The Effects of Mono-(2-Ethylhexyl) Phthalate (MEHP) on Human Estrogen Receptor (hER) and Androgen Receptor (hAR) by YES/YAS In Vitro Assay
Abstract
:1. Introduction
2. Results
2.1. Estrogenic and Androgenic Activity
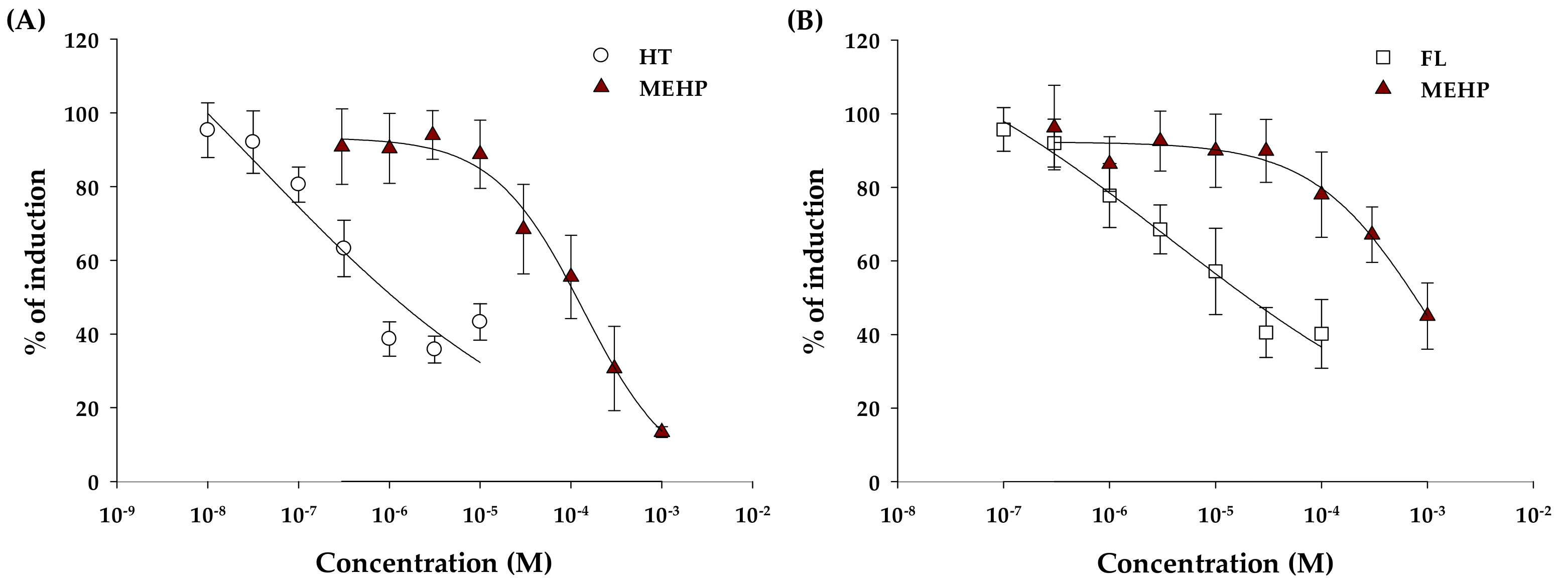
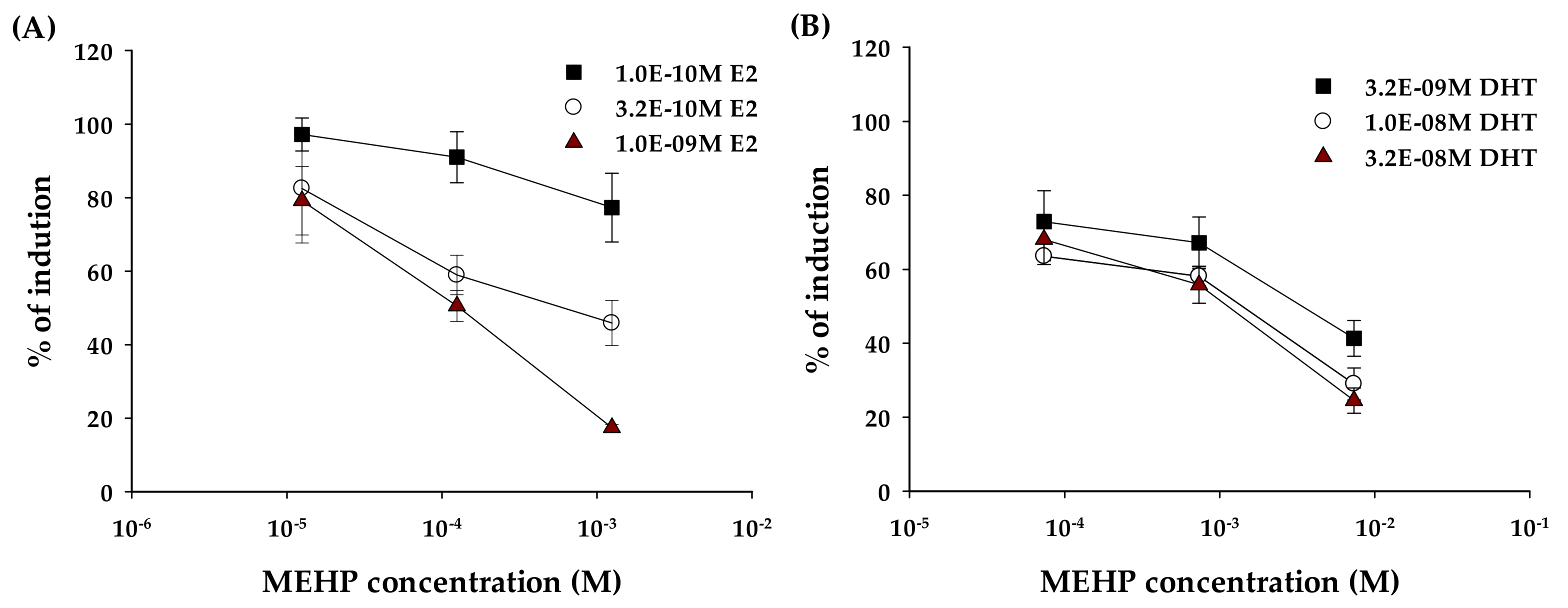
2.2. Anti-Estrogenic and Anti-Androgenic Activity
2.3. Uptake of MEHP in Yeast Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. YES and YAS Assay
4.3. Uptake of MEHP in Yeast Cells
4.3.1. Pretreatment
4.3.2. LC-MS/MS Analysis
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harris, C.A.; Henttu, P.; Parker, M.G.; Sumpter, J.P. The estrogenic activity of phthalate esters in vitro. Environ. Health Perspect. 1997, 105, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Diaz, S.; Mitchell, A.A.; Kelley, K.E.; Calafat, A.M.; Hauser, R. Medications as a potential source of exposure to phthalates in the U.S. population. Environ. Health Perspect. 2009, 117, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.E.; Hernandez-Diaz, S.; Chaplin, E.L.; Hauser, R.; Mitchell, A.A. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ. Health Perspect. 2012, 120, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Hanaoka, T.; Yoshimura, M.; Zhang, S.; Wang, P.; Tsukino, H.; Inoue, K.; Nakazawa, H.; Tsugane, S.; Takahashi, K. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): A cross-sectional study in China. Environ. Health Perspect. 2006, 114, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Czernych, R.; Chraniuk, M.; Zagozdzon, P.; Wolska, L. Characterization of estrogenic and androgenic activity of phthalates by the XenoScreen YES/YAS in vitro assay. Environ. Toxicol. Pharmacol. 2017, 53, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hygiene Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Koch, H.M.; Bolt, H.M.; Preuss, R.; Angerer, J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch. Toxicol. 2005, 79, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.S. Environmental anti-androgens and male reproductive health: Focus on phthalates and testicular dysgenesis syndrome. Reproduction 2004, 127, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.E., Jr.; Ostby, J.; Furr, J.; Price, M.; Veeramachaneni, D.N.; Parks, L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. Official J. Soc. Toxicol. 2000, 58, 350–365. [Google Scholar] [CrossRef]
- Parks, L.G.; Ostby, J.S.; Lambright, C.R.; Abbott, B.D.; Klinefelter, G.R.; Barlow, N.J.; Gray, L.E., Jr. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. Official J. Soc. Toxicol. 2000, 58, 339–349. [Google Scholar] [CrossRef]
- Rusyn, I.; Peters, J.M.; Cunningham, M.L. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit. Rev. Toxicol. 2006, 36, 459–479. [Google Scholar] [CrossRef]
- Singh, S.; Li, S.S. Phthalates: Toxicogenomics and inferred human diseases. Genomics 2011, 97, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Gruber, L.; Seckin, E.; Raab, U.; Zimmermann, S.; Kiranoglu, M.; Schlummer, M.; Schwegler, U.; Smolic, S.; Volkel, W. Phthalates and their metabolites in breast milk-Results from the Bavarian Monitoring of Breast Milk (BAMBI). Environ. Int. 2011, 37, 715–722. [Google Scholar] [CrossRef]
- Silva, M.J.; Reidy, J.A.; Samandar, E.; Herbert, A.R.; Needham, L.L.; Calafat, A.M. Detection of phthalate metabolites in human saliva. Arch. Toxicol. 2005, 79, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.P.; Calafat, A.M.; Silva, M.J.; Mendola, P.; Fenton, S.E. Concentrations of phthalate metabolites in milk, urine, saliva, and Serum of lactating North Carolina women. Environ. Health Perspect. 2009, 117, 86–92. [Google Scholar] [CrossRef]
- Koch, H.M.; Bolt, H.M.; Angerer, J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch. Toxicol. 2004, 78, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Jorgensen, N.; Andersson, A.M. Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. J. Analy. Toxicol. 2010, 34, 400–410. [Google Scholar] [CrossRef]
- Kardas, F.; Bayram, A.K.; Demirci, E.; Akin, L.; Ozmen, S.; Kendirci, M.; Canpolat, M.; Oztop, D.B.; Narin, F.; Gumus, H.; et al. Increased serum phthalates (MEHP, DEHP) and bisphenol A concentrations in children with autism spectrum disorder: The role of endocrine disruptors in Autism Etiopathogenesis. J. Child Neurol. 2016, 31, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Iida, M.; Kobayashi, S.; Jin, K.; Matsuda, T.; Kojima, H. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors alpha and beta, and androgen receptor. Toxicology 2005, 210, 223–233. [Google Scholar] [CrossRef]
- Ohtani, Y.; Shimada, Y.; Shiraishi, F.; Kozawa, K. Estrogen-antagonist activities of phthalic acid mono-n-butyl ester and phthalic acid mono-2-ethylhexyl ester. Environ. Sci. Int. J. Environ. Physiol. Toxicol. 2005, 12, 207–212. [Google Scholar]
- Fic, A.; Zegura, B.; Gramec, D.; Masic, L.P. Estrogenic and androgenic activities of TBBA and TBMEPH, metabolites of novel brominated flame retardants, and selected bisphenols, using the XenoScreen XL YES/YAS assay. Chemosphere 2014, 112, 362–369. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Fang, T.; Papadopoulos, V. Disruption of ergosterol and tryptophan biosynthesis, as well as cell wall integrity pathway and the intracellular pH homeostasis, lead to mono-(2-ethylhexyl)-phthalate toxicity in budding yeast. Chemosphere 2018, 206, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.S.; Magpantay, F.M.; Chickarmane, V.; Haskell, C.; Tania, N.; Taylor, J.; Xia, M.; Huang, R.; Rotroff, D.M.; Filer, D.L.; et al. Integrated model of chemical perturbations of a biological pathway using 18 in vitro high-throughput screening assays for the estrogen receptor. Toxicol. Sci. Official J. Soc. Toxicol. 2015, 148, 137–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Ding, X.; Xie, X. Potential estrogenic activities of DBP, DEHP and their metabolites in immature rats using the uterotrophic assay. J. Environ. Occup. Med. 2005, 22, 11–13. [Google Scholar]
- La Sala, G.; Farini, D.; de Felici, M. Estrogenic in vitro assay on mouse embryonic Leydig cells. Int. J. Develop. Biol. 2010, 54, 717–722. [Google Scholar] [CrossRef]
- Kleinstreuer, N.C.; Ceger, P.; Watt, E.D.; Martin, M.; Houck, K.; Browne, P.; Thomas, R.S.; Casey, W.M.; Dix, D.J.; Allen, D.; et al. Development and validation of a computational model for androgen receptor activity. Chem. Res. Toxicol. 2017, 30, 946–964. [Google Scholar] [CrossRef] [PubMed]
- Stroheker, T.; Cabaton, N.; Nourdin, G.; Regnier, J.F.; Lhuguenot, J.C.; Chagnon, M.C. Evaluation of anti-androgenic activity of di-(2-ethylhexyl)phthalate. Toxicology 2005, 208, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Suzuki, T.; Yokoyama, Y.; Kano, K.; Kano, I. Estimation of estrogenic and anti-estrogenic activities of some phthalate diesters and monoesters by MCF-7 cell proliferation assay in vitro. Biol. Pharm. Bull. 2003, 26, 1219–1224. [Google Scholar] [CrossRef]
- Stroheker, T.; Regnier, J.-F.; Lassurguere, J.; Chagnon, M.-C. Effect of in utero exposure to di-(2-ethylhexyl)phthalate: Distribution in the rat fetus and testosterone production by rat fetal testis in culture. Food Chem. Toxicol. 2006, 44, 2064–2069. [Google Scholar] [CrossRef]
- Lee, B.M.; Koo, H.J. Hershberger assay for antiandrogenic effects of phthalates. J. Toxicol. Environ. Health A 2007, 70, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Prell, E. Difluorotetrahydropyridothiazinone: A selective beta-galactosidase inhibitor. Arch. Pharm. 2010, 343, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Guven, R.G.; Kaplan, A.; Guven, K.; Matpan, F.; Dogru, M. Effects of various inhibitors on β-galactosidase purified from the thermoacidophilic Alicyclobacillus acidocaldarius subsp. Rittmannii isolated from Antarctica. Biotechnol. Bioprocess Eng. 2011, 16, 114–119. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Agonists | EC50 (nM) | 95% Confidence Interval (nM) | R2 |
|---|---|---|---|
| E2 | 1.26 | 1.16–1.39 | 0.9842 |
| MEHP (ER) a | - | - | - |
| DHT | 16.3 | 13.1–20.5 | 0.9496 |
| MEHP (AR) a | - | - | - |
| Antagonist | IC50 (μM) | 95% Confidence Interval (μM) | R2 |
|---|---|---|---|
| HT | 1.11 | 0.73–1.73 | 0.8546 |
| MEHP (ER) | 125 | 90.0–151 | 0.9068 |
| FL | 20.3 | 13.6–32.2 | 0.8654 |
| MEHP (AR) | 736 | 604–1740 | 0.7665 |
| RT | Precursor Ion (m/z) | Product Ion (m/z) | |
|---|---|---|---|
| MEHP | 5.765 | 277 | 134.2, 127.2 |
| 13C2-MEHP | 5.762 | 281 | 137.0, 79.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Park, C.G.; Kim, S.H.; Kim, Y.J. The Effects of Mono-(2-Ethylhexyl) Phthalate (MEHP) on Human Estrogen Receptor (hER) and Androgen Receptor (hAR) by YES/YAS In Vitro Assay. Molecules 2019, 24, 1558. https://doi.org/10.3390/molecules24081558
Kim D-H, Park CG, Kim SH, Kim YJ. The Effects of Mono-(2-Ethylhexyl) Phthalate (MEHP) on Human Estrogen Receptor (hER) and Androgen Receptor (hAR) by YES/YAS In Vitro Assay. Molecules. 2019; 24(8):1558. https://doi.org/10.3390/molecules24081558
Chicago/Turabian StyleKim, Da-Hye, Chang Gyun Park, Sang Hun Kim, and Young Jun Kim. 2019. "The Effects of Mono-(2-Ethylhexyl) Phthalate (MEHP) on Human Estrogen Receptor (hER) and Androgen Receptor (hAR) by YES/YAS In Vitro Assay" Molecules 24, no. 8: 1558. https://doi.org/10.3390/molecules24081558
APA StyleKim, D.-H., Park, C. G., Kim, S. H., & Kim, Y. J. (2019). The Effects of Mono-(2-Ethylhexyl) Phthalate (MEHP) on Human Estrogen Receptor (hER) and Androgen Receptor (hAR) by YES/YAS In Vitro Assay. Molecules, 24(8), 1558. https://doi.org/10.3390/molecules24081558





