Propylene Polymerization Catalyzed by Metallocene/Methylaluminoxane Systems on Rice Husk Ash
Abstract
:1. Introduction
2. Results and Discussion
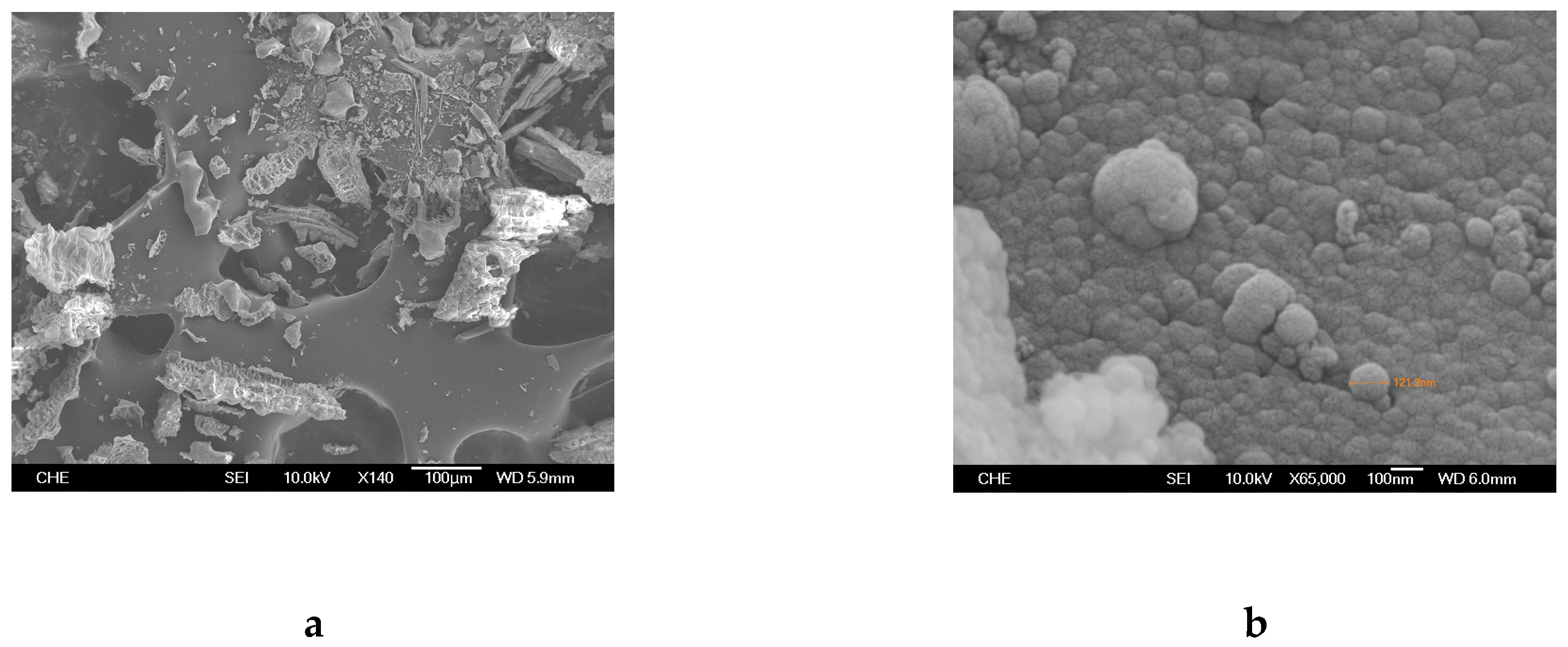
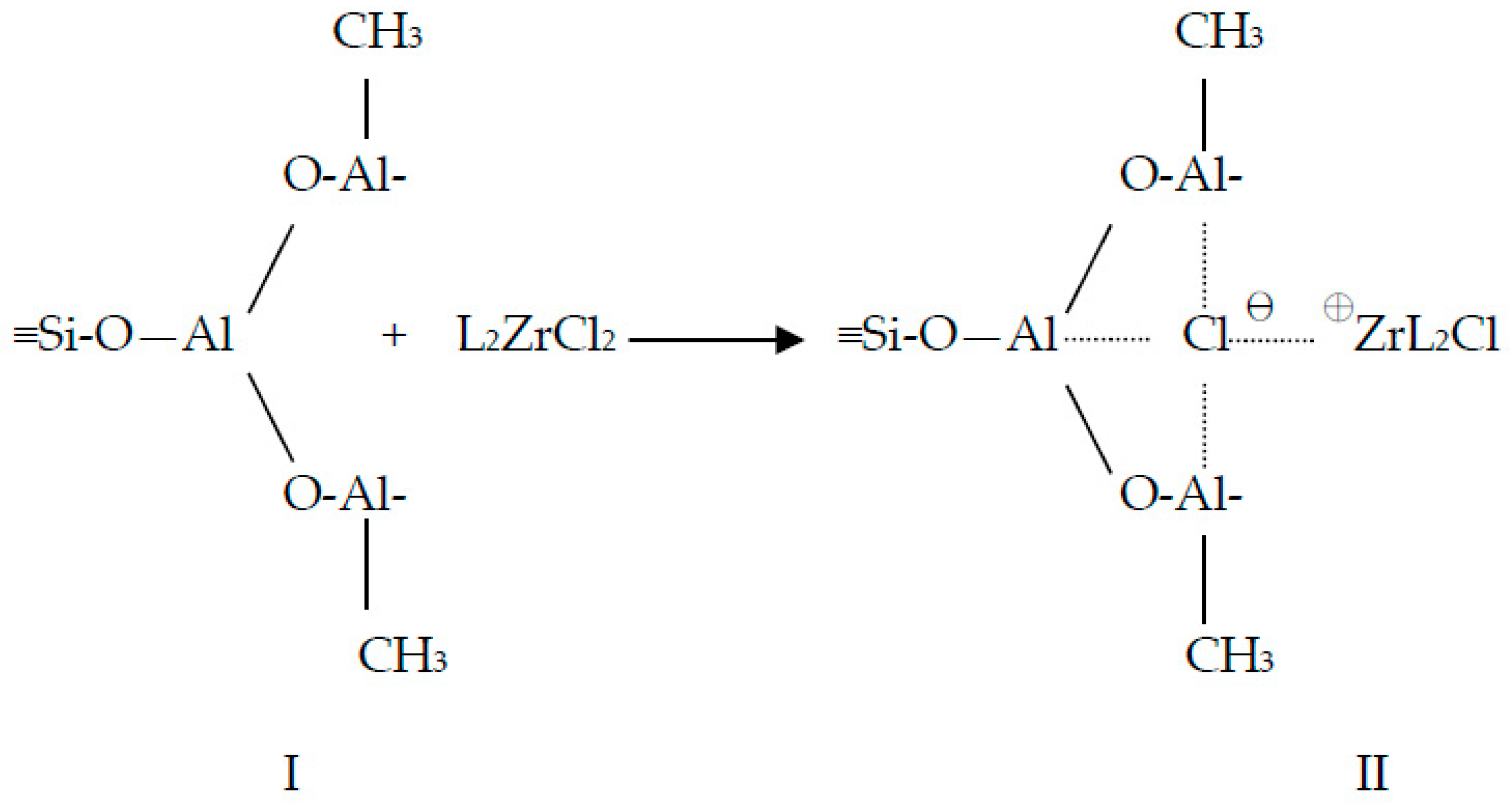
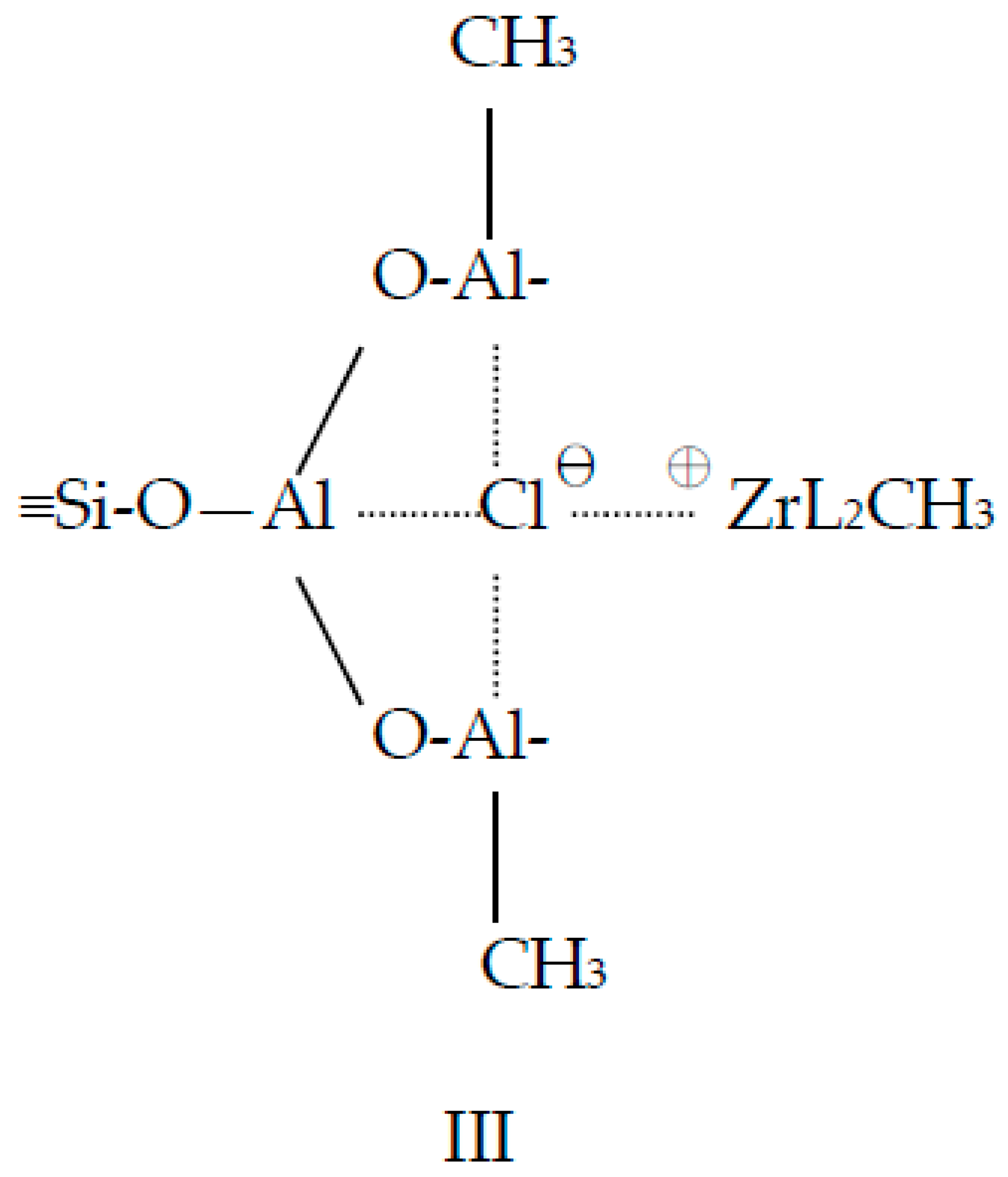
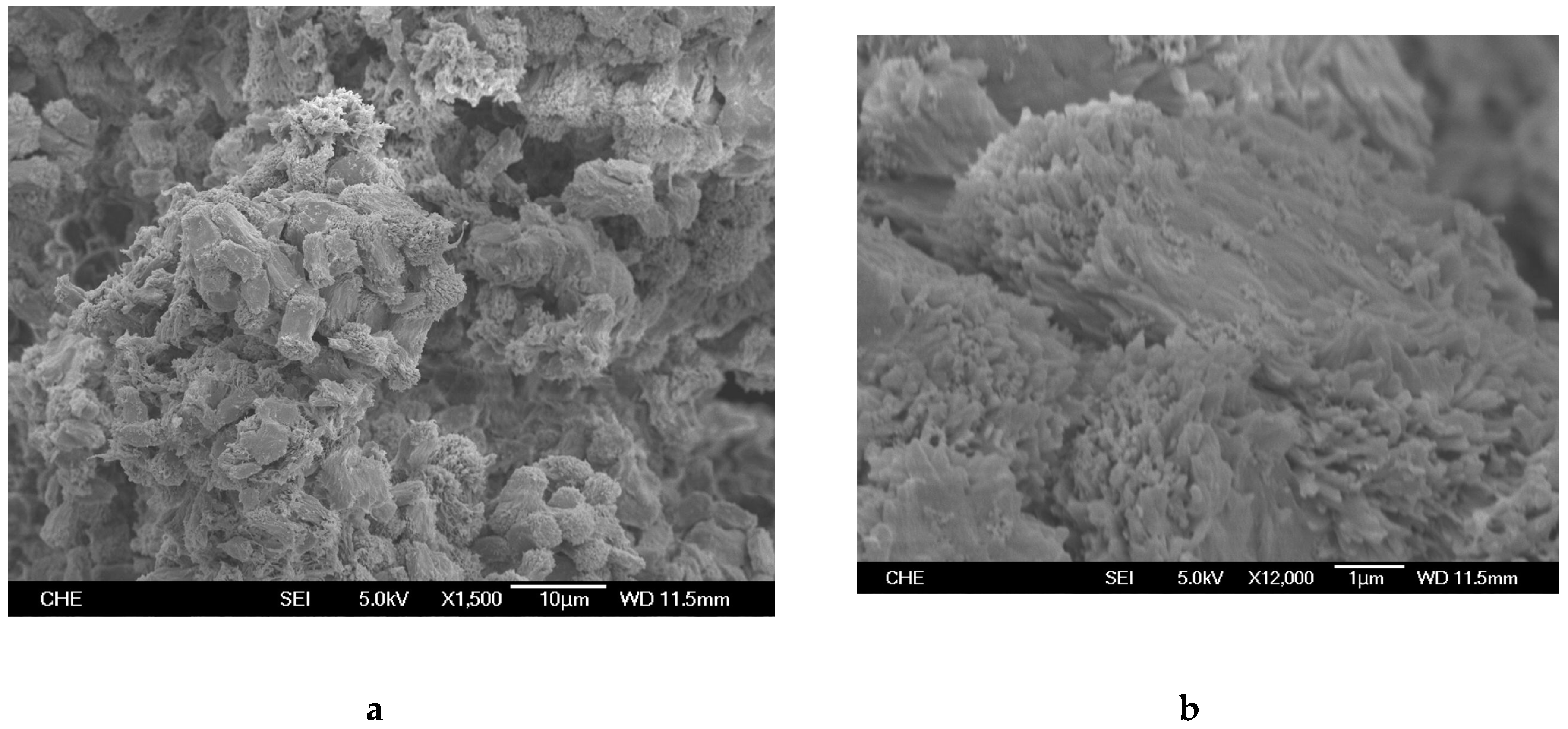
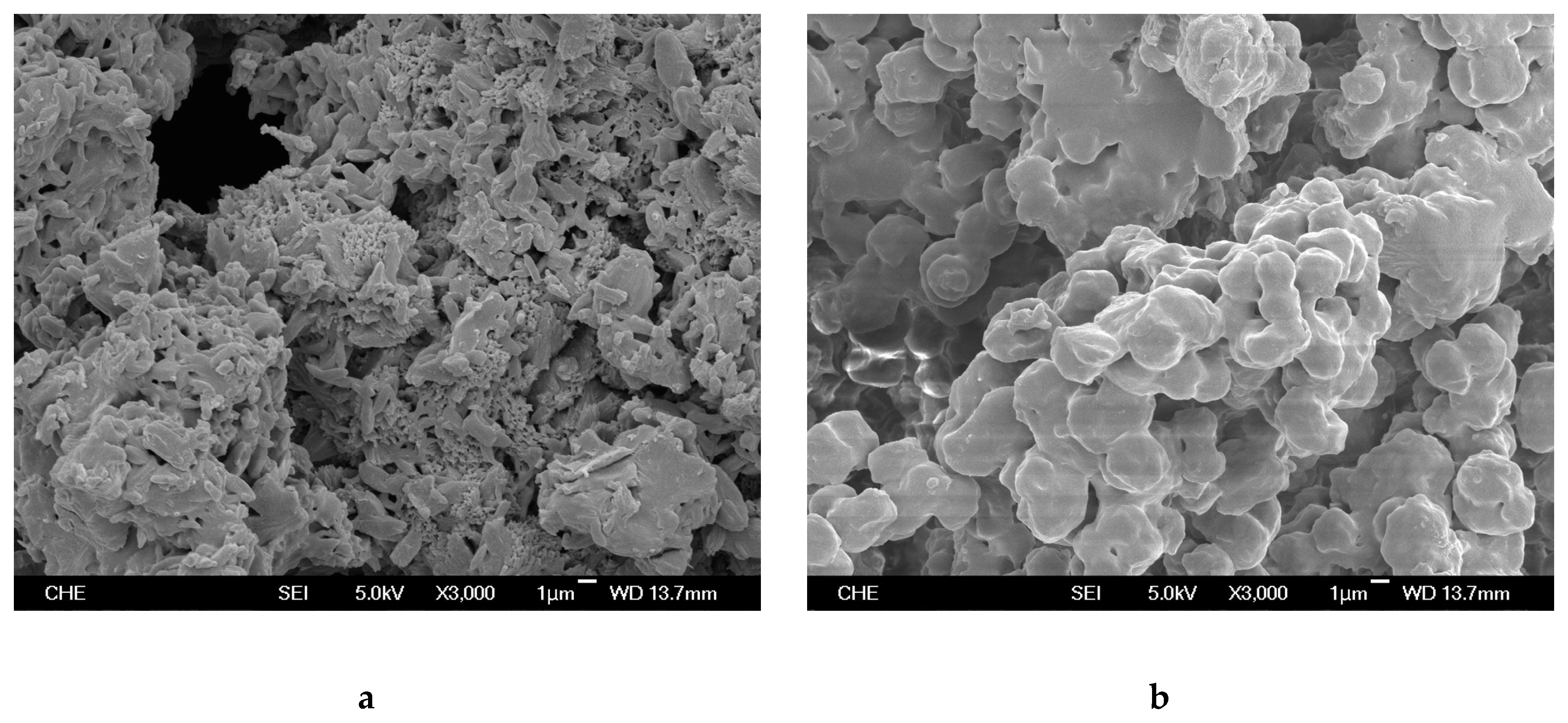
2.1. Rice Husk Ash (RHA) and Catalyst
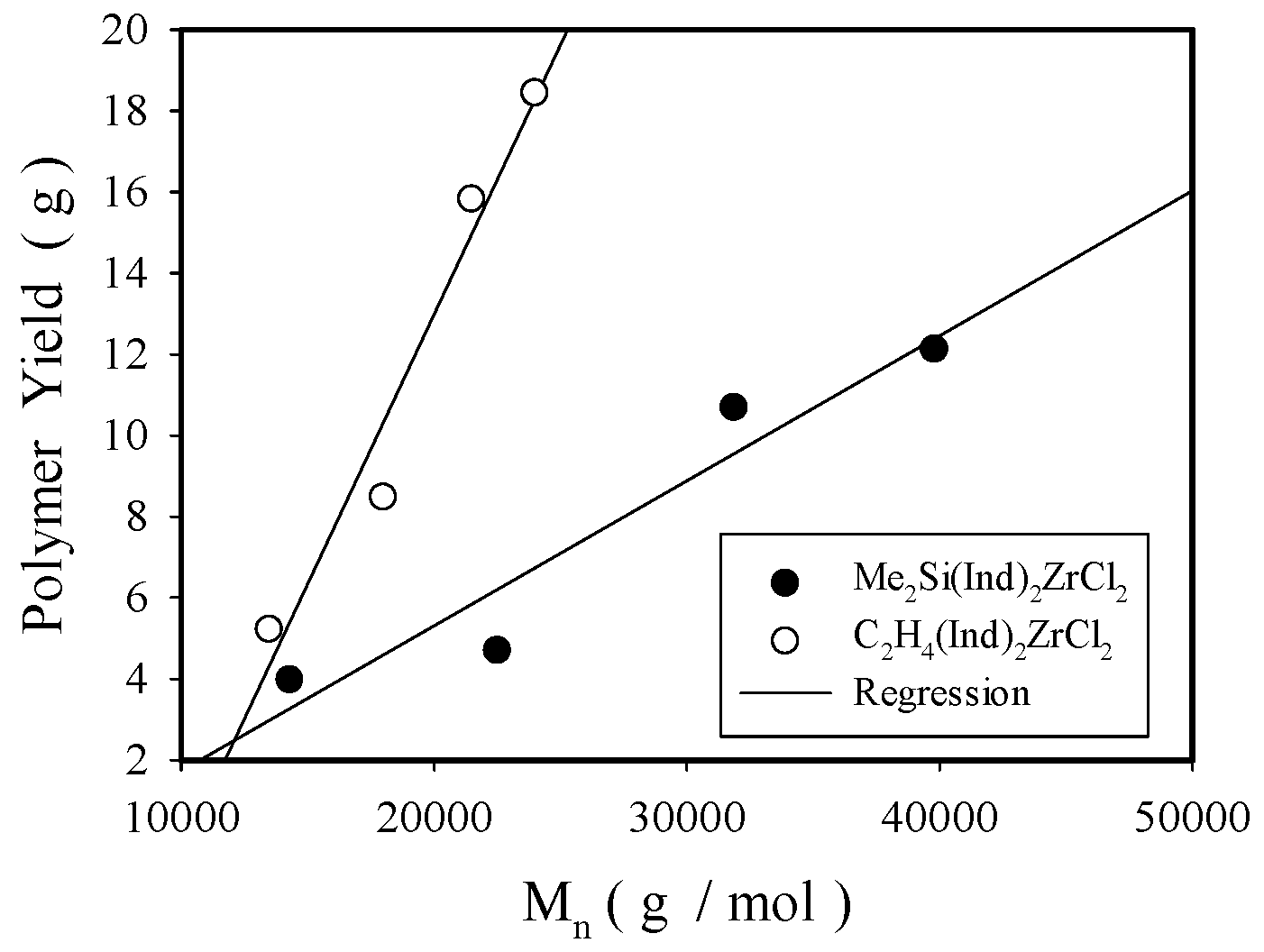
2.2. Propylene Polymerization and Polymer Characterization
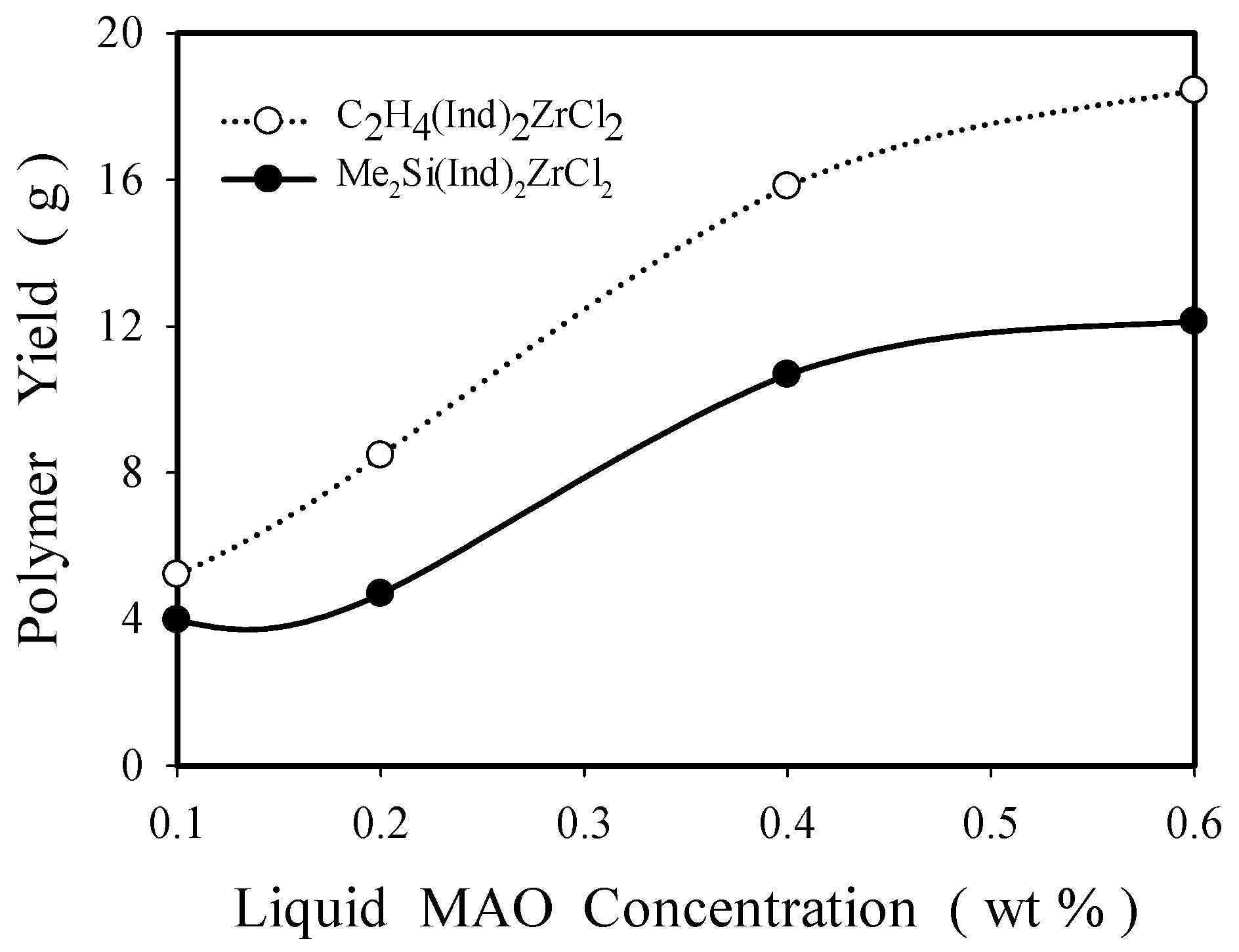
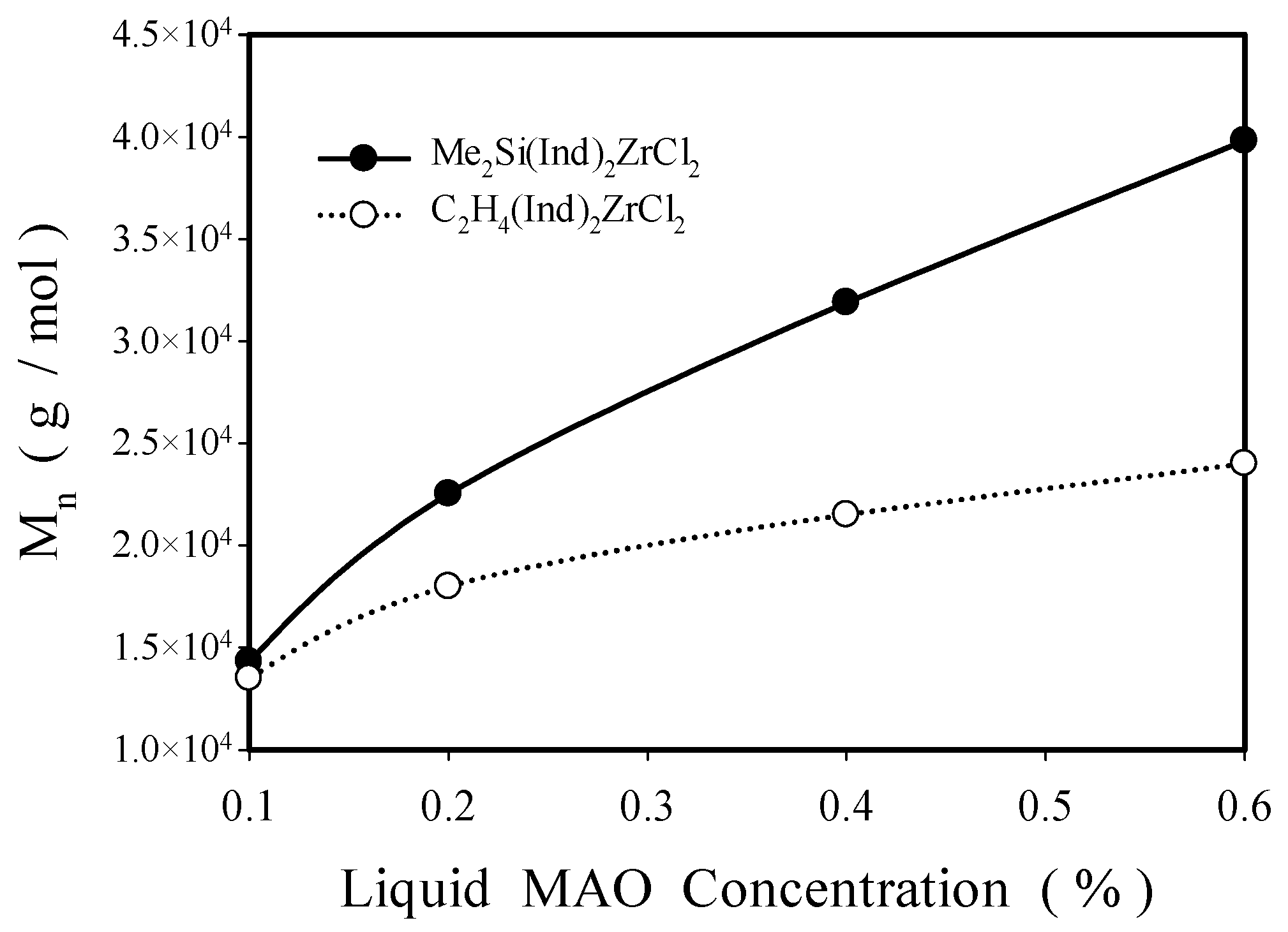
2.2.1. Effect of Solution MAO Concentration
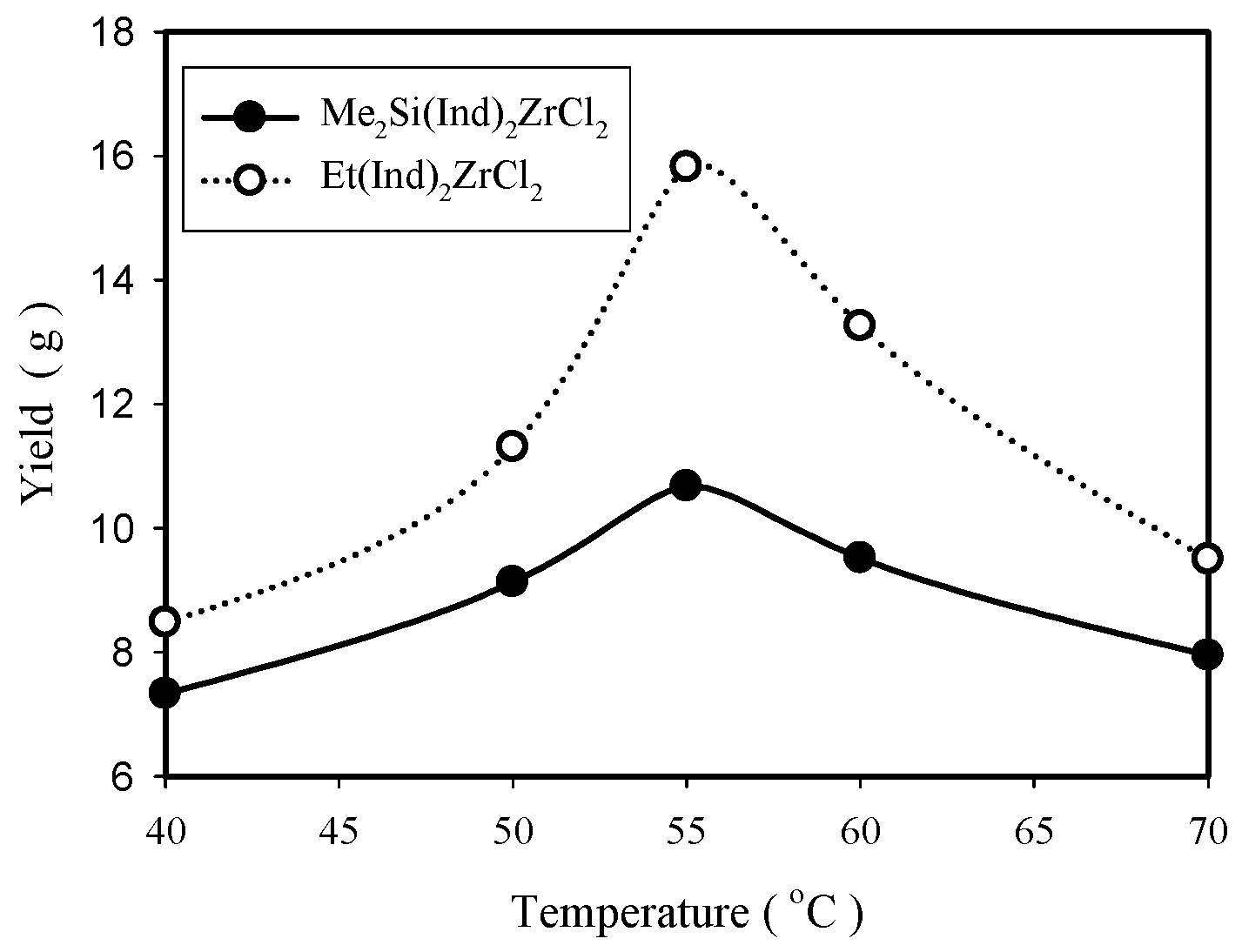
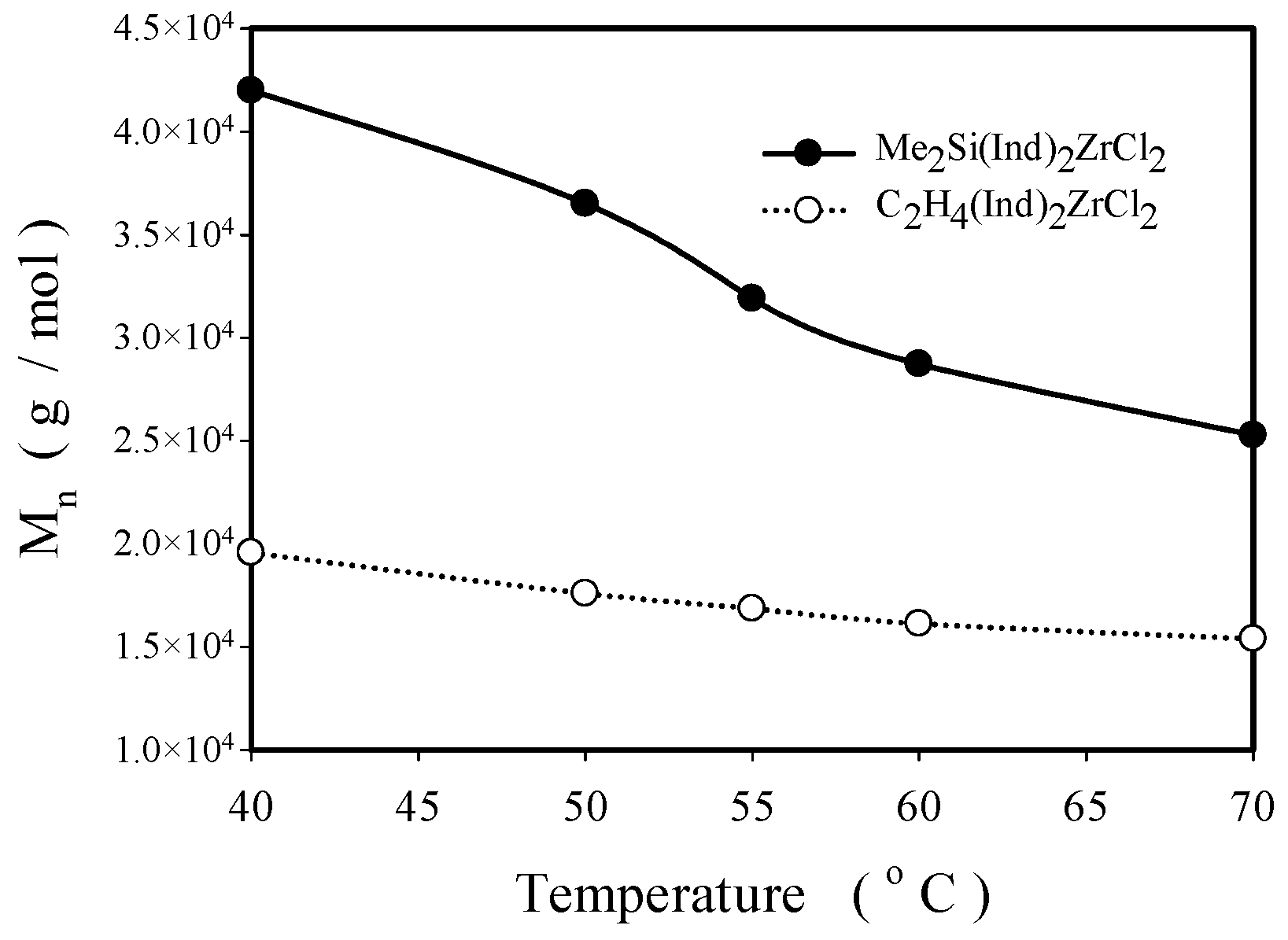
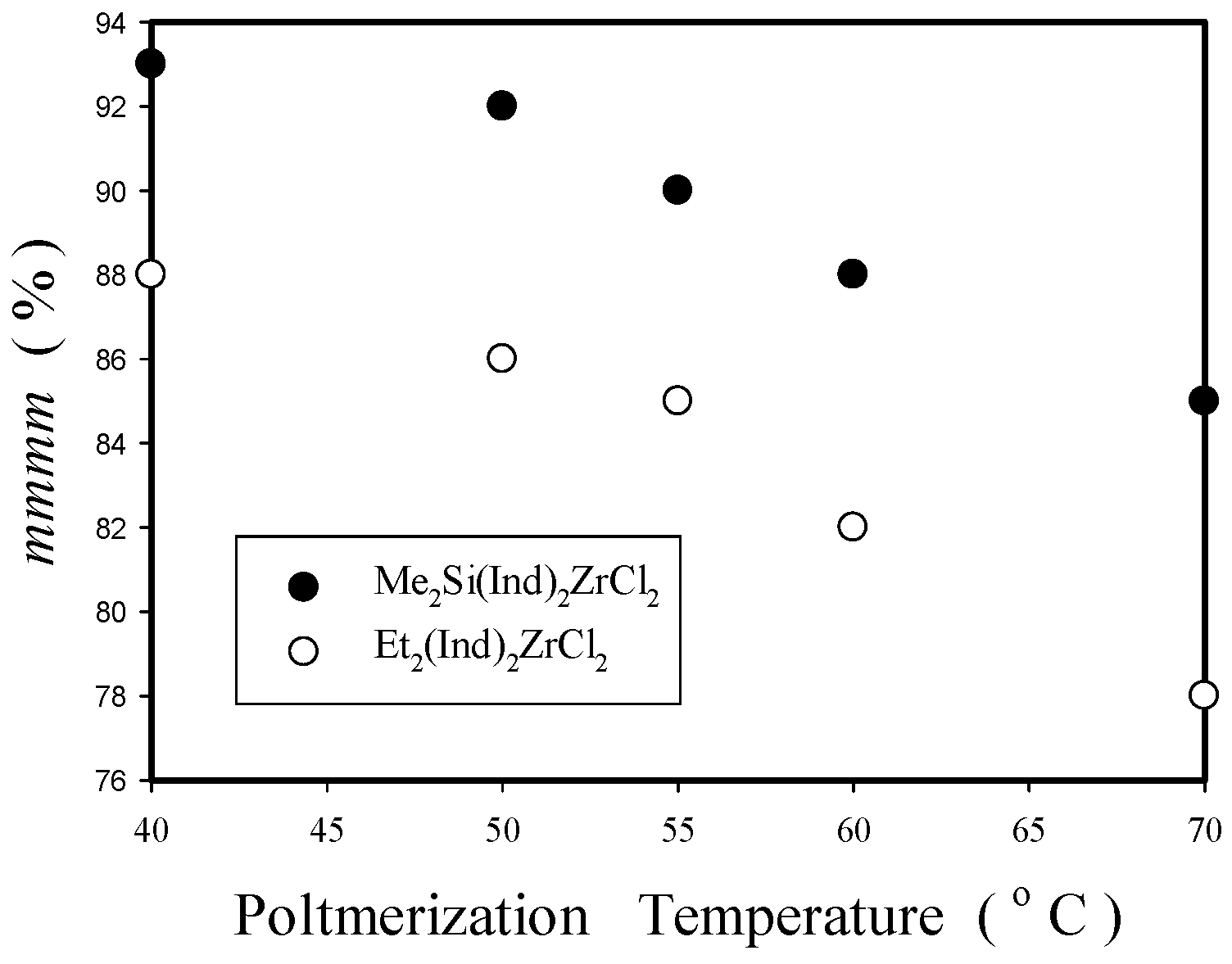
2.2.2. Effect of Polymerization Temperature
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Propylene Polymerization
3.3. Catalyst and Polymer Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Research and Markets, Polypropylene—Global Market Outlook (2017–2026). Available online: https://www.researchandmarkets.com/research/t5495s/global?w=4 (accessed on 14 February 2019).
- Lieberman, R.B.; Stewart, C. Propylene Polymers. In Encyclopedia of Polymer Engineering and Science; Mark, H.F., Ed.; Wiley: New York, NY, USA, 2004; Volume 11, pp. 287–358. [Google Scholar]
- Pasquini, N.; Moore, E.P. Introduction. In Polypropylene Handbook; Pasquini, N., Ed.; Hanser: Munich, Germany, 2005; pp. 3–13. [Google Scholar]
- Calamur, N. Propylene. In Encyclopedia of Chemical Technology; Kroschwitz, J.I., Howe-Grant, M., Eds.; Wiley: New York, NY, USA, 1996; Volume 20, pp. 263–264. [Google Scholar]
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The influence of Ziegler-Natta and metallocene catalysts on polyolefin structure, properties, and processing ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef] [PubMed]
- Samruk-Kazyna’s Research & Knowledge Management, Global Polypropylene Market Outlook, (2017). Available online: https://www.sk.kz/upload/iblock/713/713c4a54b6fdb2183807bba0dc84cbb3.pdf (accessed on 14 February 2019).
- Kaminsky, W.; Sinn, H. Methylaluminoxane: Key component for new polymerization catalysts. Adv. Polym. Sci. 2013, 258, 1–28. [Google Scholar]
- Fink, G. Contributions to the Ziegler-Natta Catalysis: An anthology. Adv. Polym. Sci. 2013, 257, 1–36. [Google Scholar]
- Fink, G. Polymerization on molecular catalysts. In Handbook of Heterogeneous Catalysis; Ertl, G., Knozinger, H., Schuth, F., Weitkamo, J., Eds.; Wiley-VCH: Weinheim, Germany, 2008; Volume 8, pp. 3792–3827. [Google Scholar]
- Fink, G.; Steinmetz, B.; Zechlin, J.; Przyblya, C.; Tesche, B. Propene polymerization with silica-supported metallocene/MAO Catalysts. Chem. Rev. 2000, 100, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.; Appaturi, J.N.; Iqbal, A. The utilization of rice husk silica as a catalyst: Review and recent progresses. Catal. Today 2012, 190, 2–14. [Google Scholar] [CrossRef]
- Jams, J.; Rao, M.S. Characterization of silica in rice husk ash. Am. Ceram. Soc. Bull. 1986, 65, 1177–1180. [Google Scholar]
- Adam, F.; Kandasamy, K.; Balakrishnan, S. Iron incorporated heterogeneous catalyst from rice hush ash. Colloid Interface Sci. 2006, 304, 137–143. [Google Scholar] [CrossRef]
- Jamnongphol, S.; Jaturapiree, A.; Sukrat, K.; Saowapark, T.; Chaichana, E.; Jongsomjit, B. Rice husk-derived silica as a support for zirconcene/MAO catalyst in ethylene polymerization. Waste Biomass Valor 2018, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.X.; Rausch, M.D.; Chien, J.C.W. Heptane-soluble homogeneous zirconocene catalyst: Synthesis of a single diastereomer, polymerization catalysis, and effect of silica supports. J. Polym. Sci. A. Polym. Chem. 1995, 33, 2093–2108. [Google Scholar] [CrossRef]
- Li, K.T.; Ko, F.S. Dimethylsilylbis (1-indenyl) zirconium dichloride/methylaluminoxane catalyst supported on nanosized silica for propylene polymerization. J. Appl. Polym. Sci. 2008, 107, 1387–1394. [Google Scholar] [CrossRef]
- Li, K.T.; Kao, Y.T. Nanosized silica-supported metallocene/MAO catalyst for propylene polymerization. J. Appl. Polym. Sci. 2006, 101, 2573–2580. [Google Scholar] [CrossRef]
- Zhuravlev, L.T. Concentration of hydroxyl groups on the surface of amorphous silicas. Langmuir 1987, 3, 316–318. [Google Scholar] [CrossRef]
- Quijada, R.; Dupont, J.; Miranda, M.S.; Scipioni, R.B.; Galland, G.B. Copolymerization of ethylene with 1-hexehe and 1-octene: Correlation between type of catalyst and comonomer incorporated. Macromol. Chem. Phys. 1995, 196, 3991–4000. [Google Scholar]
- Bonine, F.; Fraaije, V.; Fink, G. Propylene polymerization through supported metallocene/MAO catalyst: Kinetic analysis and modeling. J. Polym. Sci. A. Polym. Chem. 1995, 33, 2393–2402. [Google Scholar] [CrossRef]
- Stehling, U.; Diehold, J.; Kirsten, R.; Roll, W.; Brintzinger, H.; Jungling, S.; Mulhaupt, R.; Langhauser, F. ansa-Zirconocene Polymerization catalysts with anelated ring ligands—Effects on catalytic activity and polymer chain length. Organometallics 1994, 13, 964–970. [Google Scholar] [CrossRef]
- Li, K.T.; Yang, C.N. Uniform rod-like self-assembly of polymer nanofibrils produced via propylene polymerization on Stober silica nuclei supported metallocene catalysts. Mater. Today Commum. 2019, 19, 80–86. [Google Scholar] [CrossRef]
- Ghiotto, F.; Pateraki, C.; Severn, J.R.; Friederichs, N.; Bochmann, M. Rapid evaluation of catalysts and MAO activators by kinetics: What controls polymer molecular weight and activity in metallocene/MAO catalysts? Dalton Trans. 2013, 42, 9040–9048. [Google Scholar] [CrossRef]
- Hung, J.; Rempel, G.L. Kinetic study of propylene polymerization using Et[H4Ind]2ZrCl2/methylalumoxane catalysts. Ind. Eng. Chem. Res. 1997, 36, 1151–1157. [Google Scholar] [CrossRef]
- Schaer, E.; Ravetti, R.; Plasari, E. Study of silica particle aggregation in a batch agitated vessel. Chem. Eng. Process. 2001, 40, 277–293. [Google Scholar] [CrossRef]
- Fontana, C.M.; Kidder, G.A. Kinetics of the polymerization of propylene with aluminum bromide –hydrogen bromide catalyst. J. Am. Chem. Soc. 1948, 70, 3745–3751. [Google Scholar] [CrossRef]
- Tennekes, H.; Lumley, J.L. A First Course in Turbulence; The MIT Press: Cambridge, MA, USA, 1972. [Google Scholar]
- Batchelor, G.K. Small-scale variation of convected quantities like temperature in turbulent fluid Part 1. General discussion and the case of small conductivity. J. Fluid Mechanics 1959, 5, 113–133. [Google Scholar] [CrossRef]
- Baldyga, J.; Bournes, J.R. Principles of micromixing. In Encyclopedia of Fluid Mechanics; Cheremisinoff, N.P., Ed.; Gulf Pub. Co.: Houston, TX, USA, 1986; Volume 1, pp. 148–201. [Google Scholar]
- Li, K.T.; Toor, H.L. Turbulent reactive mixing with a series-parallel reaction: Effect of mixing on yield. AIChE J. 1986, 32, 1312–1320. [Google Scholar] [CrossRef]
- Li, K.T. On the film thickness in the film theory. J. Chin. I. Ch.E. 1995, 26, 269–270. [Google Scholar]
- Fogler, S.H. Elements of Chemical Reaction Engineering, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2006; Chapter 11. [Google Scholar]
- Rieger, B.; Mu, X.; Mallin, D.T.; Rausch, M.D.; Chien, J.C.W. Degree of stereochemical control of rac-Et[Ind]2ZrCl2/MAO catalyst and properties of anissotatic polypropylene. Macromolecules 1990, 23, 3559–3568. [Google Scholar] [CrossRef]
- Patel, M.; Karera, A.; Prasanna, P. Effect of thermal and chemical treatment on carbon and silica contents in rice husk. J. Mater. Sci. 1987, 22, 2457–2464. [Google Scholar] [CrossRef]
- Chang, F.W.; Kuo, M.S.; Tsay, M.T.; Hsieh, M.C. Hydrogenation of CO2 over nickel catalysts on rice husk ash –alumina prepared by incipient wetness impregnation. Appl. Catal. A Gen. 2003, 247, 309–320. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |



| Temp. (°C) | MAO (wt%) | Yield (g) | Mn (g/mol) | Mw (g/mol) | PDI = Mw/Mn | mmmm (%) |
|---|---|---|---|---|---|---|
| 55 | 0.1 | 4.0 | 14,315 | 49,141 | 3.4 | 90 |
| 55 | 0.2 | 4.7 | 22,523 | 51,436 | 2.3 | 90 |
| 55 | 0.4 | 10.7 | 31,893 | 81,239 | 2.5 | 90 |
| 55 | 0.6 | 12.1 | 39,818 | 109,027 | 2.7 | 90 |
| 40 | 0.4 | 7.3 | 41,980 | 113,346 | 2.7 | 93 |
| 50 | 0.4 | 9.1 | 36,504 | 84,957 | 2.3 | 92 |
| 60 | 0.4 | 9.5 | 28,726 | 65,519 | 2.3 | 88 |
| 70 | 0.4 | 8.9 | 25,272 | 53,008 | 2.1 | 85 |
| Support | Polymerization Temperature (°C) | Activity (kg PP/mol Zr.h) | (kg/kg mol) |
|---|---|---|---|
| (1) RHA (this work) | 55 | 4142 | 81,239 |
| (2) Commercial micro-sized silica [17] | 55 | 440 | 72,470 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.-T.; Yang, C.-N. Propylene Polymerization Catalyzed by Metallocene/Methylaluminoxane Systems on Rice Husk Ash. Molecules 2019, 24, 1467. https://doi.org/10.3390/molecules24081467
Li K-T, Yang C-N. Propylene Polymerization Catalyzed by Metallocene/Methylaluminoxane Systems on Rice Husk Ash. Molecules. 2019; 24(8):1467. https://doi.org/10.3390/molecules24081467
Chicago/Turabian StyleLi, Kuo-Tseng, and Cheng-Ni Yang. 2019. "Propylene Polymerization Catalyzed by Metallocene/Methylaluminoxane Systems on Rice Husk Ash" Molecules 24, no. 8: 1467. https://doi.org/10.3390/molecules24081467
APA StyleLi, K.-T., & Yang, C.-N. (2019). Propylene Polymerization Catalyzed by Metallocene/Methylaluminoxane Systems on Rice Husk Ash. Molecules, 24(8), 1467. https://doi.org/10.3390/molecules24081467






