.83 | 2.01 ± 0.39 | 7.45 ± 3.59 | ||
| 50 mL/g | 6.68 ± 3.02 | 2.58 ± 1.51 | 14.21 ± 7.58 |
| Sample | Leaves | Scrap | Dust | Midrib |
|---|---|---|---|---|
| Optimal UAE parameters for chlorogenic acid | 44.5 °C, 17.23 min, 11 mL/g, 40.46% ethanol-water ratio | 46.69 °C, 15.19 min, 10 mL/g, 40% ethanol-water ratio | 53.59 °C, 38.31 min, 10 mL/g, 55.43% ethanol-water ratio | 30.14 °C, 38.31 min, 11 mL/g, 44.83% ethanol-water ratio |
| Predicted chlorogenic acid content (μg/mL) | 276.734 | 78.49 | 49.44 | 12.24 |
| Optimal UAE parameters for rutin | 44.71 °C, 42.76 min, 49.73 mL/g, 76.83% ethanol water ratio | 69.72 °C, 26.23 min, 49.8 mL/g, 59.12% ethanol water ratio | 37.78 °C, 43.02 min, 49.88 mL/g, 58.43% ethanol water ratio | 69.27 °C, 39.716 min, 49.06 mL/g, 74.44% ethanol water ratio |
| Predicted rutin content (μg/mL) | 71.83 | 23.87 | 17.52 | 6.0185 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banožić, M.; Banjari, I.; Jakovljević, M.; Šubarić, D.; Tomas, S.; Babić, J.; Jokić, S. Optimization of Ultrasound-Assisted Extraction of Some Bioactive Compounds from Tobacco Waste. Molecules 2019, 24, 1611. https://doi.org/10.3390/molecules24081611
Banožić M, Banjari I, Jakovljević M, Šubarić D, Tomas S, Babić J, Jokić S. Optimization of Ultrasound-Assisted Extraction of Some Bioactive Compounds from Tobacco Waste. Molecules. 2019; 24(8):1611. https://doi.org/10.3390/molecules24081611
Chicago/Turabian StyleBanožić, Marija, Ines Banjari, Martina Jakovljević, Drago Šubarić, Srećko Tomas, Jurislav Babić, and Stela Jokić. 2019. "Optimization of Ultrasound-Assisted Extraction of Some Bioactive Compounds from Tobacco Waste" Molecules 24, no. 8: 1611. https://doi.org/10.3390/molecules24081611
APA StyleBanožić, M., Banjari, I., Jakovljević, M., Šubarić, D., Tomas, S., Babić, J., & Jokić, S. (2019). Optimization of Ultrasound-Assisted Extraction of Some Bioactive Compounds from Tobacco Waste. Molecules, 24(8), 1611. https://doi.org/10.3390/molecules24081611








