1. Introduction
There has been a significant increase in the use and consumption of complementary medicines from traditional herbal sources in recent years. Many of these medicines often have multiple herbal components, and each of these components have associated pharmacological claims. The active ingredients within each medicinal herb can differ due to different sources of production. It is important for these types of manufactured formulas to have a standardised procedure for quality control (QC) in order to ensure the efficacy of the product [
1,
2,
3,
4,
5,
6]. In recent years, the method validation of phyto-markers has been the industry standard for the quality control of medicinal herbal products. This often involves the quantitation of one or two analytes to assess the chemical variability of a commercial herbal product. This type of analysis is woefully insufficient when it is used for the QC of chemical formulations with multiple herbal products [
7,
8,
9,
10,
11]. A more comprehensive and rigorous method is required. This study demonstrates how the QC of an eight-herb Traditional Chinese medicine (TCM) formulation known as Qi Ju Di Huang Wan (QJDHW) [
12,
13,
14,
15] used in the treatment of hypertension can be achieved using an analytical method validation, a principal component analysis (PCA), and a hierarchical cluster analysis (HCA).
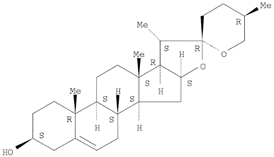
The target analyte for each herb in the QJDHW formulation was selected using the Herbal Chemical Marker Ranking System (Herb MaRS) based on bioactivity against hypertension [
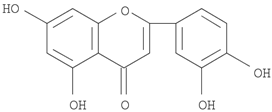
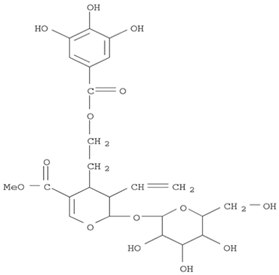
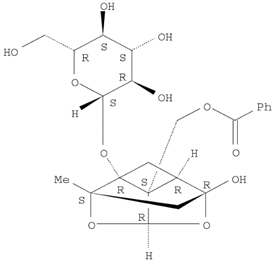
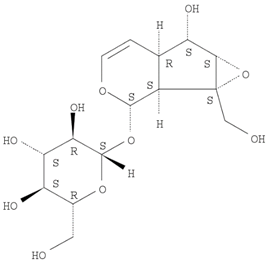
16]. The Herb MaRS ranking system was developed at the National Institute of Complementary Medicine (NICM) and assesses the bioactivity, physiological activity, and the bioavailability of each analyte present in any herb or herbal formulation. The chemical structures of the target analytes are shown in
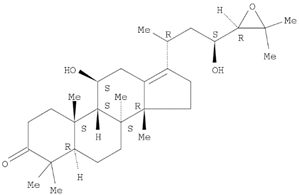
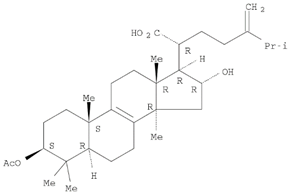
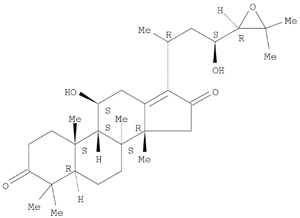
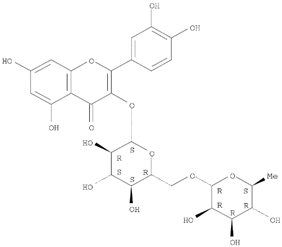
Table 1 and the herbal composition in the QJDHW formula is shown in
Table 2.
QJDHW is typically consumed as an aqueous alcohol extract and has been shown to have a significant effect in decreasing the concentration of angiotensin in plasma and myocardium, reducing the endothelin (ET) content and improving the kidney blood stream in the rat tail murine model for essential hypertension [
17,
18]. In 2010, a systematic review of randomized controlled trials on the effectiveness and safety of QJDHW for the treatment of essential hypertension (10 randomized trials involving 1024 patients) suggested that QJDHW, when combined with antihypertensive drugs, is more effective in lowering blood pressure in the treatment of essential hypertension than antihypertensive drugs alone [
19]. There are currently no clinical trials that have reported severe adverse events related to QJDHW use.
Commercially available QJDHW was acquired from eleven different sources. An analytical method validation was performed using UPLC (Ultra-Performance liquid Chromatography, Waters Corporation, Milford, MA, USA) ESI-MS/MS (Electrospray ionisation mass spectrometry, Waters Corporation). The quantitative variability of the formulation was then assessed using the eleven different sources. The data obtained from the chromatographic spectra were then statistically analysed using PCA and HCA to assess the qualitative chemical variability of QJHDW. In this manner, the chemical variability of commercially available QJHDW can be understood, and further improvements in QC can be implemented.
2. Methods
2.1. Instrumentation
A Waters ACQUITY UPLC system (Waters Corporation) coupled to a Waters Xevo TQ MS triple quadrupole mass spectrometer fitted with a Z-SprayTM source was used in the analytical method development. Electrospray ionisation ((+)/(−) ESI-MS/MS) scanning mode and argon collision gas was used to identify each analyte in the herb against a commercially purchased analytical standard. Chromatographic separation was achieved using an Acquity BEH C18 (150 mm × 2.10 mm, 1.7 μm packing) column. The injection volume was set at 3 µL, and the column heater temperature was set at 28 °C at the start of each run. The results of the analyses were processed using Waters MassLynxTM version 4.1 (Waters Corporation).
An Adam AFA-210LC analytical balance (Adam Equipment Co., Perth, WA, Australia) and a Sartorius SE-2 micro analytical balance (Sartorius Australia, Melbourne, VIC, Australia) were used to weigh the samples and standards. The Powersonic 420 ultrasonic bath (Thermoline Scientific, Sydney, NSW, Australia) and Beckmann GP centrifuge from Beckmann Coulter (Beckmann Coulter, Sydney, NSW, Australia) were used in the extraction of the analytes from the herbal formulation. The extraction solutions were then passed through a Millipore 0.22 μm centrifuge filter with a microporous membrane purchased from EMD Millipore (Millipore, Billerica, MA, USA).
2.2. Reagents, Chemicals, and Samples
LC grade acetonitrile (Mallinckrodt Chemicals Ltd., Chesterfield, UK) and LR grade ethanol (95%), methanol, and formic acid (90%) (Biolab, Adelaide, SA, Australia) were purchased. The gases used in the method validation were ultrahigh purity grade air, argon, helium, hydrogen, and nitrogen (Coregas, Sydney, NSW, Australia). Purified water (>18 MΩ cm) was obtained from an Elga Purelab Prima and Purelab Ultra high purity water system (Biolab, Adelaide, SA, Australia).
The analytical standards alisol C (98.6%), alisol B (96.0%), catalpol (98.0%), rutin (98.0%), luteolin (98.0%), and diosgenin (97.0%) were primary grade and purchased from Sigma-Aldrich (Sigma-Aldrich, Australia). The analytical standards paeoniflorin (98.7%), pachymic acid (97.9%), and cornuside (98%) were secondary grade and purchased from Phytomarker (Phytomarker Ltd., Tianjin, China). The primary grade standards have purity and spectroscopic characterisations while the secondary grade standards have purity by LC-PDA (Photo diode-array detection) only. The calibration curves were prepared with a standard purity adjustment.
Eleven samples of the Qi Ju Di Huang Wan herbal formula was obtained from suppliers in the Australian marketplace. There were five suppliers who provided the eleven samples. Most of these batch samples were donated, and the commercial donors requested to remain anonymous. Sample A-III was used for the method validation.
2.3. Sample Extraction and LC Mobile Phase Preparation
The dried aqueous extract in pill form of the herbal formulation was decapsulated and passed through a ≤200 μm sieve. Approximately 0.5 g of each sample was weighed into a 10 mL conical flask, 10 mL 70%
v/
v aqueous methanol was added, and the mixture was sonicated for 1 h. The sample was then centrifuged at 4000 rpm (3466×
g) for 10 min to pellet out the insoluble excipient. The supernatant was then passed through a 0.2 μm polyvinylidene difluoride (PVDF) membrane filter into 2 mL autosampler vials with glass insert for LC-MS analysis. The resultant liquid was stored at 4 °C and discarded after 48 h because the peak area of the analytes decreased by ≥2% after this time. Mobile phase A (0.1% aqueous formic acid) was prepared by the addition of 900 mL of water to a 1000 mL volumetric flask followed by 1.1 mL formic acid before making up to volume with water. Mobile phase B was acetonitrile. The mobile phase program is shown in
Table 3. The mobile phases were degassed by sonication for 5 min and filtered through a 0.45 μm PVDF membrane filter before use.
2.4. Preparation of Stock Calibration Solution Using Analytical Standards
Two mixed stock standard solutions were prepared. The first mixed standard solution consisted of alisol B, pachymic acid, alisol C, rutin, catalpol, luteolin, and diosgenin. The second solution contained cornuside and paeoniflorin. This was done since the concentrations of cornuside and paeoniflorin were higher than the other analytes, and they showed a better solubility in ethanol than in methanol.
Individual solutions of 1000 μg/mL alisol B, pachymic acid, alisol C, rutin, catalpol, luteolin, and diosgenin were prepared by weighing 5.0 mg of each standard into a 5 mL volumetric flask and adding 3 mL of methanol. The solutions were then sonicated for 5 min or until the solid had dissolved. The solutions were cooled to room temperature and made up to volume with methanol.
The first mixed standard stock solution containing 40 μg/mL alisol B, 40 μg/mL pachymic acid, 25 μg/mL alisol C, 150 μg/mL rutin, 20 μg/mL catalpol, 70 μg/mL luteolin, and 20 μg/mL diosgenin was prepared by adding 1.50, 0.70, 0.40, 0.40, 0.25, 0.20, and 0.20 mL of the respective individual standard solutions into a 5 mL volumetric flask and made up to volume with methanol. The solution was then diluted 20-fold to give an intermediate mixed standard solution. This was done by diluting 50 μL of the original mixed stock solution into 1000 μL with methanol.
The second mixed standard stock solution containing 25,000 μg/mL cornuside, and 25,000 μg/mL paeoniflorin was prepared by weighing 125 mg of the respective standards into a 5 mL volumetric flask and adding 3 mL ethanol before sonication for 5 min. The solution was then cooled to room temperature and made up to volume with ethanol. The solution was diluted 40-fold to give an intermediate mixed standard calibration solution. This was prepared by diluting 25 μL of the mixed standard stock solution into 1000 μL 95% aqueous methanol.
These intermediate mixed standards were diluted as shown in
Table 4 to give the mixed working standard solutions used to construct the calibration curve.
2.5. Recovery Studies
To determine the analyte extraction efficiency of the method, an accurate weight of approx. 0.5 g of each herbal sample was transferred into 10-mL volumetric flasks. Then, the two spiking stock solutions for all the analytes were added to each sample for the 50%, 100%, and 200% recovery levels. The concentrations of the mixed spiking solutions were such that, for the 100% spike level, the resultant peak area and height would double that of the unspiked sample. Seven replicates were carried out for each spike level to give a total of twenty-one samples for all three spike levels. The spiking solvent was evaporated overnight in a fume hood.
2.6. MS Conditions
The MS source conditions were set as follows: Nitrogen was the desolvation gas (800 L/h heated to 350 °C) and argon as the collision induced dissociation gas (0.15 mL/min) gave rise to a collision cell pressure of 4.3 × 10
−6 Bar. The scan time was 0.05 s. The extractor cone voltage was 2 V, and the cone gas flow was 20 L/h. The source temperature was 150 °C, the capillary voltage was –3.2 kV in the (+) ESI mode and 2.00 kV in the (−) ESI mode. Two MRM (Multiple Reaction Monitoring) transitions (or product
m/
z’s) were chosen for each target analyte, with the most abundant transition used as the quantifier and the other transition used as the qualifier. The ESI polarity, precursor, and product ions were monitored, and the argon collision voltages required to achieve the transitions and the dwell times used are summarised in
Table 5. The quadrupoles Q1 and Q3 operated with a peak width of 3 AMU and a scan time of 2 s.
2.7. Chemometric Analysis
The qualitative variability of the targeted analytes in the herbal formulation was studied using principal component analysis (PCA) and hierarchical cluster analysis (HCA) [
20,
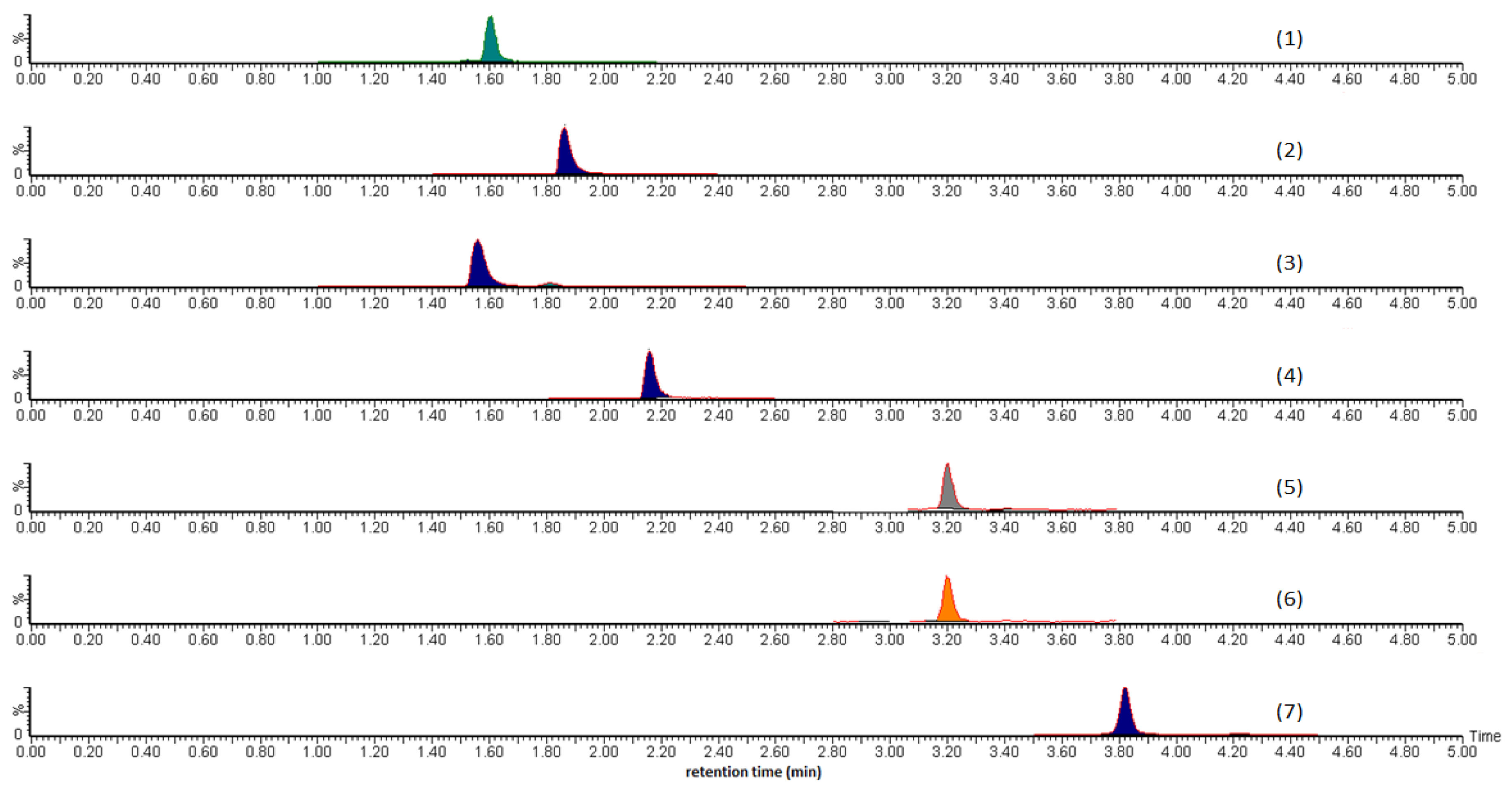
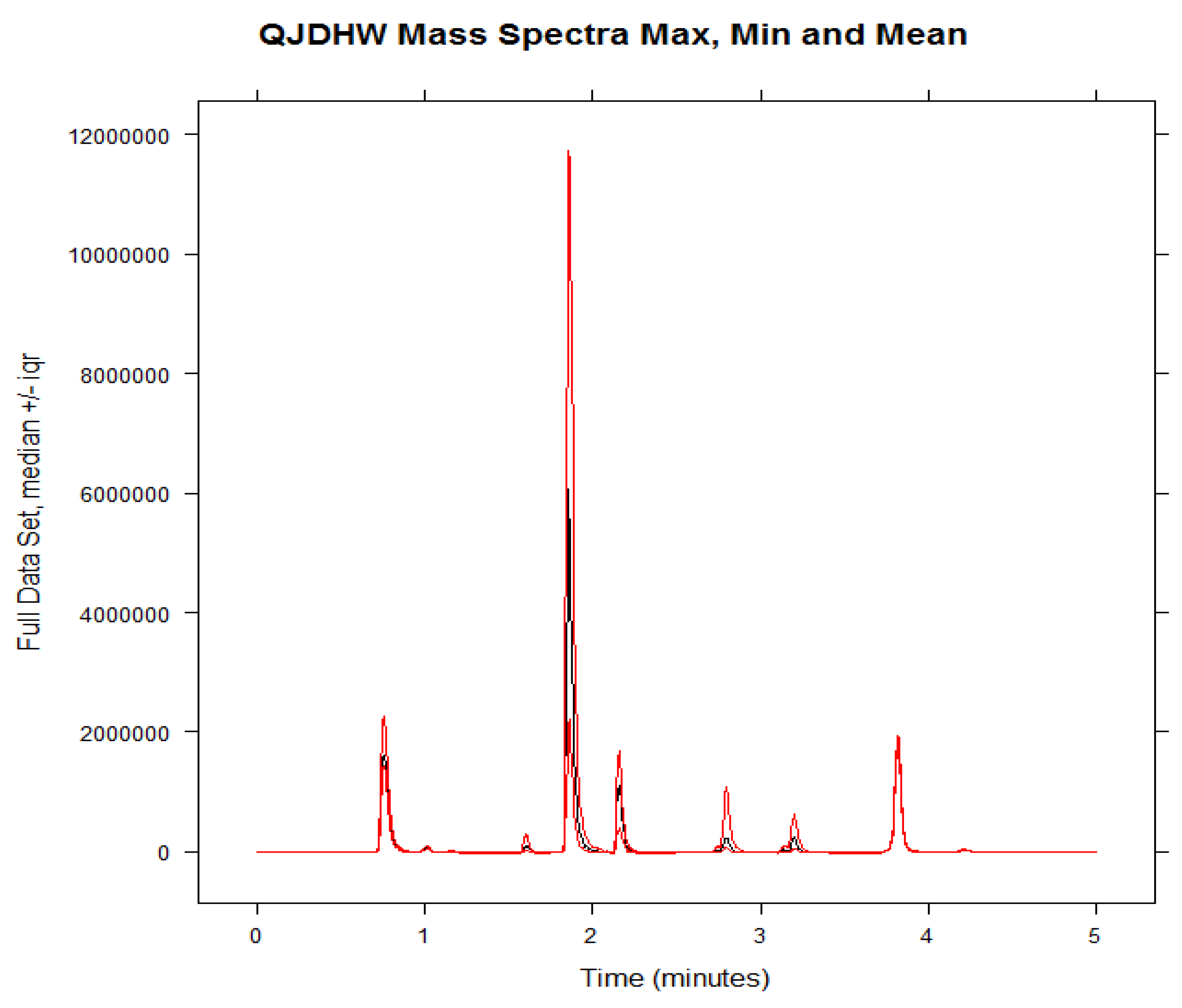
21]. The software used was R (v.2.14.2) for data processing and statistical analysis. The raw chromatographic data of each analyte present in each herbal sample was converted into eleven separate comma-separated value (CSV) files. The paeoniflorin peak at approx. 1.6 min was shifted to approx. 0.8 min, and the alisol C peak at 3.2 min was shifted to 2.8 min to prevent peak overlap. The LC-MS chromatographic profiles obtained were then meshed into a single R.data file and placed in a neat stack plot.
The R.data file was then loaded and accessed using the R (v.2.14.2) “chemometrics” package written by Varmuza and Filzmoser developed for PCA analysis [
22]. The graphics wrapper for the data was provided by the ChemoSpec package written by Hanson [
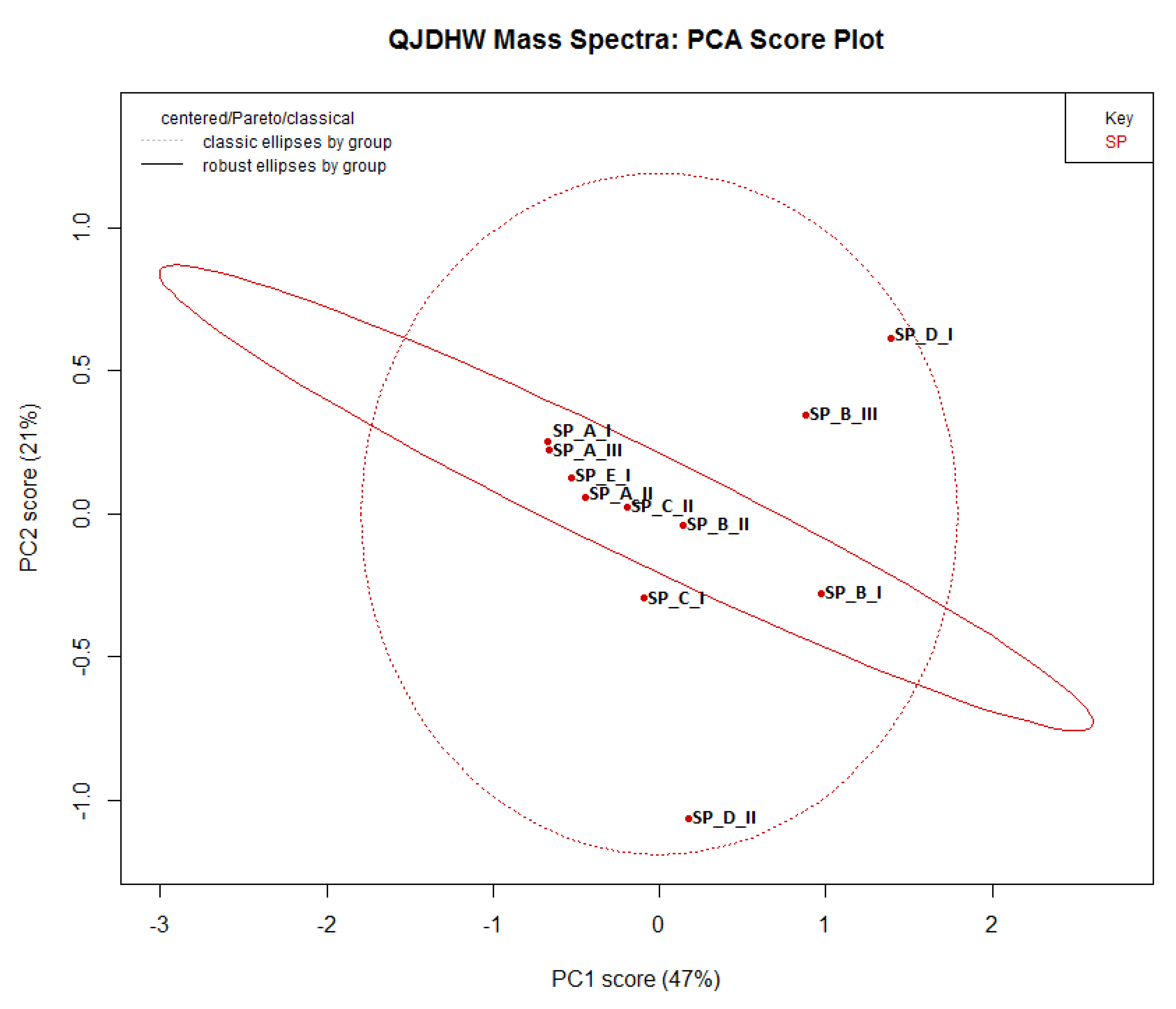
23]. The baseline of the dataset was then corrected to reduce the influence of noise present in the samples. The data was then normalized to negate the small differences due to changes in the concentration during the preparation of seven replicates. Finally, the data was binned to compensate for the effect of narrow peaks having shifting retention times. The region of interest containing the relevant peaks between 0.5 min and 4.0 min was selected for the analysis. The two options available for PCA were either classical or robust. While the classical method characterised a good deal of variance in the data set due to “outliers”, the robust method downplayed this aspect and used median absolute deviation to study variance. Classical PCA was chosen since the outliers needed to contribute to the variance to better understand the underlying variability and the quality of an herbal product. The “Pareto” scaling option was chosen to explain the variance because it is a compromise between “noscaling” which weighs peaks according to size and the “autoscaling” option which weights all peaks equally.
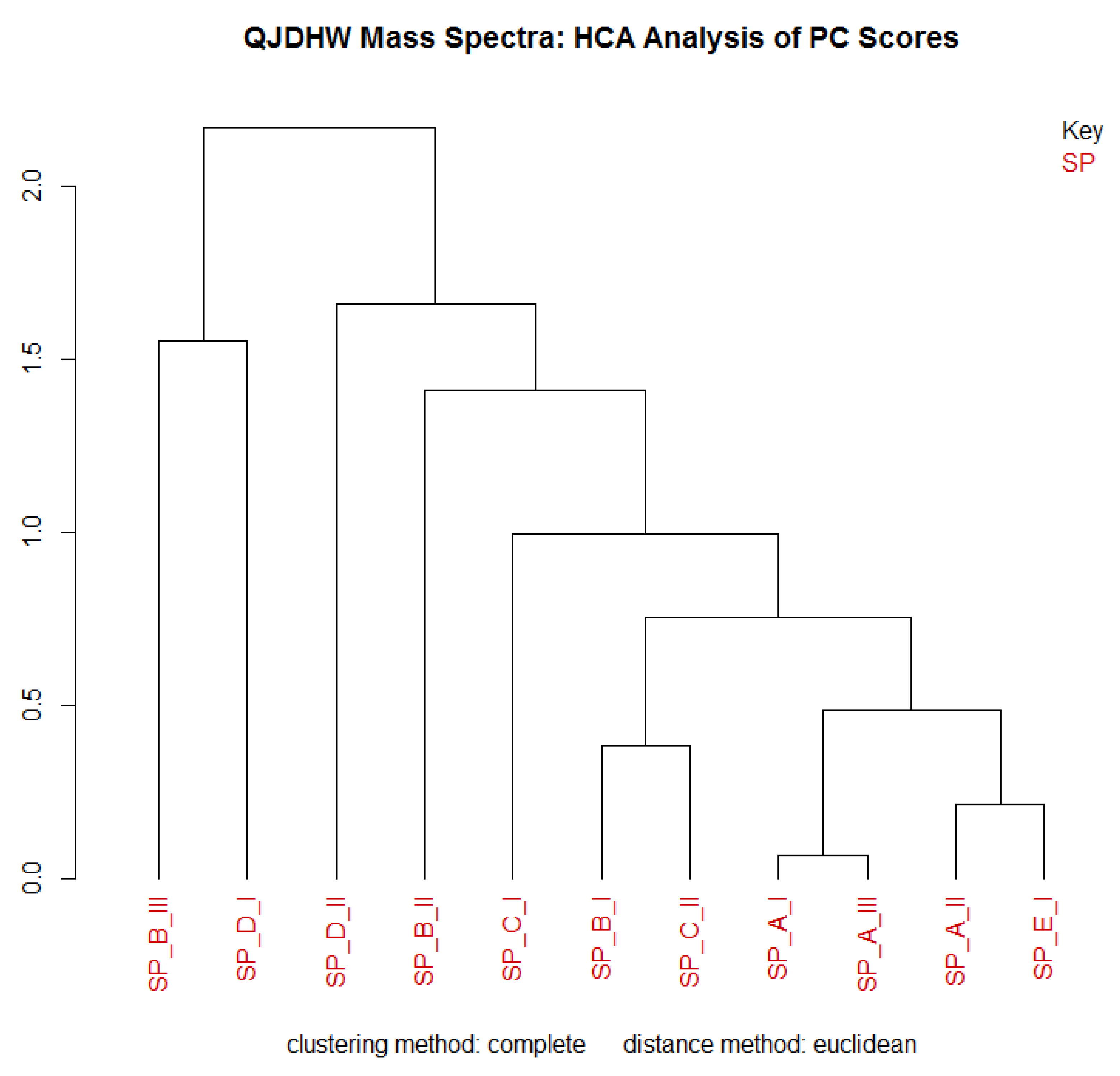
The specific reason for this choice was due to the cornuside peak having a much larger peak despite having a similar concentration to alisol C and pachymic acid in the samples. Both the robust and classical ellipses were shown in the PCA plot and the robust ellipse was chosen to identify potential outliers since it provided more definitive grouping of the samples due to its use of median absolute deviation. HCA was then performed on the data to corroborate the variance present in the PCA plot.