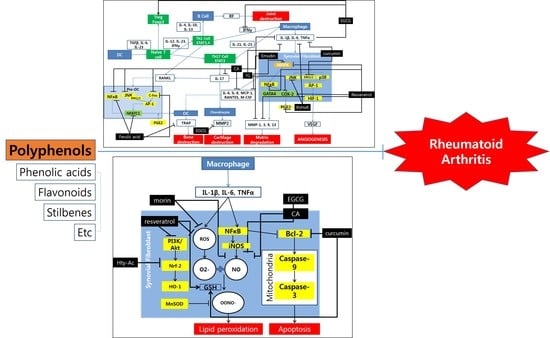
Could Polyphenols Help in the Control of Rheumatoid Arthritis?
Abstract
1. Introduction
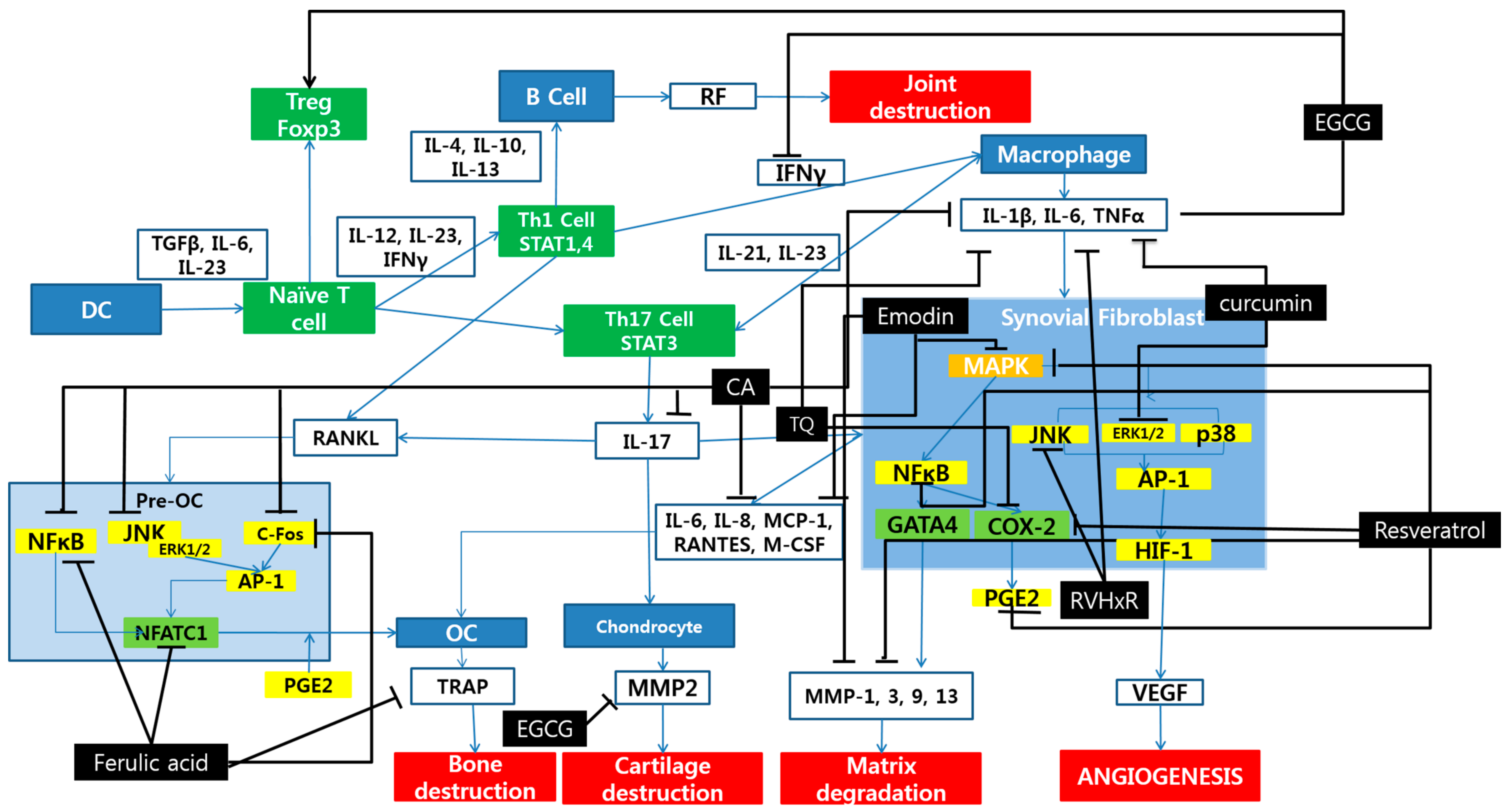
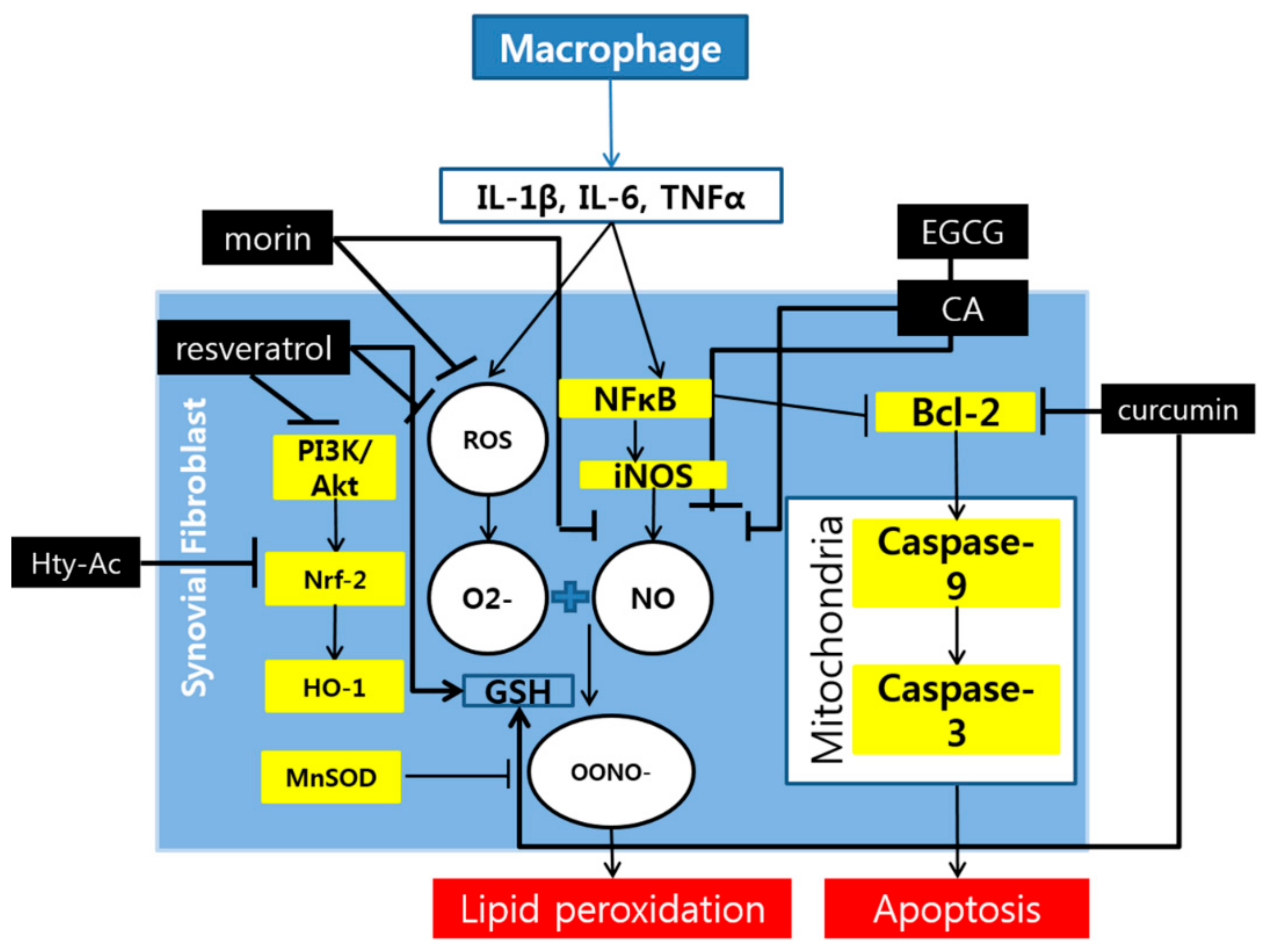
2. Polyphenols and Rheumatoid Arthritis
2.1. Phenolic Acids
2.2. Stilbenes
2.3. Flavonoids
2.4. Other Compounds
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Viatte, S.; Plant, D.; Raychaudhuri, S. Genetics and epigenetics of rheumatoid arthritis. Nat. Rev. Rheumatol 2013, 9, 141–153. [Google Scholar] [CrossRef]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51 (Suppl. 5), v3–v11. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Doss, H.M.; Samarpita, S.; Ganesan, R.; Rasool, M. Ferulic acid, a dietary polyphenol suppresses osteoclast differentiation and bone erosion via the inhibition of RANKL dependent NF-kappaB signalling pathway. Life Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Paskova, L.; Kuncirova, V.; Ponist, S.; Mihalova, D.; Nosal, R.; Harmatha, J.; Hradkova, I.; Cavojsky, T.; Bilka, F.; Siskova, K.; et al. Effect of N-Feruloylserotonin and Methotrexate on Severity of Experimental Arthritis and on Messenger RNA Expression of Key Proinflammatory Markers in Liver. J. Immunol. Res. 2016, 2016, 7509653. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, H.H.; Kim, J.E.; Kim, J.A.; Kim, Y.H.; Jun, C.D.; Kim, S.H. Allose gallates suppress expression of pro-inflammatory cytokines through attenuation of NF-kappaB in human mast cells. Planta Med. 2007, 73, 769–773. [Google Scholar] [CrossRef]
- Kwak, S.C.; Lee, C.; Kim, J.Y.; Oh, H.M.; So, H.S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic acid inhibits osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor kappa-B ligand-induced nuclear factor of activated T cells c1 expression. Biol. Pharm. Bull. 2013, 36, 1779–1786. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Neog, M.K.; Joshua Pragasam, S.; Krishnan, M.; Rasool, M. p-Coumaric acid, a dietary polyphenol ameliorates inflammation and curtails cartilage and bone erosion in the rheumatoid arthritis rat model. Biofactors 2017, 43, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, E.G.; Lee, H.; Cho, I.J.; Choi, Y.J.; Sung, M.S.; Yoo, H.G.; Yoo, W.H. Kaempferol inhibits IL-1beta-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int. J. Mol. Med. 2013, 32, 971–977. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodriguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxid. Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, X.; Cao, W.; Lu, J.; Wang, X.; Wang, G.; Wang, Z.; Chen, X. Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats treatment with resveratrol. Sci. Rep. 2016, 6, 32928. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Hsu, L.F.; Lee, C.W.; Chiang, Y.C.; Lee, M.H.; How, J.M.; Wu, C.M.; Huang, C.L.; Lee, I.T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-kappaB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.W.; Gao, J.S.; Li, L.; Xie, X. Resveratrol inhibits TNF-alpha-induced IL-1beta, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway. Rheumatol. Int. 2013, 33, 1829–1835. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Xuzhu, G.; Komai-Koma, M.; Leung, B.P.; Howe, H.S.; McSharry, C.; McInnes, I.B.; Xu, D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann. Rheum. Dis. 2012, 71, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wahba, M.G.; Messiha, B.A.; Abo-Saif, A.A. Protective effects of fenofibrate and resveratrol in an aggressive model of rheumatoid arthritis in rats. Pharm. Biol. 2016, 54, 1705–1715. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kim, K.; Vance, T.M.; Chun, O.K. Greater flavonoid intake is associated with improved CVD risk factors in US adults. Br. J. Nutr. 2016, 115, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Burke, A.C.; Huff, M.W. Citrus Flavonoids as Regulators of Lipoprotein Metabolism and Atherosclerosis. Annu. Rev. Nutr. 2016, 36, 275–299. [Google Scholar] [CrossRef]
- Kometani, T.; Fukuda, T.; Kakuma, T.; Kawaguchi, K.; Tamura, W.; Kumazawa, Y.; Nagata, K. Effects of alpha-glucosylhesperidin, a bioactive food material, on collagen-induced arthritis in mice and rheumatoid arthritis in humans. Immunopharmacol. Immunotoxicol. 2008, 30, 117–134. [Google Scholar] [CrossRef]
- He, Y.H.; Zhou, J.; Wang, Y.S.; Xiao, C.; Tong, Y.; Tang, J.C.; Chan, A.S.; Lu, A.P. Anti-inflammatory and anti-oxidative effects of cherries on Freund’s adjuvant-induced arthritis in rats. Scand. J. Rheumatol. 2006, 35, 356–358. [Google Scholar] [CrossRef]
- Kim, J.E.; Son, J.E.; Jung, S.K.; Kang, N.J.; Lee, C.Y.; Lee, K.W.; Lee, H.J. Cocoa polyphenols suppress TNF-alpha-induced vascular endothelial growth factor expression by inhibiting phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase kinase-1 (MEK1) activities in mouse epidermal cells. Br. J. Nutr. 2010, 104, 957–964. [Google Scholar] [CrossRef]
- Ahmed, S.; Pakozdi, A.; Koch, A.E. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2006, 54, 2393–2401. [Google Scholar] [CrossRef]
- Yun, H.J.; Yoo, W.H.; Han, M.K.; Lee, Y.R.; Kim, J.S.; Lee, S.I. Epigallocatechin-3-gallate suppresses TNF-alpha -induced production of MMP-1 and -3 in rheumatoid arthritis synovial fibroblasts. Rheumatol. Int. 2008, 29, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jung, Y.O.; Ryu, J.G.; Oh, H.J.; Son, H.J.; Lee, S.H.; Kwon, J.E.; Kim, E.K.; Park, M.K.; Park, S.H.; et al. Epigallocatechin-3-gallate ameliorates autoimmune arthritis by reciprocal regulation of T helper-17 regulatory T cells and inhibition of osteoclastogenesis by inhibiting STAT3 signaling. J. Leukoc. Biol. 2016, 100, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Yan, M.; Kim, S.B.; Ravikumar, S.; Kwon, S.R.; Vanarsa, K.; Kim, H.Y.; Davis, L.S.; Mohan, C. Green Tea Epigallocatechin-3-Gallate Suppresses Autoimmune Arthritis Through Indoleamine-2,3-Dioxygenase Expressing Dendritic Cells and the Nuclear Factor, Erythroid 2-Like 2 Antioxidant Pathway. J. Inflamm. 2015, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Leichsenring, A.; Backer, I.; Furtmuller, P.G.; Obinger, C.; Lange, F.; Flemmig, J. Long-Term Effects of (−)-Epigallocatechin Gallate (EGCG) on Pristane-Induced Arthritis (PIA) in Female Dark Agouti Rats. PLoS ONE 2016, 11, e0152518. [Google Scholar] [CrossRef] [PubMed]
- Morinobu, A.; Biao, W.; Tanaka, S.; Horiuchi, M.; Jun, L.; Tsuji, G.; Sakai, Y.; Kurosaka, M.; Kumagai, S. (−)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008, 58, 2012–2018. [Google Scholar] [CrossRef]
- Oka, Y.; Iwai, S.; Amano, H.; Irie, Y.; Yatomi, K.; Ryu, K.; Yamada, S.; Inagaki, K.; Oguchi, K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J. Pharmacol. Sci. 2012, 118, 55–64. [Google Scholar] [CrossRef]
- Lee, J.D.; Huh, J.E.; Jeon, G.; Yang, H.R.; Woo, H.S.; Choi, D.Y.; Park, D.S. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritis fibroblast-like synovial cells and in vivo models. Int. Immunopharmacol. 2009, 9, 268–276. [Google Scholar] [CrossRef]
- Rathi, B.; Bodhankar, S.; Mohan, V.; Thakurdesai, P. Ameliorative Effects of a Polyphenolic Fraction of Cinnamomum zeylanicum L. Bark in Animal Models of Inflammation and Arthritis. Sci. Pharm. 2013, 81, 567–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Jin, S.; He, D.; Zhao, S.; Liu, S. Genistein modulate immune responses in collagen-induced rheumatoid arthritis model. Maturitas 2008, 59, 405–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, J.; He, P.; Li, W.; Zhang, Q.; Li, N.; Sun, T. Genistein inhibit cytokines or growth factor-induced proliferation and transformation phenotype in fibroblast-like synoviocytes of rheumatoid arthritis. Inflammation 2012, 35, 377–387. [Google Scholar] [CrossRef]
- Umar, S.; Kumar, A.; Sajad, M.; Zargan, J.; Ansari, M.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Hesperidin inhibits collagen-induced arthritis possibly through suppression of free radical load and reduction in neutrophil activation and infiltration. Rheumatol. Int. 2013, 33, 657–663. [Google Scholar] [CrossRef]
- Decendit, A.; Mamani-Matsuda, M.; Aumont, V.; Waffo-Teguo, P.; Moynet, D.; Boniface, K.; Richard, E.; Krisa, S.; Rambert, J.; Merillon, J.M.; et al. Malvidin-3-O-beta glucoside, major grape anthocyanin, inhibits human macrophage-derived inflammatory mediators and decreases clinical scores in arthritic rats. Biochem. Pharmacol. 2013, 86, 1461–1467. [Google Scholar] [CrossRef]
- Tsubaki, M.; Takeda, T.; Kino, T.; Itoh, T.; Imano, M.; Tanabe, G.; Muraoka, O.; Satou, T.; Nishida, S. Mangiferin suppresses CIA by suppressing the expression of TNF-alpha, IL-6, IL-1beta, and RANKL through inhibiting the activation of NF-kappaB and ERK1/2. Am. J. Transl. Res. 2015, 7, 1371–1381. [Google Scholar] [PubMed]
- Sultana, F.; Neog, M.K.; Rasool, M. Targeted delivery of morin, a dietary bioflavanol encapsulated mannosylated liposomes to the macrophages of adjuvant-induced arthritis rats inhibits inflammatory immune response and osteoclastogenesis. Eur. J. Pharm. Biopharm. 2017, 115, 229–242. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Wei, T.; Gao, J.; He, H.; Chang, X.; Yan, T. Effects of Naringenin on inflammation in complete freund’s adjuvant-induced arthritis by regulating Bax/Bcl-2 balance. Inflammation 2015, 38, 245–251. [Google Scholar] [CrossRef]
- Umar, S.; Hedaya, O.; Singh, A.K.; Ahmed, S. Thymoquinone inhibits TNF-alpha-induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation. Toxicol. Appl. Pharmacol. 2015, 287, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Tekeoglu, I.; Dogan, A.; Ediz, L.; Budancamanak, M.; Demirel, A. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother. Res. 2007, 21, 895–897. [Google Scholar] [CrossRef]
- Vaillancourt, F.; Silva, P.; Shi, Q.; Fahmi, H.; Fernandes, J.C.; Benderdour, M. Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J. Cell Biochem. 2011, 112, 107–117. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Alcaraz, M.J.; Sanchez-Hidalgo, M.; Fernandez-Bolanos, J.G.; Alarcon-de-la-Lastra, C.; Ferrandiz, M.L. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Lubberts, E.; Alarcon-de-la-Lastra, C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagen-induced arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef]
- Ramadan, G.; Al-Kahtani, M.A.; El-Sayed, W.M. Anti-inflammatory and anti-oxidant properties of Curcuma longa (turmeric) versus Zingiber officinale (ginger) rhizomes in rat adjuvant-induced arthritis. Inflammation 2011, 34, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Kloesch, B.; Becker, T.; Dietersdorfer, E.; Kiener, H.; Steiner, G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int. Immunopharmacol. 2013, 15, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Moon, D.O.; Choi, I.W.; Choi, B.T.; Nam, T.J.; Rhu, C.H.; Kwon, T.K.; Lee, W.H.; Kim, G.Y.; Choi, Y.H. Curcumin induces apoptosis and inhibits prostaglandin E(2) production in synovial fibroblasts of patients with rheumatoid arthritis. Int. J. Mol. Med. 2007, 20, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sun, Y.; Liu, Z.; Zhang, M.; Li, C.; Cai, H. The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des. Dev. Ther. 2015, 9, 4931–4942. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Noh, E.M.; Moon, S.J.; Kim, J.M.; Kwon, K.B.; Park, B.H.; You, Y.O.; Hwang, B.M.; Kim, H.J.; Kim, B.S.; et al. Emodin suppresses inflammatory responses and joint destruction in collagen-induced arthritic mice. Rheumatology 2013, 52, 1583–1591. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, K.; Qiu, Y.; Yan, F.; Lin, C. Therapeutic effect of emodin on collagen-induced arthritis in mice. Inflammation 2013, 36, 1253–1259. [Google Scholar] [CrossRef]
- Ha, M.K.; Song, Y.H.; Jeong, S.J.; Lee, H.J.; Jung, J.H.; Kim, B.; Song, H.S.; Huh, J.E.; Kim, S.H. Emodin inhibits proinflammatory responses and inactivates histone deacetylase 1 in hypoxic rheumatoid synoviocytes. Biol. Pharm. Bull. 2011, 34, 1432–1437. [Google Scholar] [CrossRef]
- Fleury, G.; Mania, S.; Hannouche, D.; Gabay, C. The perioperative use of synthetic and biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Swiss Med. Wkly. 2017, 147, w14563. [Google Scholar] [CrossRef]
- Cho, S.-K.; Bae, S.-C. Pharmacologic treatment of rheumatoid arthritis. J. Korean Med. Assoc. 2017, 60. [Google Scholar] [CrossRef]
- Soto, M.; Falqué, E.; Domínguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef]
| Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|
| Ferulic acid | Grains (rice, wheat and oats), vegetables, fruits, nuts | monocyte/macrophage cells/Rat | 25, 50, 100 μM/24 h | ↓ NFATc1, c-Fos, NF-κB, TRAP, MMP-9, Cathepsin | [10] |
| Natural polyphenol N-feruloylserotonin (N-f-5HT) | Leuzea carthamoides | AA | 3 mg/kg/28 days | ↓ CRP, LOX, TNF-α, iNOS, IL-1β | [11] |
| Gallotanins | Euphorbia | HMC-1/human | 10 mg/mL/30 min | ↓ TNF- α, IL-1β, IL-6, NF-κB | [12] |
| Kaempferol (3,5,7,4′-tetrahydroxy-flavone) | Gallic acid | RASFs/human | 100 µM/ 2 days | ↓ IL-1β, MMPs, COX, PGE2 | [16] |
| Chlorogenic acid (CGA) | Gardenia jasminoides | osteoclast/ BMMs | 10, 25, 50 μg/mM/4 days | ↓ NF-κB, P38, Akt, ERK | [13] |
| p-Coumaric Acid (CA) | Gnetm cleistostachyum | AIA | 100 mg/kg/8 days | ↓TNF-α, CIC ↑ IgG | [14] |
| p-Coumaric Acid (CA) | Gnetm cleistostachyum | AIA | 100 mg/kg/16 days | ↓ TNF-α, IL-1β, IL-6, MCP-1, RANKL, TRAP, IL-1β, IL-6, IL-17, iNOS, COX-2, NF-κB-p65, p-NF-κB-p65, NFATc-1, c-Fos, JNK, p-JNK, ERK1/2 ↑OPG | [15] |
| Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|
| Resveratrol | Red grapes | FLSs/AA | 5, 15, 45 mg/kg/12 days | ↑ MtROS ↓ Beclin1, LC3A/B, MnSOD | [18] |
| Resveratrol | Red grapes | FLSs/Human | 50 μg/24 h | ↓ COX-2, PGE2, NADPH oxidase, ROS, Akt, p38, MAPK, ERK1/2, NF-κB | [19] |
| Resveratrol | Red grapes | FLSs/Human | 6.25, 12.5, 25, 50 µM/1 h | ↓ IL-1β, MMP-3, P-Akt, PI3K-Akt | [20] |
| Resveratrol | Red grapes | Human * randomized controlled clinical trial | 1000 mg/day/3 month | ↓ RF, MMP-3, TNF-α, IL-6, | [21] |
| Resveratrol | Red grapes |
|
|
| [22] |
| Resveratrol | Red grapes | CFA induced rat | 10 mg/kg/day/7 days | RF, MMP-3, COMP, IgG, ANA, TNF-a, MPO, MDA ↑ IL-10, GSH | [23] |
| Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|
| A-glucosylhesperidin | Citrus fruit | CIA rat | 3 mg/0.3 mL/3 times a week, 31 days | ↓ TNFα | [27] |
| Anthocyanin | Cherries | AIA rat (Male Sprague Dawley) | 10, 20, 40 mg/kg/14 days | ↓TNFα, PGE2, MDA ↑ SOD | [28] |
| Cocoa polyphenol (epicatechin, catechins, flavonol glycosides and procyanidin) | Cocoa | JB6 P+ mouse epidermal cells | 0, 10, 20 μM /mL/1 h | ↓ VEGF, NF-kB, AP-1 ↓ p-Akt, p-p70S6K, p- ERK, p- p90RSK, p- MKK4, p-JNK, p- PI3K | [29] |
| Epigallocatechin-3-gallate (EGCG) | Green tea (Camellia sinensis) | RASFs | 10, 20, 30, 40, 50 μM/12 h | ↓ENA-78, RANTES, GRO-alpha, MMP-2 | [30] |
| Epigallocatechin-3-gallate (EGCG) | Green tea | CIA rat (DBA/1J) | 20, 30, 40, 50 mg/kg/3 weeks | ↓ IgG2a, IL-1β, IL-6, TNFα, TRAP, IL-17, VEGF, nitrotyrosine, iNOS, p-STAT3, c-Fos, NFATc1, CTSK, MMP9, p-STAT3 727, IL-17, CCL6, AHR, IL-21, p-STAT3 705, p-ERK, RANK, CTR ↑ IL-10, TGF- β, SOCS3, Foxp3 | [32] |
| Epigallocatechin gallate | Green tea | PIA rats (Dark Agouti) | 10 mg/kg/5 days | ↓ MPO | [34] |
| Epigallocatechin-3-gallate (EGCG) | Green tea (Camellia sinensis) |
| 20 μM, 50 μM/15 days | ↓ CTR, carbonic anhydrase II, cathepsin K, alpha-v integrin, β-3 integrin, NF-ATc1 | [35] |
| Epigallocatechin-3-gallate (EGCG) | Green tea | CIA rat (DBA/1J) | 10 mg/kg/3 weeks | ↓ IL-6, TNFα, IFN-γ ↑anti-CII specific IgG1 antibodies | [33] |
| Epigallocatechin-3-gallate (EGCG) | Camellia sinensis | Osteoclast precursors cells mature osteoclasts | 10, 100 μM/7 days | ↓ Multinucleated osteoclast formation, MMP-9, MMP-2 | [36] |
| Epigallocatechin 3-gallate (EGCG) | Green tea (Camellia sinensis) | RASFs | 125, 250, 500 nM/24 h | ↓ MAPK, MMP-1, MMP-3, p-ERK1/2, p-JNK, p-p38, AP-1 | [31] |
| Fisetin | Rhus verniciflua Stokes | RA FLs | 0.1, 1, 10 μg/mL/72 h | ↓ TNFα, IL-6, IL-8, MCP-1, VEGF | [37] |
| Flavonol-rich residual layer of hexane fraction (RVHxR) | Rhus verniciflua Stokes | RA FLs | 0.1, 1, 10 μg/mL/72 h | ↓ TNFα, IL-6, IL-8, MCP-1, VEGF ↓ p-ERK, p-JNK, ↑ p- p38-MAPK | [37] |
| Gallic acid | Cinnamomum zeylanicum Bark |
|
|
| [38] |
| Genistein | CIA rats | 1 mL/kg/42 days | ↓ IFN-γ, Th1/Th2, T-bet ↑ GATA-3, IL-4 | [39] | |
| Genistein | Soybean | RA FLS | 10 μg/mL/24 h | ↓ MMP-9 | [40] |
| Hesperidin | CIA rat (Wistar rat) | 160 mg/kg / 22 days | ↓ ELA, TBARS, nitrite ↑ GSH, SOD, catalase | [41] | |
| Malvidin-3-O-β-glucoside | Red grape skinExtract powder |
|
|
| [42] |
| Mangiferin | Thymelaeaceae family (e.g., Phaleria cumingii) | CIA rat (DBA/1) | 100 and 400 mg/kg/14 days and 27 days | ↓ NF-κB, ERK1/2,IL-1β, IL-6, TNF-α, RANKL | [43] |
| Morin (ML-morin) | Fruits, vegetables, tea | Spleen and synovial macrophages | 10 mg/kg/3 days | ↓ ROS, NO, iNOS, NF-κB-p65, TNF-α, IL-1 β, IL-6, MCP-1, VEGF, RANKL, STAT-3 | [44] |
| Naringin | Grape, citrus fruit | AIA rat (Female Sprague-Dawley) |
| ↓ TNFα, IL-1β, IL-6, Bcl-2 ↑ Bax | [45] |
| Theaflavin-3,3′-digallate (TFDG) | Camellia sinensis | osteoclast precursors cells mature osteoclasts | 10, 100 μM/7 days | ↓ Multinucleated osteoclast formation, MMP-9, MMP-2 | [36] |
| Thymoquinone | Nigella sativa | RA synovium | 1, 2, 3, 4, 5 μM/2 h | ↓ IL-6, IL-8, ICAM-1, VCAM-1, Cad-11, p38, JNK | [46] |
| Thymoquinone | Nigella sativa | CIA rat (Sprague-Dawley Wistar rat) | 2.5 mg/kg/5 days 5 mg/kg/5 days | ↓ IL-1β | [47] |
| Thymoquinone | Nigella sativa |
|
|
| [48] |
| Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|
| EVOO-polyphenol extract (PE) | EVOO | CIA in DBA-1/J | 100, 200 mg/kg/13 days | ↓ TNF-α, IL-1β, IL-6, PEG2, p38, JNK, p65, lκB- α | [49] |
| Hydroxytyrosol acetate (Hty-Ac) | EVOO | CIA in DBA-1/J | 0.05%/42 days | ↓ IgG1, IgG2a, COMP, MMP-3, TNF-Q, IFN-S, IL-1R, IL-6, IL-17A, Nrf2, HO-1 | [50] |
| Curcuminoid |
| AIA | 200 mg/kg/28 days | ↑ TNF-α, IL-1β, IL-6, IL-4, IL-10, SOD, CAT, GSH ↓ LPO, ALAT, ALP | [51] |
| Curcumin | Turmeric rhizome |
| 12.5, 25, 50 μM/6 h |
| [52] |
| Curcumin oil-water nanoemulsions (CM-Ns) | Herb turmeric | AIA | 50 mg/kg/14 days | ↓ NF-κB, TNF-α, IL-1β | [54] |
| Curcumin | Rhizome of Curcuma longa | FLS/Patient | 0, 25, 50, 75, 100 μM/24 h | ↓ Bcl-2, COX-2↑caspase-3, caspase-9 | [53] |
| Emodin | Rheum palmatum | CIA DBA/1 J | 10 mg/kg/11 days | ↓ NF-κB, MMP, M-CSF | [55] |
| Emodin | Rheum palmatum | CIA | 5, 10, 20 mg/kg/21 days | ↓ TNF-α, IL-6, PGE2 | [56] |
| Emodin | Rheum palmatum | Synovial membrane/Humans | 0.1, 1, 10 μM/24 h | ↓ HDAC, HDAC1, VEGF, COX-2, HIF-1a, MMP-1, MMP-13, NF-κB, MAPK | [57] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, S.; Kwon, D.; Um, E.; Kim, B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules 2019, 24, 1589. https://doi.org/10.3390/molecules24081589
Sung S, Kwon D, Um E, Kim B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules. 2019; 24(8):1589. https://doi.org/10.3390/molecules24081589
Chicago/Turabian StyleSung, Siyun, Doyoung Kwon, Eunsik Um, and Bonglee Kim. 2019. "Could Polyphenols Help in the Control of Rheumatoid Arthritis?" Molecules 24, no. 8: 1589. https://doi.org/10.3390/molecules24081589
APA StyleSung, S., Kwon, D., Um, E., & Kim, B. (2019). Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules, 24(8), 1589. https://doi.org/10.3390/molecules24081589