Characterization of Tapeworm Metabolites and Their Reported Biological Activities
Abstract
1. Introduction
2. Results
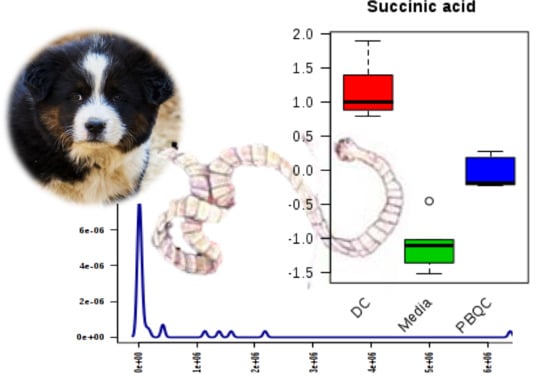
2.1. Polar Metabolites of D. caninum ESP Identified Using GC-MS
Statistical Analysis Using MetaboAnalyst 4.0 Software
2.2. Non-Polar Metabolites of D. Caninum Identified Using GC-MS and LC-MS
2.3. Literature Review Reveals Bioactive Small Molecules Present in the D. caninum ESP
3. Discussion
4. Materials and Methods
4.1. GC-MS Analysis of ESP Samples of D. caninum
4.1.1. Sample Preparation and Cryomill Extraction Methods
4.1.2. Derivatization and Analysis of Polar Fraction Using Targeted GC-MS Technique
4.1.3. Analysis of Non-Polar Fraction Using Targeted GC-MS Approach
4.2. LC-MS Identification of SCFA
4.3. Data Analyses and Statistical Interpretation
4.4. Literature Searches and Their Content Analyses Focusing on Anti-Inflammatory Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Taeniasis/Cysticercosis: Fact Sheet. World Health Organisation, Avenue Appia 20, 1202 Geneva. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/taeniasis-cysticercosis (accessed on 20 March 2019).
- CDC. Dipylidium Caninum; Centre for Disease Control and Prevention, Global Health, Division of Parasitic Diseases and Malaria, 2017. Available online: https://www.cdc.gov/dpdx/dipylidium/index.html (accessed on 20 March 2019).
- Coakley, G.; Buck, A.H.; Maizels, R.M. Host parasite communications—Messages from helminths for the immune system parasite communication and cell-cell interactions. Mol. Biochem. Parasitol. 2016, 208, 33–40. [Google Scholar] [CrossRef]
- Pan, W.; Hao, W.T.; Shen, Y.J.; Li, X.Y.; Wang, F.F.S.; Sun, F.-F.; Yin, J.H.; Zhang, J.; Tang, R.X.; Cao, J.-P.; et al. The excretory-secretory products of Echinococcus granulosus protoscoleces directly regulate the differentiation of B10, B17 and Th17 cells. Parasit. Vectors 2017, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Nono, J.K.; Pletinckx, K.; Lutz, M.B.; Brehm, K. Excretory/secretory-products of Echinococcus multilocularis larvae induce apoptosis and tolerogenic properties in dendritic cells in vitro. PLoS Negl. Trop. Dis. 2012, 6, e1516. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Lopes, F.; Leung, G.; Jayme, T.S.; Matisz, C.E.; Burkhard, R.; Carneiro, M.; Workentine, M.L.; Wang, A.; Petri, B.; et al. Macrophages treated with antigen from the tapeworm Hymenolepis diminuta condition CD25+ T cells to suppress colitis. FASEB J. 2019, 33, 5676–5689. [Google Scholar] [CrossRef] [PubMed]
- Graves, N.; Venu, V.P.; Yipp, B.G.; Petri, B.; Hirota, S.; Gilleard, J.; McKay, D.M.; Lopes, F. A trypsin-sensitive proteoglycan from the tapeworm Hymenolepis diminuta inhibits murine neutrophil chemotaxis in vitro by suppressing p38 MAP kinase activation. J. Innate Immun. 2019, 11, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Lopes, F.; Leung, G.; Mancini, N.L.; Matisz, C.E.; Wang, A.; Thomson, E.A.; Graves, N.; Gilleard, J.; McKay, D.M. Treatment with cestode parasite antigens esults in recruitment of CCR2+ myeloid cells, the adoptive transfer of which ameliorates colitis. Infect. Immun. 2016, 84, 3471–3483. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.O.; Esteves, C.Z.; Oliveira, D.N.; Guerreiro, T.M.; Melo, C.F.O.R.; Catharino, R.R. Mass Spectrometry and Metabolomics—New Approaches for Helminth Biochemical Studies in Human Helminthiasis; Rodrigo, L., Ed.; InTechOpen Limited: London, UK, 2017. [Google Scholar]
- Shepherd, C.; Navarro, S.; Wangchuk, P.; Wilson, D.; Daly, N.L.; Loukas, A. Identifying the immunomodulatory components of helminths. Parasit Immunol. 2015, 37, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Langley, P.A.; Huyton, P. Sex pheromone of the tsetse fly: isolation, identification, and synthesis of contact aphrodisiacs. Science 1978, 201, 750–753. [Google Scholar] [CrossRef]
- Doonan, J.; Lumb, F.E.; Pineda, M.K.; Tarafdar, A.; Crowe, J.; Khan, A.M.; Suckling, C.J.; Harnett, M.M.; Harnett1, W. Protection against arthritis by the parasitic worm product ES-62, and its drug-like small molecule analogues, is associated with inhibition of osteoclastogenesis. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Wangchuk, P.; Shepherd, C.; Constantinoiu, C.; Ryan, R.Y.M.; Kouremenos, K.A.; Becker, L.; Buitrago, G.; Giacomin, P.; Wilson, D.; Daly, N.; et al. Hookworm-derived metabolites suppress pathology in a mouse model of colitis and inhibit secretion of key inflammatory cytokines in primary human leukocytes. Infect. Immun. 2019. [Google Scholar] [CrossRef]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.A.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal. Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Lin, C.; Zheng, Y.; Hou, J.; Li, D. The cardioprotective effects of citric Acid and L-malic acid on myocardial ischemia/reperfusion injury. Evid. Based Complement. Altern. Med. 2013, 2013, 820695. [Google Scholar] [CrossRef]
- Unnikrishnan, M.K.; Rao, M.N. Antiinflammatory activity of methionine, methionine sulfoxide and methionine sulfone. Agents Actions 1990, 31, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Kim, H.Y.; Lee, H.J.; Shim, I.; Hahm, D.H. Wound healing activity of gamma-aminobutyric Acid (GABA) in rats. J. Microbiol. Biotechnol. 2007, 17, 1661–1669. [Google Scholar]
- Szél, E.; Polyánka, H.; Szabó, K.; Hartmann, P.; Degovics, D.; Balázs, B.; Nemeth, I.B.; Korponyai, C.; Csanyi, E.; Kaszaki, J.; et al. Anti-irritant and anti-inflammatory effects of glycerol and xylitol in sodium lauryl sulphate-induced acute irritation. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, O.; Kosugi, T.; Roncal, C.; Mu, W.; Heinig, M.; Cirillo, P.; Sanchez-Lozada, L.G.; Johnson, R.J.; Nakagawa, T. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J. Am. Soc. Nephrol. 2008, 19, 1712–1720. [Google Scholar] [CrossRef]
- Ahmad, M.; Baba, W.N.; Gani, A.; Wani, T.A.; Gani, A.; Masoodi, F.A. Effect of extraction time on antioxidants and bioactive volatile components of green tea (Camellia sinensis), using GC/MS. Cogent Food Sci. Agric. 2015, 1, 1106387. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Kuda, O. Bioactive metabolites of docosahexaenoic acid. Biochimie 2017, 136, 12–20. [Google Scholar] [CrossRef]
- Tedelind, S.; Westverg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Tsai, T.H.; Chuang, L.T.; Li, Y.Y.; Zouboulis, C.C.; Tsai, P.J. Antibacterial and anti-inflammatory properties of capric acid against propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.V.; Saric, J.; Keiser, J.; Wu, J.; Utzinger, J.; Holmes, E. Advances in metabolic profiling of experimental nematode and trematode infections. Adv. Parasitol. 2010, 73, 373–404. [Google Scholar]
- Beames, C.G.J. Neutral lipids of Ascaris lumbricoides with special reference to the esterified fatty acids. Exp. Parasitol. 1965, 16, 291–299. [Google Scholar] [CrossRef]
- Kaplan, F.; Srinivasan, J.; Mahanti, P.; Ajredini, R.; Durak, O.; Nimalendran, R.; Sternberg, P.W.; Teal, P.E.; Schroeder, F.C.; Edison, A.S.; et al. Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage. PLoS ONE 2011, 6, e17804. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The human metabolome database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef]
- Belzer, C.; Chia, L.W.; Aalvink, S.; Chamlaqain, B.; Piironen, V.; Knol, J.; de Vos, W.M. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B12 production by intestinal symbionts. MBio 2017, 8, e00770-17. [Google Scholar] [CrossRef]
- Loukas, A.; Hotez, P.J.; Diemert, D.; Yazdanbakhsh, M.; McCarthy, J.S.; Correa-Oliveira, R.; Croese, J.; Bethony, J.M. Hookworm infection. Nat. Rev. Dis. Primers. 2016, 2, 16088. [Google Scholar] [CrossRef]
- Maizels, R.M. Parasitic helminth infections and the control of human allergic and autoimmune disorders. Clin. Microbiol. Infect. 2016, 22, 481–486. [Google Scholar] [CrossRef]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of host immunity by helminths: The expanding repertoire of parasite effector molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35, S35–S38. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef]
- Overgaard, A.J.; Weir, J.M.; De Souza, D.P.; Tull, D.; Haase, C.; Meikle, P.J.; Pociot, F. Lipidomic and metabolomic characterization of a genetically modified mouse model of the early stages of human type 1 diabetes pathogenesis. Metabolomics 2016, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lin, K.; Sequeia, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2017, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Excretory-secretory products of tapeworms/samples are available from the authors at Australian Institute of Tropical Health and Medicine, James Cook University, Cairns, Australia. |
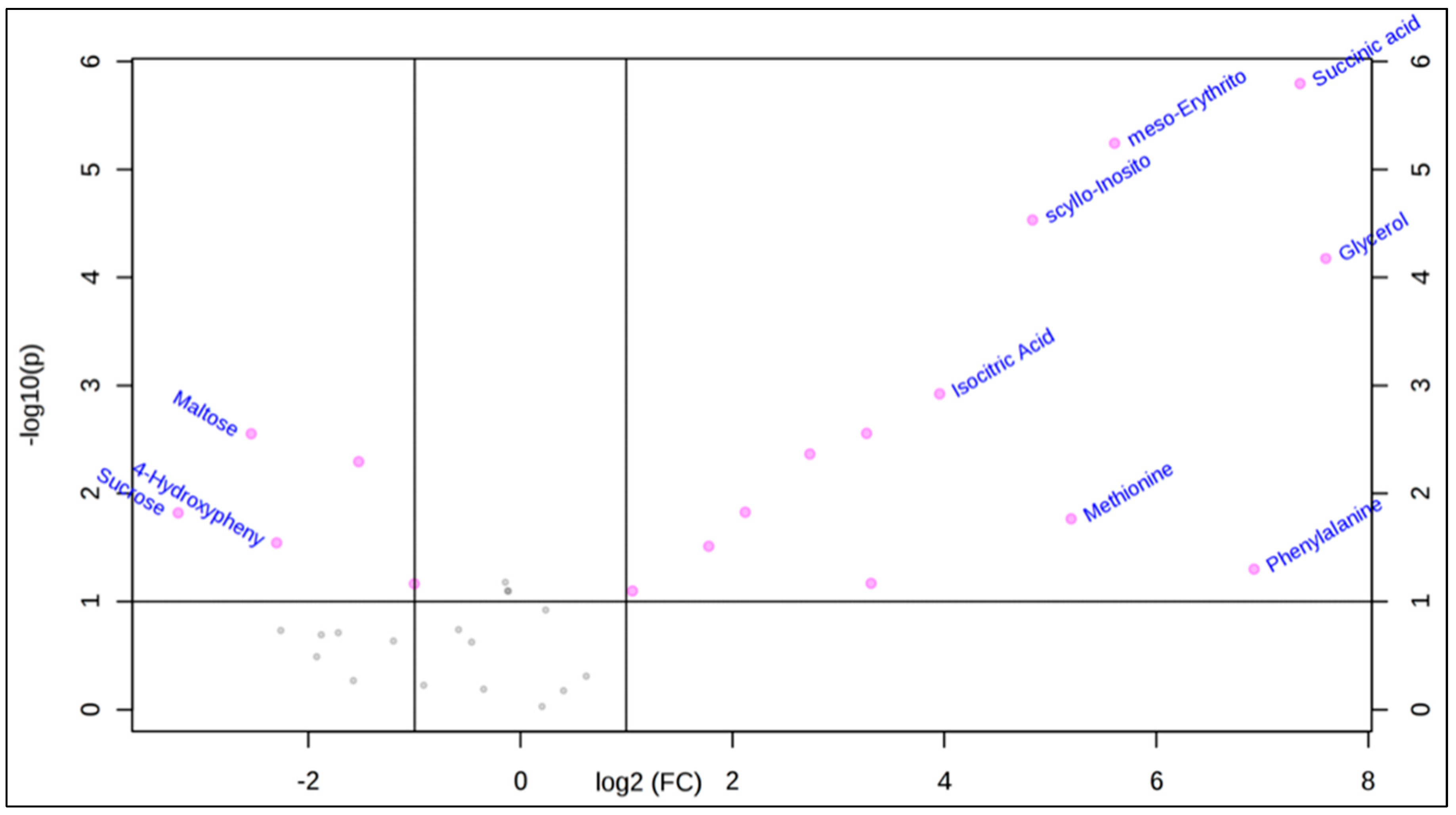

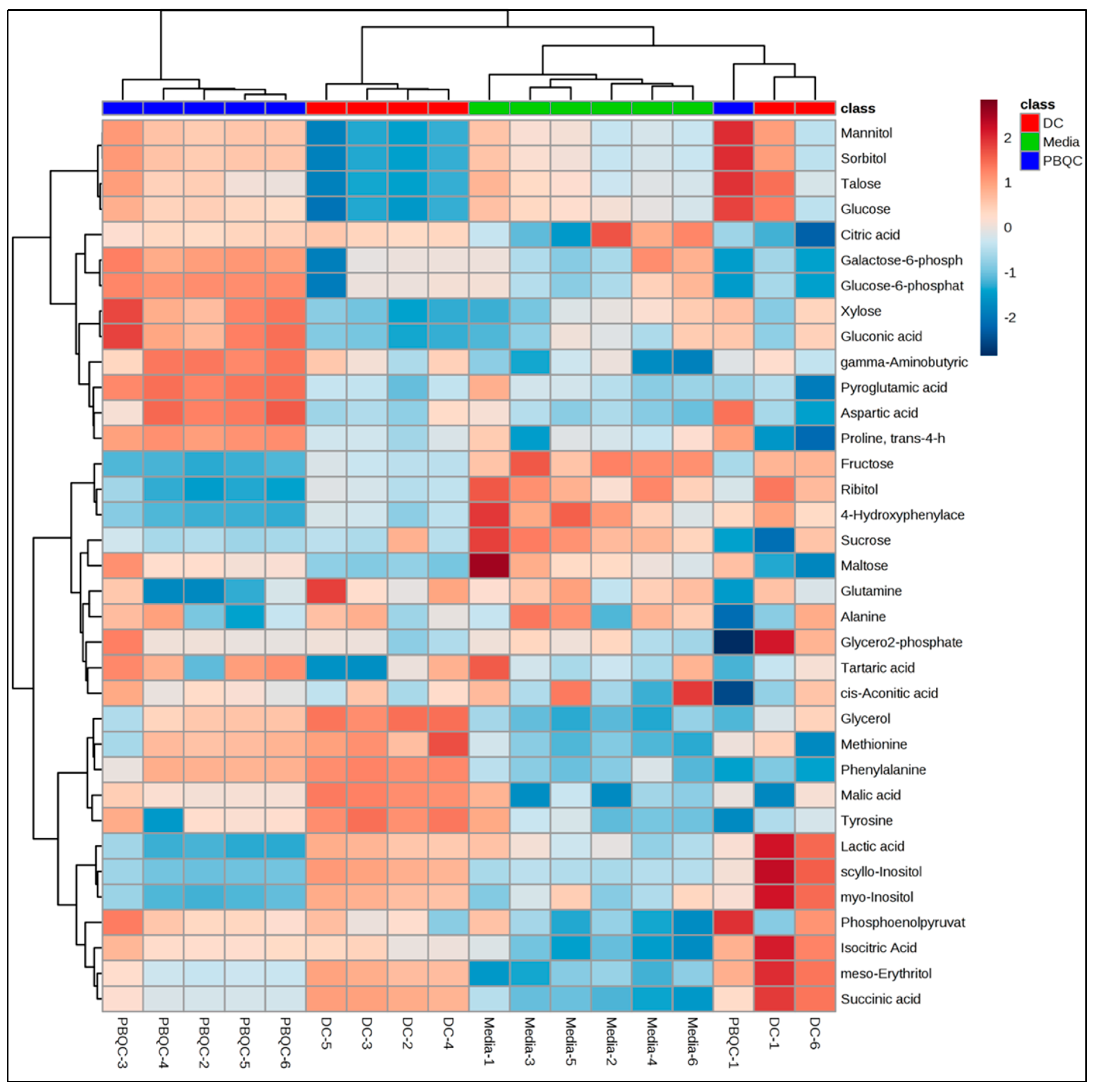
| Compounds | Rt * | Peak Area | Log 2 (FC) ** | Mass (m/z, M+) | Chemical Class *** | KEGG ID **** | Reported Bioactivities |
|---|---|---|---|---|---|---|---|
| Lactic acid | 6.73 | 4,919,948 | 2.73 | 90 | Organic acid | C00186 | Anti-inflammatory [14] |
| Alanine | 6.92 | 39,820 | ns | 89 | Amino acid | C00041 | N/A |
| Succinic acid | 8.11 | 14,637,634 | 7.35 | 118 | Organic acid | C00042 | N/A |
| Malic acid | 8.97 | 80,527 | 3.30 | 134 | Organic acid | C03668 | Anti-platelet & anti-inflammatory [15] |
| Meso-Erythritol | 9.02 | 24,013 | 5.60 | 122 | Sugar alcohol | C00503 | N/A |
| Aspartic acid | 9.20 | 217 | ns | 133 | Amino acid | C00402 | N/A |
| Methionine | 9.22 | 7437 | 5.19 | 149 | Amino acid | C00073 | Anti-inflammatory [16] |
| trans-4-Hydroxyproline | 9.23 | 1874 | ns | 131 | Amino acid | C01157 | N/A |
| Pyroglutamic acid | 9.27 | 28,950 | ns | 129 | Amino acid lactam | C01879 | N/A |
| GABA | 9.28 | 2666 | 2.12 | 103 | Fatty acid derivative | D00058 | Wound healing & neurotransmitter [17] |
| Phenylalanine | 9.43 | 45,266 | 6.92 | 165 | Amino acid | C00079 | N/A |
| Phosphoenol-pyruvate | 9.53 | 261 | 1.05 | 168 | Phosphate ester | C00074 | N/A |
| Tartaric acid | 9.73 | 311 | ns | 150 | Organic acid | C02107 | N/A |
| Xylose | 9.86 | 5285 | ns | 150 | Sugar | C00181 | N/A |
| Ribitol | 10.08 | 5464 | −1.00 | 152 | Sugar alcohol | C00474 | N/A |
| Glycerol-2-phosphate | 10.11 | 6003 | ns | 172 | Glycerophosphate | C02979 | N/A |
| Glycerol | 10.13 | 47,846 | 7.59 | 92 | Sugar alcohol | C00116 | Anti-inflammatory [18] |
| cis-Aconitic acid | 10.27 | 173 | ns | 174 | Organic acid | C00417 | N/A |
| Citric acid | 10.53 | 757 | ns | 192 | Organic acid | C00158 | Cardioprotective [15] |
| Isocitric acid | 10.54 | 872 | 3.95 | 192 | Organic acid | C00311 | N/A |
| Fructose | 10.73 | 17,559 | −1.52 | 180 | Sugar | C02336 | Pro-inflammatory [19] |
| Talose | 10.80 | 70,147 | ns | 180 | Sugar | N/A | N/A |
| Glucose | 10.83 | 66,453 | ns | 180 | Sugar | C00031 | N/A |
| Mannitol | 10.94 | 9570 | ns | 182 | Sugar alcohol | C00392 | N/A |
| Tyrosine | 10.96 | 89,216 | 1.77 | 181 | Amino acid | C00082 | N/A |
| Sorbitol | 10.98 | 9527 | ns | 182 | Sugar alcohol | C00794 | N/A |
| Glutamine | 11.21 | 657 | ns | 146 | Organic acid | C00064 | |
| Gluconic acid | 11.27 | 5178 | ns | 196 | Organic acid | C00257 | N/A |
| Scyllo-Inositol | 11.38 | 92,797 | 4.83 | 180 | Sugar alcohol | C06153 | N/A |
| 4-Hydroxy-Phenylacetic acid | 11.42 | 678 | −2.30 | 152 | Benzenoid | C00642 | N/A |
| Myo-Inositol | 11.67 | 430,582 | 3.26 | 180 | Sugar alcohol | C00137 | N/A |
| Galactose-6-Phosphate | 12.32 | 119 | ns | 260 | Sugar phosphate | N/A | N/A |
| Glucose-6-Phosphate | 12.41 | 193 | ns | 260 | Sugar phosphate | C00092 | N/A |
| Sucrose | 13.40 | 8792 | −3.23 | 342 | Disaccharide | C00089 | N/A |
| Maltose | 13.61 | 5089 | −2.54 | 342 | Sugar | C00208 | N/A |
| Fatty Acid Names. (Lipid Number) | Rt * | Peak Areas | Mass (m/z, M+) | KEGG ID ** | Reported Biological Properties |
|---|---|---|---|---|---|
| Undecanoic acid (C11:0) | 7.35 | 5503 | 186 | C17715 | N/A |
| Decanoic acid (C10:0) | 7.67 | 5507 | 172 | C01571 | N/A |
| Tridecylic acid (C13:0) | 9.03 | 21,853 | 214 | N/A | N/A |
| Dodecanoic acid (C12:0) | 9.22 | 767 | 200 | C02679 | Anti-inflammatory & antibacterial [20] |
| Tetradecanoic acid (C14:0) | 11.29 | 1864 | 228 | C06424 | N/A |
| Pentadecylic acid (C15:0) | 12.53 | 3431 | 242 | C16537 | N/A |
| Hexadecanoic acid (C16:0) | 13.84 | 16,171 | 256 | C00249 | Anti-inflammatory [21] |
| Heptadecanoic acid (C17:0) | 15.25 | 1238 | 270 | N/A | N/A |
| Octadecanoic acid (C18:0) | 15.25 | 32,883 | 284 | C01530 | Anti-inflammatory [22] |
| Oleic acid (C18:1, 9Z- cis) | 17.16 | 1219 | 282 | C00712 | N/A |
| Arachidonic acid (C20:4) | 18.15 | 2076 | 304 | C00219 | N/A |
| Linoleic acid (C18:2) | 18.33 | 5336 | 280 | C01595 | N/A |
| Eicosanoic acid (C20:0) | 19.60 | 5981 | 312 | C06425 | N/A |
| Docosahexaenoic acid (C22:6) | 26.84 | 6960 | 328 | C06429 | Anti-inflammatory [23] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wangchuk, P.; Constantinoiu, C.; Eichenberger, R.M.; Field, M.; Loukas, A. Characterization of Tapeworm Metabolites and Their Reported Biological Activities. Molecules 2019, 24, 1480. https://doi.org/10.3390/molecules24081480
Wangchuk P, Constantinoiu C, Eichenberger RM, Field M, Loukas A. Characterization of Tapeworm Metabolites and Their Reported Biological Activities. Molecules. 2019; 24(8):1480. https://doi.org/10.3390/molecules24081480
Chicago/Turabian StyleWangchuk, Phurpa, Constantin Constantinoiu, Ramon M. Eichenberger, Matt Field, and Alex Loukas. 2019. "Characterization of Tapeworm Metabolites and Their Reported Biological Activities" Molecules 24, no. 8: 1480. https://doi.org/10.3390/molecules24081480
APA StyleWangchuk, P., Constantinoiu, C., Eichenberger, R. M., Field, M., & Loukas, A. (2019). Characterization of Tapeworm Metabolites and Their Reported Biological Activities. Molecules, 24(8), 1480. https://doi.org/10.3390/molecules24081480