Evaluation of Polyphenolic Compounds and Pharmacological Activities in Hairy Root Cultures of Ligularia fischeri Turcz. f. spiciformis (Nakai)
Abstract
1. Introduction
2. Results and Discussion
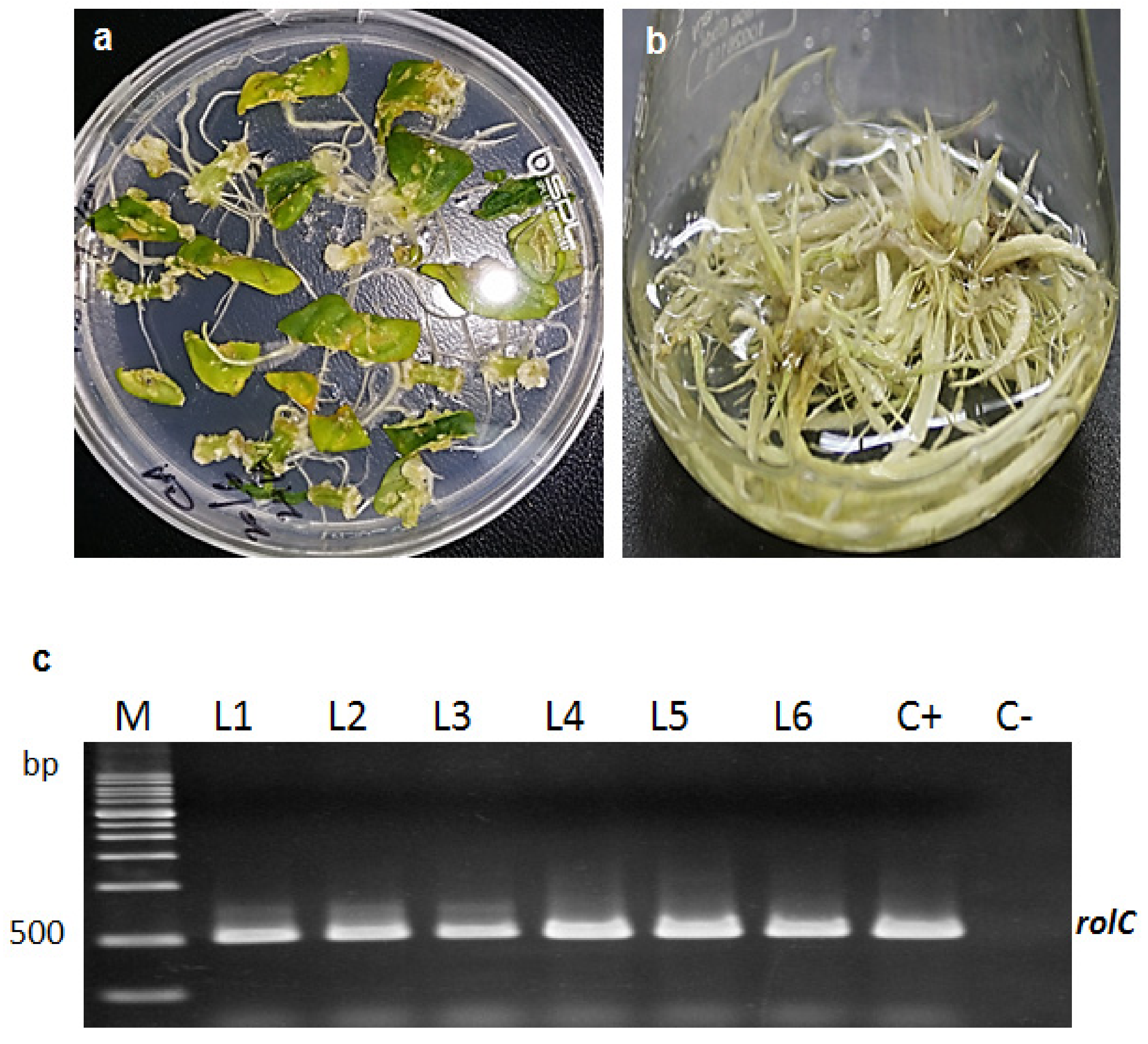
2.1. Formation of Transgenic Hairy Root Cultures (HRCs)
2.2. Factors Influencing the Growth Kinetics in Biomass Accumulation
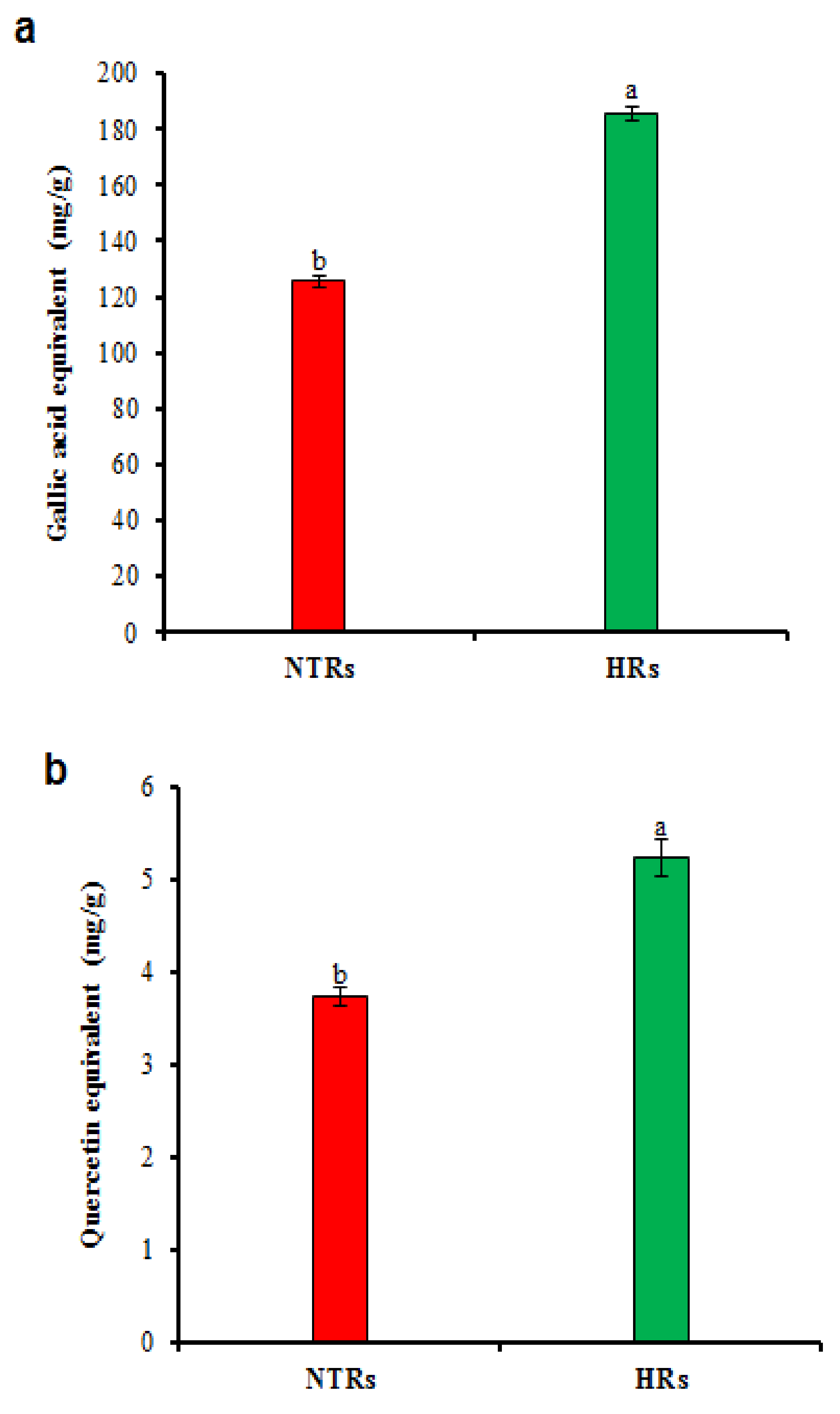
2.3. Polyphenolic Contents
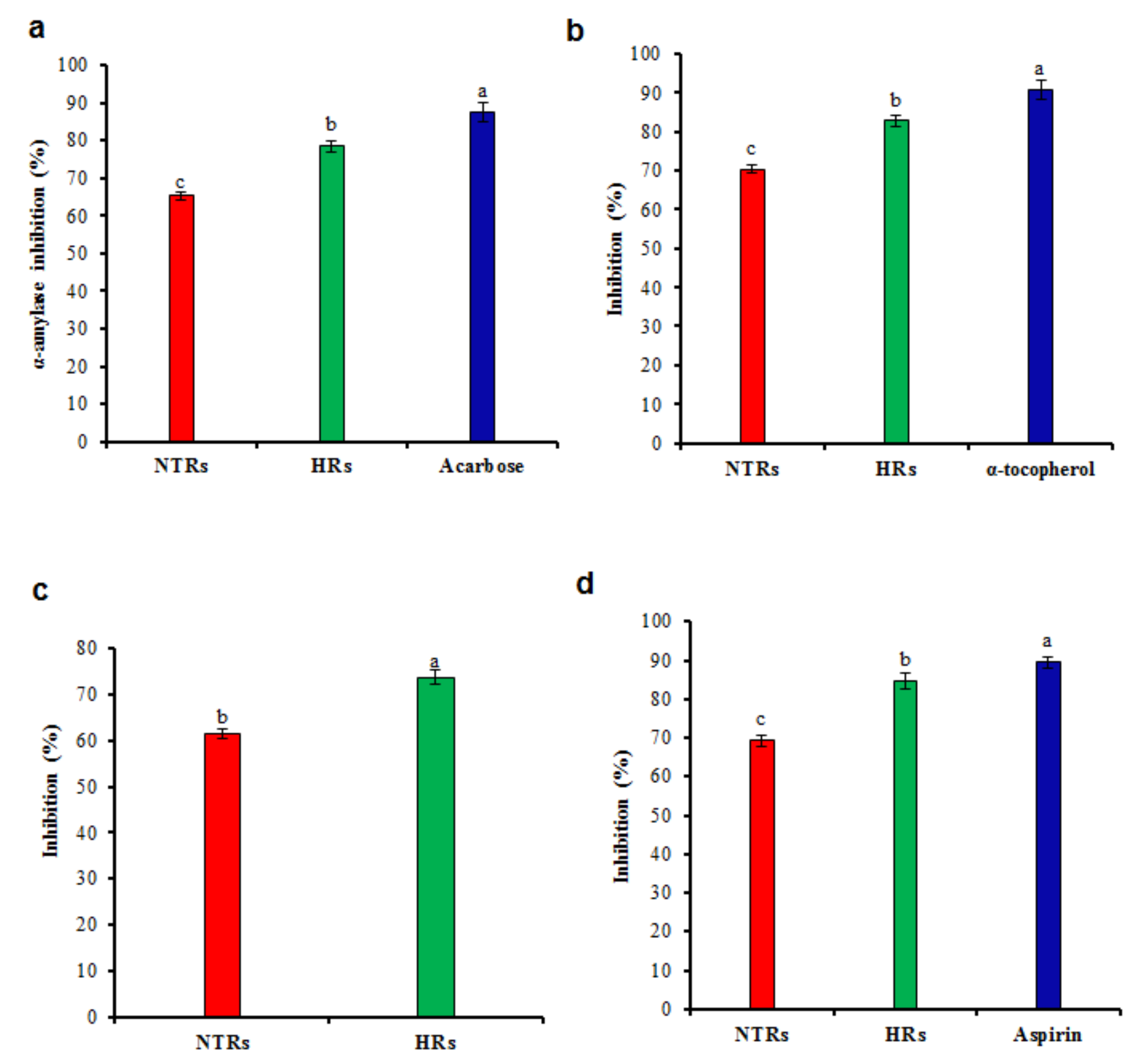
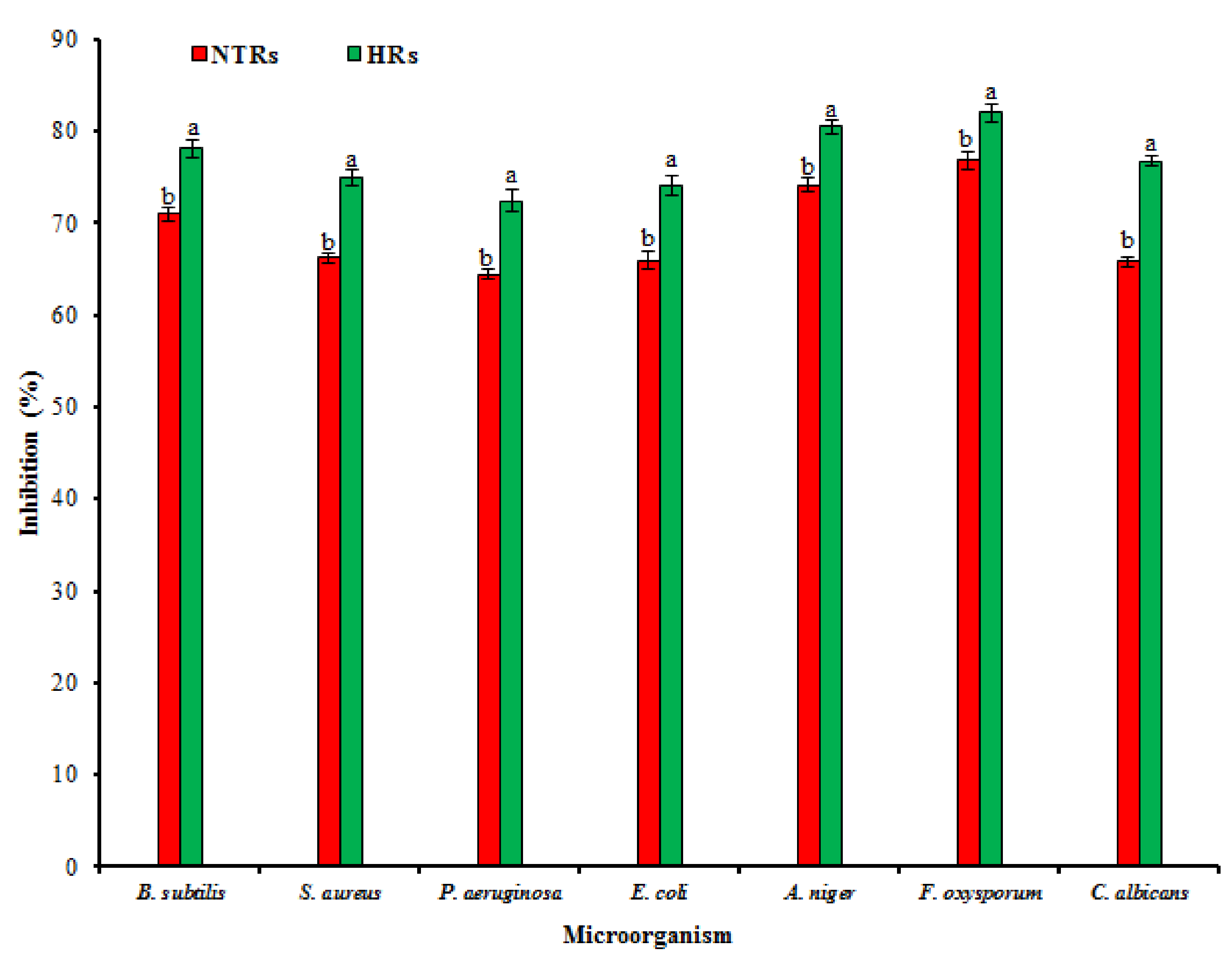
2.4. Pharmacological Activities
3. Materials and Methods
3.1. Induction and Proliferation of Hairy Root Cultures (HRCs)
3.2. Growth Kinetics of HRCs
3.3. Quantitative Analysis of Phenolic Compounds
3.3.1. Total Phenolic Compounds (TPC)
3.3.2. Total Flavonoid Contents (TFC)
3.3.3. Extraction of Phenolic Compounds
3.3.4. UHPLC Analysis of Phenolic Components
3.4. Determination of Pharmacological Activities
3.4.1. Preparation of Extracts
3.4.2. Antioxidant Activities
Radical Scavenging Capacity
Nitric Oxide Scavenging Capacity
Reductive Potential
Phosphomolybdenum Method
Chelating Effects on Ferrous Ions
3.4.3. Antidiabetic Activity
Inhibition of α-amylase Activity
Non-Enzymatic Glycosylation of Hemoglobin Activity
3.4.4. Anti-inflammatory Activity
Lipoxygenase Assay
Albumin Denaturation Inhibition Assay
3.4.5. Antimicrobial Activity
3.4.6. Anticancer Activity
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gobu, F.R.; Chen, J.J.; Zeng, J.; Wei, W.J.; Wang, W.F.; Lin, C.J.; Gao, K. Isolation, structure elucidation, and immunosuppressive activity of diterpenoids from Ligularia fischeri. J. Nat. Prod. 2017, 80, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Wang, C.F.; Liu, T.; Zhang, Y.B.; Liu, Y.Z.; Zhang, Z.Z. Chemical constituents from the roots of Ligularia fischeri Turcz. Nat. Prod. Res. Dev. 2011, 23, 1014–1016. [Google Scholar]
- Hong, S.; Joo, T.; Jhoo, J.W. Antioxidant and anti-inflammatory activities of 3,5-dicaffeoylquinic acid isolated from Ligularia fischeri leaves. Food Sci. Biotechnol. 2015, 24, 257–263. [Google Scholar] [CrossRef]
- Rekha, K.; Sivasubramanian, C.; Thiruvengadam, M. Evaluation of polyphenol composition and biological activities of two samples from summer and winter seasons of Ligularia fischeri var. Spiciformis Nakai. Acta Biol. Hung. 2015, 66, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: Systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Ren-Wang, J.; Kit-Man, L.; Po-Ming, H.; Thomas, C.W.; Mak, K.S.; Kwok-Pui, F. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar]
- Thiruvengadam, M.; Praveen, N.; Kim, E.H.; Kim, S.H.; Chung, I.M. Production of anthraquinones, phenolic compounds and biological activities from hairy root cultures of Polygonum multiflorum Thunb. Protoplasma 2014, 251, 555–566. [Google Scholar] [CrossRef]
- Modarres, M.; Esmaeilzadeh Bahabadi, S.; Taghavizadeh Yazdi, M.E. Enhanced production of phenolic acids in cell suspension culture of Salvia leriifolia Benth. Using growth regulators and sucrose. Cytotechnology 2018, 70, 741–750. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Teles, R.B.; Diniz, T.C.; Costa Pinto, T.C.; de Oliveira Júnior, R.G.; Gama E Silva, M.; de Lavor, É.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as therapeutic agents in Alzheimer’s and Parkinson’s diseases: A systematic review of preclinical evidences. Oxid. Med. Cell Longev. 2018, 2018, 7043213. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thwe, A.; Valan Arasu, M.; Li, X.; Park, C.H.; Kim, S.J.; Al-Dhabi, N.A.; Park, S.U. Effect of different Agrobacterium rhizogenes strains on hairy root induction and phenylpropanoid biosynthesis in tartary buckwheat (Fagopyrum tataricum Gaertn). Front. Microbiol. 2016, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Nagella, P.; Thiruvengadam, M.; Jung, S.J.; Murthy, H.N.; Chung, I.M. Establishment of Gymnema sylvestre hairy root cultures for the production of gymnemic acid. Acta Physiol. Plant 2013, 35, 3067–3073. [Google Scholar] [CrossRef]
- Dilshad, E.; Cusido, R.M.; Ramirez Estrada, K.; Bonfil, L.M.; Mirza, B. Genetic transformation of Artemisia carvifolia Buch with rol genes enhances artemisinin accumulation. PLoS ONE 2015, 10, e0140266. [Google Scholar] [CrossRef]
- Ismail, H.; Dilshad, E.; Tahir Waheed, M.; Sajid, M.; Kayani, K.; Mirza, B. Transformation of Lactuca sativa L. with rolC gene results in increased antioxidant potential and enhanced analgesic, anti-inflammatory and antidepressant activities in vivo. 3 Biotech 2016, 6, 215. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rekha, K.; Chung, I.M. Induction of hairy roots by Agrobacterium rhizogenes-mediated transformation of spine gourd (Momordica dioica Roxb. ex. willd) for the assessment of phenolic compounds and biological activities. Sci. Hortic. 2016, 198, 132–141. [Google Scholar] [CrossRef]
- Panda, B.M.; Mehta, U.J.; Hazra, S. Optimizing culture conditions for establishment of hairy root culture of Semecarpus anacardium L. 3 Biotech 2017, 7, 21. [Google Scholar] [CrossRef][Green Version]
- Ho, T.T.; Lee, J.D.; Jeong, C.S.; Paek, K.Y.; Park, S.Y. Improvement of biosynthesis and accumulation of bioactive compounds by elicitation in adventitious root cultures of Polygonum multiflorum. Appl. Microbiol. Biotechnol. 2018, 102, 199–209. [Google Scholar] [CrossRef]
- Tavassoli, P.; Afshar, A.S. Influence of different Agrobacterium rhizogenes strains on hairy root induction and analysis of phenolic and flavonoid compounds in marshmallow (Althaea officinalis L.). 3 Biotech 2018, 8, 351. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Trivedi, M.; Guang, Z.C.; Guo, G.Q.; Zheng, G.C. Genetic transformation of Gentiana macrophylla with Agrobacterium rhizogenes: growth and production of secoiridoid glucoside gentiopicroside in transformed hairy root cultures. Plant Cell Rep. 2007, 26, 199–210. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Vermeichik, G.N.; Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Fedoreyev, S.A.; Zhuraulev, Y.N. Individual and combined effect of the rol A, B and C genes on anthraquinone production in Rubia cordifolia transformed calli. Biotechnol. Bioeng. 2008, 100, 118–125. [Google Scholar] [CrossRef]
- Bonhomme, V.; Laurain-Mattar, D.; Fliniaux, M.A. Effects of the rolC gene on hairy root: Induction development and tropane alkaloid production by Atropa belladonna. J. Nat. Prod. 2000, 63, 1249–1252. [Google Scholar] [CrossRef]
- Vereshchagina, Y.V.; Bulgakov, V.P.; Grigorchuk, V.P.; Rybin, V.G.; Veremeichik, G.N.; Tchernoded, G.K.; Gorpenchenko, T.Y.; Koren, O.G.; Phan, N.H.; Minh, N.T.; et al. The rolC gene increases caffeoylquinic acid production in transformed artichoke cells. Appl. Microbiol. Biotechnol. 2014, 98, 7773–7780. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Chung, I.M.; Thiruvengadam, M. Evaluation of phenolic compounds, antioxidant and antimicrobial activities from transgenic hairy root cultures of gherkin (Cucumis anguria L.). S. Afr. J. Bot. 2015, 100, 80–86. [Google Scholar] [CrossRef]
- Kundu, S.; Salma, U.; Ali, M.N.; Hazra, A.K.; Mandal, N. Development of transgenic hairy roots and augmentation of secondary metabolites by precursor feeding in Sphagneticola calendulacea (L.) Pruski. Ind. Crops Prod. 2018, 121, 206–215. [Google Scholar] [CrossRef]
- Tusevski, O.; Simic, S.G. Phenolic acids and flavonoids in Hypericum perforatum L. hairy roots. Int. J. Pharm. Bio. Sci. 2013, 4, 737–748. [Google Scholar]
- Singh, H.; Dixit, S.; Verma, P.C.; Singh, P.K. Evaluation of total phenolic compounds and insecticidal and antioxidant activities of tomato hairy root extract. J. Agric. Food Chem. 2014, 62, 2588–2594. [Google Scholar] [CrossRef]
- Gabr, A.M.M.; Mabrok, H.B.; Abdel-Rahim, E.A.; El-Bahr, M.K.; Smetanska, I. Determination of lignans, phenolic acids and antioxidant capacity in transformed hairy root culture of Linum usitatissimum. Nat. Prod. Res. 2017, 32, 1867–1871. [Google Scholar] [CrossRef]
- Skała, E.; Kicel, A.; Olszewska, M.A.; Kiss, A.K.; Wysokińska, H. Establishment of hairy root cultures of Rhaponticum carthamoides (Willd.) Iljin for the production of biomass and caffeic acid derivatives. Biomed. Res. Int. 2015, 2015, 181098. [Google Scholar] [CrossRef]
- Fu, X.; Yin, Z.P.; Chen, J.G.; Shangguan, X.C.; Wang, X.; Zhang, Q.F.; Peng, D.Y. Production of chlorogenic acid and its derivatives in hairy root cultures of Stevia rebaudiana. J. Agric. Food Chem. 2015, 63, 262–268. [Google Scholar] [CrossRef]
- Zheleznichenko, T.; Banaev, E.; Asbaganov, S.; Voronkova, M.; Kukushkina, T.; Filippova, E.; Mazurkova, N.; Shishkina, L.; Novikova, T. Nitraria schoberi L. hairy root culture as a source of compounds with antiviral activity against influenza virus subtypes А (H5N1) and А (H3N2). 3 Biotech 2018, 8, 260. [Google Scholar] [CrossRef]
- Park, N.I.; Tuan, P.A.; Li, X.; Kim, Y.K.; Yang, T.J.; Park, S.U. An efficient protocol for genetic transformation of Platycodon grandiflorum with Agrobacterium rhizogenes. Mol. Biol. Rep. 2011, 38, 2307–2313. [Google Scholar] [CrossRef]
- Kim, Y.K.; Li, X.; Xu, H.; Park, N.I.; Uddin, M.R.; Pyon, J.Y.; Park, S.U. Production of phenolic compounds in hairy root culture of tartary buckwheat (Fagopyrum tataricum Gaertn). J. Crop Sci. Biotechnol. 2009, 12, 53–58. [Google Scholar] [CrossRef]
- Sharafi, A.; Sohi, H.H.; Mousavi, A.; Azadi, P.; Dehsara, B.; Khalifani, B.H. Enhanced morphinan alkaloid production in hairy root cultures of Papaver bracteatum by over-expression of salutaridinol 7-O-acetyltransferase gene via Agrobacterium rhizogenes mediated transformation. World J. Microbiol. Biotechnol. 2013, 29, 2125–2131. [Google Scholar] [CrossRef]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of glucosinolates, phenolic compounds and associated gene expression profiles of hairy root cultures in turnip (Brassica rapa ssp. rapa). 3 Biotech 2016, 6, 175. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Anbumegala, M.; Surendran, R.; Arun, M.; Shanmugam, G. Elite hairy roots of Raphanus sativus (L.) as a source of antioxidants and flavonoids. 3 Biotech 2018, 8, 128. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elkelish, A.; Elansary, H.O.; Ali, H.M.; Elshikh, M.; Witczak, J.; Ahmad, M. Genetic transformation and hairy root induction enhance the antioxidant potential of Lactuca serriola L. Oxid. Med. Cell Longev. 2017, 2017, 5604746. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyn, I.; Grzegorczyk-Karolak, I.; Frydrych, B.; Królicka, A.; Wysokińska, H. Hairy roots of Dracocephalum moldavica: Rosmarinic acid content and antioxidant potential. Acta Physiol. Plant. 2013, 35, 2095–2103. [Google Scholar] [CrossRef]
- Fathi, R.; Mohebodini, M.; Chamani, E. High-efficiency Agrobacterium rhizogenes-mediated genetic transformation in Cichorium intybus L. via removing macronutrients. Ind. Crops Prod. 2019, 128, 572–580. [Google Scholar] [CrossRef]
- Nopo-Olazabal, C.; Hubstenberger, J.; Nopo-Olazabal, L.; Medina-Bolivar, F. Antioxidant activity of selected stilbenoids and their bioproduction in hairy root cultures of muscadine grape (Vitis rotundifolia Michx.). J. Agric. Food Chem. 2013, 61, 11744–11758. [Google Scholar] [CrossRef]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.K.; Ravishankar Rai, V.; Ramachandra, Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400. [Google Scholar] [CrossRef]
- Tripathi, Y.B.; Singh, V.P. Role of Tamra bhasma, an Ayurvedic preparation, in the management of lipid peroxidation in liver of albino rats. Indian J. Exp. Biol. 1996, 34, 66–70. [Google Scholar]
- Xia, X.; Weng, J. Targeting metabolic syndrome: Candidate natural agents. J. Diabetes 2010, 2, 243–249. [Google Scholar] [CrossRef]
- Kalita, D.; Holm, D.G.; LaBarbera, D.V.; Petrash, J.M.; Jayanty, S.S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS ONE 2018, 13, e0191025. [Google Scholar] [CrossRef]
- Shah, S.M.; Ashraf, M.; Ahmad, I.; Arshad, S.; Yar, M.; Latif, A. Anti-lipoxygenase activity of some indigenous medicinal plants. J. Med. Plants Res. 2013, 7, 219–222. [Google Scholar]
- Pilaisangsuree, V.; Somboon, T.; Tonglairoum, P.; Keawracha, P.; Wongsa, T.; Kongbangkerd, A.; Limmongkon, A. Enhancement of stilbene compounds and anti-inflammatory activity of methyl jasmonate and cyclodextrin elicited peanut hairy root culture. Plant Cell Tissue Organ Cult. 2018, 132, 165–179. [Google Scholar] [CrossRef]
- Sitarek, P.; Rijo, P.; Garcia, C.; Skała, E.; Kalemba, D.; Białas, A.J.; Szemraj, J.; Pytel, D.; Toma, M.; Wysokińska, H.; et al. Antibacterial, anti-inflammatory, antioxidant, and antiproliferative properties of essential oils from hairy and normal roots of Leonurus sibiricus L. and their chemical composition. Oxid. Med. Cell Longev. 2017, 2017, 7384061. [Google Scholar] [CrossRef]
- Moreno-Anzúrez, N.E.; Marquina, S.; Alvarez, L.; Zamilpa, A.; Castillo-España, P.; Perea-Arango, I.; Torres, P.N.; Herrera-Ruiz, M.; Díaz García, E.R.; García, J.T.; et al. Cytotoxic and anti-inflammatory campesterol derivative from genetically transformed hairy roots of Lopezia racemosa Cav. (Onagraceae). Molecules 2017, 22, 118. [Google Scholar] [CrossRef]
- Hanafy, M.S.; Matter, M.A.; Asker, M.S.; Rady, M.R. Production of indole alkaloids in hairy root cultures of Catharanthus roseus L. and their antimicrobial activity. S. Afr. J. Bot. 2016, 105, 9–18. [Google Scholar] [CrossRef]
- Tusevski, B.; Vinterhalter, D.; Krstic Milosevic, M.; SokovicA Ciric, D.; Vinterhalter, S.; Zdravkovic Korac, J.; Petreska Stanoeva, M.; Stefova, S.; Simic, G. Production of phenolic compounds, antioxidant and antimicrobial activities in hairy root and shoot cultures of Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2017, 128, 589–605. [Google Scholar] [CrossRef]
- Syklowska-Baranek, K.; Pietrosiuk, A.; Gawron, A.; Kawiak, A.; Łojkowska, E.; Jeziorek, M.; Chinou, I. Enhanced production of antitumour naphthoquinones in transgenic hairy root lines of Lithospermum canescens. Plant Cell Tissue Organ Cult. 2012, 108, 213–219. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirements of tobacco tissue culture. Plant Physiol. 1965, 21, 487–492. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Haploid plants from pollen grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Kim, S.H.; Chung, I.M. Influence of amphetamine, γ-aminobutyric acid, and fosmidomycin on metabolic, transcriptional variations and determination of their biological activities in turnip (Brassica rapa ssp. rapa). S. Afr. J. Bot. 2016, 103, 181–192. [Google Scholar] [CrossRef]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Elicitation of silver nanoparticles enhanced the secondary metabolites and pharmacological activities in cell suspension cultures of bitter gourd. 3 Biotech 2018, 10, 412. [Google Scholar] [CrossRef]
- Saleem, B.; Islam, M.; Saeed, H.; Imtiaz, F.; Asghar, M.; Saleem, Z.; Mehmood, A.; Naheed, S. Investigations of Acacia modesta Wall. Leaves for in vitro anti-diabetic, proliferative and cytotoxic effects. Braz. J. Pharm. Sci. 2018, 54, e17467. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| No. | Phenolic Compounds (μg/g DM) | Control Roots (NTRs) | Hairy Roots (HRs) |
|---|---|---|---|
| Flavonols | |||
| 1 | Myricetin | 1964.35 ± 2.0 a,z | 2379.50 ± 3.0 a,y |
| 2 | Quercetin | 409.20 ± 3.5 b,z | 511.25 ± 4.0 b,y |
| 3 | Kaempferol | 87.25 ± 2.2 g,z | 115.50 ± 2.0 j,y |
| 4 | Rutin | 97.00 ± 1.0 f,z | 135.25 ± 2.0 f,y |
| 5 | Naringenin | 21.95 ± 1.0 m,z | 25.00 ± 1.0 p,y |
| 6 | Biochanin A | 4.30 ± 1.0 p,z | 10.00 ± 1.0 s,y |
| 7 | Formononetin | 12.30 ± 1.2 o,z | 21.85 ± 1.0 q,y |
| Total | 2596.35 z | 3198.35 y | |
| Hydroxycinnamic acids | |||
| 8 | Caffeic acid | 139.35 ± 2.0 d,z | 175.50 ± 2.0 d,y |
| 9 | p-Coumaric acid | 80.15 ± 1.5 h,z | 105.10 ± 1.0 k,y |
| 10 | Ferulic acid | 81.35 ± 1.0 h,z | 121.50 ± 1.5 i,y |
| 11 | m-Coumaric acid | 5.45 ± 1.1 p,z | 10.10 ± 1.0 s,y |
| 12 | o-Coumaric acid | 17.55 ± 1.0 n,y | 16.00 ± 1.0 r,z |
| 13 | Chlorogenic acid | 72.95 ± 2.0 i,z | 130.50 ± 2.0 g,y |
| Total | 396.80 z | 558.70 y | |
| Hydroxybenzoic acids | |||
| 14 | Gallic acid | 88.35 ± 1.5 g,z | 105.50 ± 1.0 k,y |
| 15 | Protocatechuic | 66.85 ± 2.0 j,z | 95.70 ± 1.0 l,y |
| 16 | β-Resorcylic | 25.35 ± 1.0 l,z | 33.50 ± 1.0 o,y |
| 17 | Vanillic acid | 26.60 ± 1.2 l,z | 41.59 ± 1.0 n,y |
| 18 | Syringic acid | 51.45 ± 1.4 k,z | 75.50 ± 1.5 m,y |
| 19 | Gentisic acid | 71.50 ± 2.0 i,z | 125.00 ± 2.0 h,y |
| 20 | Salicylic acid | 222.80 ± 2.0 c,z | 300.15 ± 3.0 c,y |
| Total | 552.90 z | 776.94 y | |
| Other phenolic compounds | |||
| 21 | Vanillin | 13.15 ± 1.0 o,z | 20.50 ± 1.5 q,y |
| 22 | Homogentisic | 18.90± 2.0 n,z | 24.00 ± 2.0 p,y |
| 23 | Resveratrol | 22.05± 1.5 m,z | 41.50 ± 1.0 n,y |
| 24 | Veratric acid | 21.70 ± 1.0 m,z | 40.20 ± 1.0 n,y |
| 25 | Pyrogallol | 122.00 ± 1.0 e,z | 145.00 ± 1.5 e,y |
| Total | 197.80 z | 271.20 y |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azam Ansari, M.; Chung, I.-M.; Rajakumar, G.; A. Alzohairy, M.; Almatroudi, A.; Gopiesh Khanna, V.; Thiruvengadam, M. Evaluation of Polyphenolic Compounds and Pharmacological Activities in Hairy Root Cultures of Ligularia fischeri Turcz. f. spiciformis (Nakai). Molecules 2019, 24, 1586. https://doi.org/10.3390/molecules24081586
Azam Ansari M, Chung I-M, Rajakumar G, A. Alzohairy M, Almatroudi A, Gopiesh Khanna V, Thiruvengadam M. Evaluation of Polyphenolic Compounds and Pharmacological Activities in Hairy Root Cultures of Ligularia fischeri Turcz. f. spiciformis (Nakai). Molecules. 2019; 24(8):1586. https://doi.org/10.3390/molecules24081586
Chicago/Turabian StyleAzam Ansari, Mohammad, Ill-Min Chung, Govindasamy Rajakumar, Mohammad A. Alzohairy, Ahmad Almatroudi, Venkatesan Gopiesh Khanna, and Muthu Thiruvengadam. 2019. "Evaluation of Polyphenolic Compounds and Pharmacological Activities in Hairy Root Cultures of Ligularia fischeri Turcz. f. spiciformis (Nakai)" Molecules 24, no. 8: 1586. https://doi.org/10.3390/molecules24081586
APA StyleAzam Ansari, M., Chung, I.-M., Rajakumar, G., A. Alzohairy, M., Almatroudi, A., Gopiesh Khanna, V., & Thiruvengadam, M. (2019). Evaluation of Polyphenolic Compounds and Pharmacological Activities in Hairy Root Cultures of Ligularia fischeri Turcz. f. spiciformis (Nakai). Molecules, 24(8), 1586. https://doi.org/10.3390/molecules24081586









