Structure–Activity Relationships of the Tetrapeptide Ac-His-Arg-(pI)DPhe-Tic-NH2 at the Mouse Melanocortin Receptors: Modification at the (pI)DPhe Position Leads to mMC3R Versus mMC4R Selective Ligands
Abstract
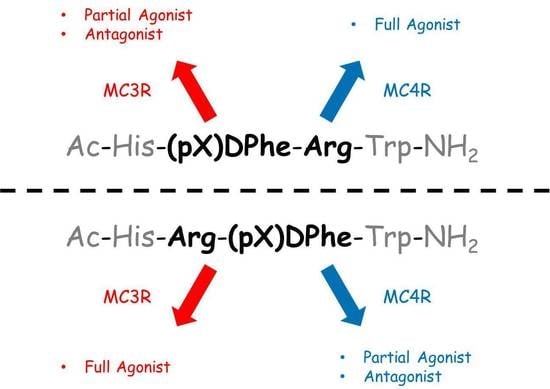
1. Introduction
2. Results
Peptide Synthesis and Pharmacological Evaluation
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Peptide Synthesis
4.3. AlphaScreen Bioassay
4.4. β-Galactosidase Assay
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chhajlani, V.; Muceniece, R.; Wikberg, J.E. Molecular cloning of a novel human melanocortin receptor. Biochem. Biophys. Res. Commun. 1993, 195, 866–873. [Google Scholar] [CrossRef]
- Chhajlani, V.; Wikberg, J.E. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992, 309, 417–420. [Google Scholar] [CrossRef]
- Mountjoy, K.G.; Robbins, L.S.; Mortrud, M.T.; Cone, R.D. The cloning of a family of genes that encode the melanocortin receptors. Science 1992, 257, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Roselli-Rehfuss, L.; Mountjoy, K.G.; Robbins, L.S.; Mortrud, M.T.; Low, M.J.; Tatro, J.B.; Entwistle, M.L.; Simerly, R.B.; Cone, R.D. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl. Acad. Sci. USA 1993, 90, 8856–8860. [Google Scholar] [CrossRef] [PubMed]
- Gantz, I.; Konda, Y.; Tashiro, T.; Shimoto, Y.; Miwa, H.; Munzert, G.; Watson, S.J.; DelValle, J.; Yamada, T. Molecular cloning of a novel melanocortin receptor. J. Biol. Chem. 1993, 268, 8246–8250. [Google Scholar] [PubMed]
- Gantz, I.; Miwa, H.; Konda, Y.; Shimoto, Y.; Tashiro, T.; Watson, S.J.; DelValle, J.; Yamada, T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 1993, 268, 15174–15179. [Google Scholar] [PubMed]
- Gantz, I.; Shimoto, Y.; Konda, Y.; Miwa, H.; Dickinson, C.J.; Yamada, T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem. Biophys. Res. Commun. 1994, 200, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Griffon, N.; Mignon, V.; Facchinetti, P.; Diaz, J.; Schwartz, J.C.; Sokoloff, P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem. Biophys. Res. Commun. 1994, 200, 1007–1014. [Google Scholar] [CrossRef]
- Haynes, R.C. The activation of adrenal phosphorylase by the adreno-corticotropic hormone. J. Biol. Chem. 1958, 233, 1220–1222. [Google Scholar] [PubMed]
- Schioth, H.B.; Chhajlani, V.; Muceniece, R.; Klusa, V.; Wikberg, J.E. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 1996, 59, 797–801. [Google Scholar] [CrossRef]
- Butler, A.A.; Kesterson, R.A.; Khong, K.; Cullen, M.J.; Pelleymounter, M.A.; Dekoning, J.; Baetscher, M.; Cone, R.D. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 2000, 141, 3518–3521. [Google Scholar] [CrossRef]
- Chen, A.S.; Marsh, D.J.; Trumbauer, M.E.; Frazier, E.G.; Guan, X.M.; Yu, H.; Rosenblum, C.I.; Vongs, A.; Feng, Y.; Cao, L.H.; et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000, 26, 97–102. [Google Scholar] [CrossRef]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef]
- Fan, W.; Boston, B.A.; Kesterson, R.A.; Hruby, V.J.; Cone, R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 1997, 385, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kelly, M.A.; Opitz-Araya, X.; Thomas, R.E.; Low, M.J.; Cone, R.D. Exocrine gland dysfunction in MC5-R-deficient mice: Evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 1997, 91, 789–798. [Google Scholar] [CrossRef]
- Nakanishi, S.; Inoue, A.; Kita, T.; Nakamura, M.; Chang, A.C.; Cohen, S.N.; Numa, S. Nucleotide sequence of cloned cDNA for bovine corticotropin-β-lipotropin precursor. Nature 1979, 278, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Eipper, B.A.; Mains, R.E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr. Rev. 1980, 1, 1–27. [Google Scholar] [CrossRef]
- Smith, A.I.; Funder, J.W. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr. Rev. 1988, 9, 159–179. [Google Scholar] [CrossRef]
- Hruby, V.J.; Wilkes, B.C.; Hadley, M.E.; Al-Obeidi, F.; Sawyer, T.K.; Staples, D.J.; Devaux, A.E.; Dym, O.; Castrucci, A.M.D.; Hintz, M.F.; et al. α-Melanotropin: The minimal active sequence in the frog-skin bioassay. J. Med. Chem. 1987, 30, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Castrucci, A.M.; Hadley, M.E.; Sawyer, T.K.; Wilkes, B.C.; Al-Obeidi, F.; Staples, D.J.; de Vaux, A.E.; Dym, O.; Hintz, M.F.; Riehm, J.P.; et al. α-Melanotropin: The minimal active sequence in the lizard skin bioassay. Gen. Comp. Endocrinol. 1989, 73, 157–163. [Google Scholar] [CrossRef]
- Kiefer, L.L.; Veal, J.M.; Mountjoy, K.G.; Wilkinson, W.O. Melanocortin receptor binding determinants in the agouti protein. Biochemistry 1998, 37, 991–997. [Google Scholar] [CrossRef]
- Tota, M.R.; Smith, T.S.; Mao, C.; MacNeil, T.; Mosley, R.T.; Van der Ploeg, L.H.T.; Fong, T.M. Molecular interaction of agouti protein and agouti-related protein with human melanocortin receptors. Biochemistry 1999, 38, 897–904. [Google Scholar] [CrossRef]
- Irani, B.G.; Xiang, Z.M.; Yarandi, H.N.; Holder, J.R.; Moore, M.C.; Bauzo, R.M.; Proneth, B.; Shaw, A.M.; Millard, W.J.; Chambers, J.B.; et al. Implication of the melanocortin-3 receptor in the regulation of food intake. Eur. J. Pharmacol. 2011, 660, 80–87. [Google Scholar] [CrossRef]
- Atalayer, D.; Robertson, K.L.; Haskell-Luevano, C.; Andreasen, A.; Rowland, N.E. Food demand and meal size in mice with single or combined disruption of melanocortin type 3 and 4 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1667–R1674. [Google Scholar] [CrossRef][Green Version]
- Rowland, N.E.; Fakhar, K.J.; Robertson, K.L.; Haskell-Luevano, C. Effect of serotonergic anorectics on food intake and induction of Fos in brain of mice with disruption of melanocortin 3 and/or 4 receptors. Pharmacol. Biochem. Behav. 2010, 97, 107–111. [Google Scholar] [CrossRef][Green Version]
- Rowland, N.E.; Schaub, J.W.; Robertson, K.L.; Andreasen, A.; Haskell-Luevano, C. Effect of MTII on food intake and brain c-Fos in melanocortin-3, melanocortin-4, and double MC3 and MC4 receptor knockout mice. Peptides 2010, 31, 2314–2317. [Google Scholar] [CrossRef][Green Version]
- Brown, K.S.; Gentry, R.M.; Rowland, N.E. Central injection in rats of α-melanocyte-stimulating hormone analog: Effects on food intake and brain Fos. Regul. Pept. 1998, 78, 89–94. [Google Scholar] [CrossRef]
- Ebihara, K.; Ogawa, Y.; Katsuura, G.; Numata, Y.; Masuzaki, H.; Satoh, N.; Tamaki, M.; Yoshioka, T.; Hayase, M.; Matsuoka, N.; et al. Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes 1999, 48, 2028–2033. [Google Scholar] [CrossRef]
- Hinney, A.; Volckmar, A.L.; Knoll, N. Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Prog. Mol. Biol. Transl. Sci. 2013, 114, 147–191. [Google Scholar]
- Greenfield, J.R.; Miller, J.W.; Keogh, J.M.; Henning, E.; Satterwhite, J.H.; Cameron, G.S.; Astruc, B.; Mayer, J.P.; Brage, S.; See, T.C.; et al. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 2009, 360, 44–52. [Google Scholar] [CrossRef]
- Dorr, R.T.; Lines, R.; Levine, N.; Brooks, C.; Xiang, L.; Hruby, V.J.; Hadley, M.E. Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sci. 1996, 58, 1777–1784. [Google Scholar] [CrossRef]
- Lansdell, M.I.; Hepworth, D.; Calabrese, A.; Brown, A.D.; Blagg, J.; Burring, D.J.; Wilson, P.; Fradet, D.; Brown, T.B.; Quinton, F.; et al. Discovery of a Selective Small-Molecule Melanocortin-4 Receptor Agonist with Efficacy in a Pilot Study of Sexual Dysfunction in Humans. J. Med. Chem. 2010, 53, 3183–3197. [Google Scholar] [CrossRef] [PubMed]
- Hadley, M.E. Discovery that a melanocortin regulates sexual functions in male and female humans. Peptides 2005, 26, 1687–1689. [Google Scholar] [CrossRef]
- Kuhnen, P.; Clement, K.; Wiegand, S.; Blankenstein, O.; Gottesdiener, K.; Martini, L.L.; Mai, K.; Blume-Peytavi, U.; Gruters, A.; Krude, H. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 2016, 375, 240–246. [Google Scholar] [CrossRef]
- Clement, K.; Biebermann, H.; Farooqi, I.S.; Van der Ploeg, L.; Wolters, B.; Poitou, C.; Puder, L.; Fiedorek, F.; Gottesdiener, K.; Kleinau, G.; et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 2018, 24, 551–555. [Google Scholar] [CrossRef]
- Ni, X.P.; Butler, A.A.; Cone, R.D.; Humphreys, M.H. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J. Hypertens. 2006, 24, 2239–2246. [Google Scholar] [CrossRef]
- Martin, W.J.; McGowan, E.; Cashen, D.E.; Gantert, L.T.; Drisko, J.E.; Hom, G.J.; Nargund, R.; Sebhat, I.; Howard, A.D.; Van der Ploeg, L.H.T.; et al. Activation of melanocortin MC4 receptors increases erectile activity in rats ex copula. Eur. J. Pharmacol. 2002, 454, 71–79. [Google Scholar] [CrossRef]
- Van der Ploeg, L.H.T.; Martin, W.J.; Howard, A.D.; Nargund, R.P.; Austin, C.P.; Guan, X.M.; Drisko, J.; Cashen, D.; Sebhat, I.; Patchett, A.A.; et al. A role for the melanocortin 4 receptor in sexual function. Proc. Natl. Acad. Sci. USA 2002, 99, 11381–11386. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tao, Y.X. Mutations in melanocortin-3 receptor gene and human obesity. Prog. Mol. Biol. Transl. Sci. 2016, 140, 97–129. [Google Scholar] [PubMed]
- Doering, S.R.; Freeman, K.T.; Schnell, S.M.; Haslach, E.M.; Dirain, M.; Debevec, G.; Geer, P.; Santos, R.G.; Giulianotti, M.A.; Pinilla, C.; et al. Discovery of mixed pharmacology melanocortin-3 agonists and melanocortin-4 receptor tetrapeptide antagonist compounds (TACOs) based on the sequence Ac-Xaa(1)-Arg-(pI)DPhe-Xaa(4)-NH2. J. Med. Chem. 2017, 60, 4342–4357. [Google Scholar] [CrossRef]
- Fleming, K.A.; Freeman, K.T.; Powers, M.D.; Santos, R.G.; Debevec, G.; Giulianotti, M.A.; Houghten, R.A.; Doering, S.R.; Pinilla, C.; Haskell-Luevano, C. Discovery of polypharmacological melanocortin-3 and -4 receptor probes and identification of a 100-fold selective nM MC3R agonist versus a µM MC4R partial agonist. J. Med. Chem. 2019, 62, 2738–2749. [Google Scholar] [CrossRef]
- Proneth, B.; Pogozheva, I.D.; Portillo, F.P.; Mosberg, H.I.; Haskell-Luevano, C. Melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH(2) modified at the para position of the benzyl side chain (DPhe): Importance for mouse melanocortin-3 receptor agonist versus antagonist activity. J. Med. Chem. 2008, 51, 5585–5593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holder, J.R.; Bauzo, R.M.; Xiang, Z.M.; Haskell-Luevano, C. Structure-activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the mouse melanocortin receptors: Part 2 modifications at the Phe position. J. Med. Chem. 2002, 45, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Carpino, L.A.; Han, G.Y. 9-Fluorenylmethoxycarbonyl function, a new base-sensitive amino-protecting group. J. Am. Chem. Soc. 1970, 92, 5748–5749. [Google Scholar] [CrossRef]
- Carpino, L.A.; Han, G.Y. The 9-fluorenylmethoxycarbonyl amino-protecting group. J. Org. Chem. 1972, 37, 3404–3409. [Google Scholar] [CrossRef]
- Chen, W.B.; Shields, T.S.; Stork, P.J.S.; Cone, R.D. A colorimetric assay for measuring activation of Gs- and Gq-coupled signaling pathways. Anal. Biochem. 1995, 226, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Ericson, M.D.; Schnell, S.M.; Freeman, K.T.; Haskell-Luevano, C. A fragment of the Escherichia coli ClpB heat-shock protein is a micromolar melanocortin 1 receptor agonist. Bioorg. Med. Chem. Lett. 2015, 25, 5306–5308. [Google Scholar] [CrossRef] [PubMed]
- Tala, S.R.; Schnell, S.M.; Haskell-Luevano, C. Microwave-assisted solid-phase synthesis of side-chain to side-chain lactam-bridge cyclic peptides. Bioorg. Med. Chem. Lett. 2015, 25, 5708–5711. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tala, S.R.; Flores, V.; Freeman, K.; Haskell-Luevano, C. Synthesis and pharmacology of α/β3-peptides based on the melanocortin agonist Ac-His-DPhe-Arg-Trp-NH2 sequence. ACS Med. Chem. Lett. 2015, 6, 568–572. [Google Scholar] [CrossRef]
- Sawyer, T.K.; Sanfilippo, P.J.; Hruby, V.J.; Engel, M.H.; Heward, C.B.; Burnett, J.B.; Hadley, M.E. 4-Norleucine, 7-D-phenylalanine-α-melanocyte-stimulating hormone—A highly potent α-melanotropin with ultralong biological-activity. Proc. Natl. Acad. Sci. USA 1980, 77, 5754–5758. [Google Scholar] [CrossRef]
- Haskell-Luevano, C.; Holder, J.R.; Monck, E.K.; Bauzo, R.M. Characterization of melanocortin NDP-MSH agonist peptide fragments at the mouse central and peripheral melanocortin receptors. J. Med. Chem. 2001, 44, 2247–2252. [Google Scholar] [CrossRef]
- Schild, H.O. pA, a new scale for the measurement of drug antagonism. Br. J. Pharmacol. Chemother. 1947, 2, 189–206. [Google Scholar] [CrossRef]
- Haghighi, S.M.; Zhou, Y.; Dai, J.X.; Sawyer, J.R.; Hruby, V.J.; Cai, M.Y. Replacement of Arg with Nle and modified D-Phe in the core sequence of MSHs, Ac-His-D-Phe-Arg-Trp-NH2, leads to hMC1R selectivity and pigmentation. Eur. J. Med. Chem. 2018, 151, 815–823. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Christensen, T. Qualitative test for monitoring coupling completeness in solid-phase peptide-synthesis using chloranil. Acta Chem. Scand. Ser. B 1979, 33, 763–766. [Google Scholar] [CrossRef]
- Lensing, C.J.; Freeman, K.T.; Schnell, S.M.; Adank, D.N.; Speth, R.C.; Haskell-Luevano, C. An in vitro and in vivo investigation of bivalent ligands that display preferential binding and functional activity for different melanocortin receptor homodimers. J. Med. Chem. 2016, 59, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
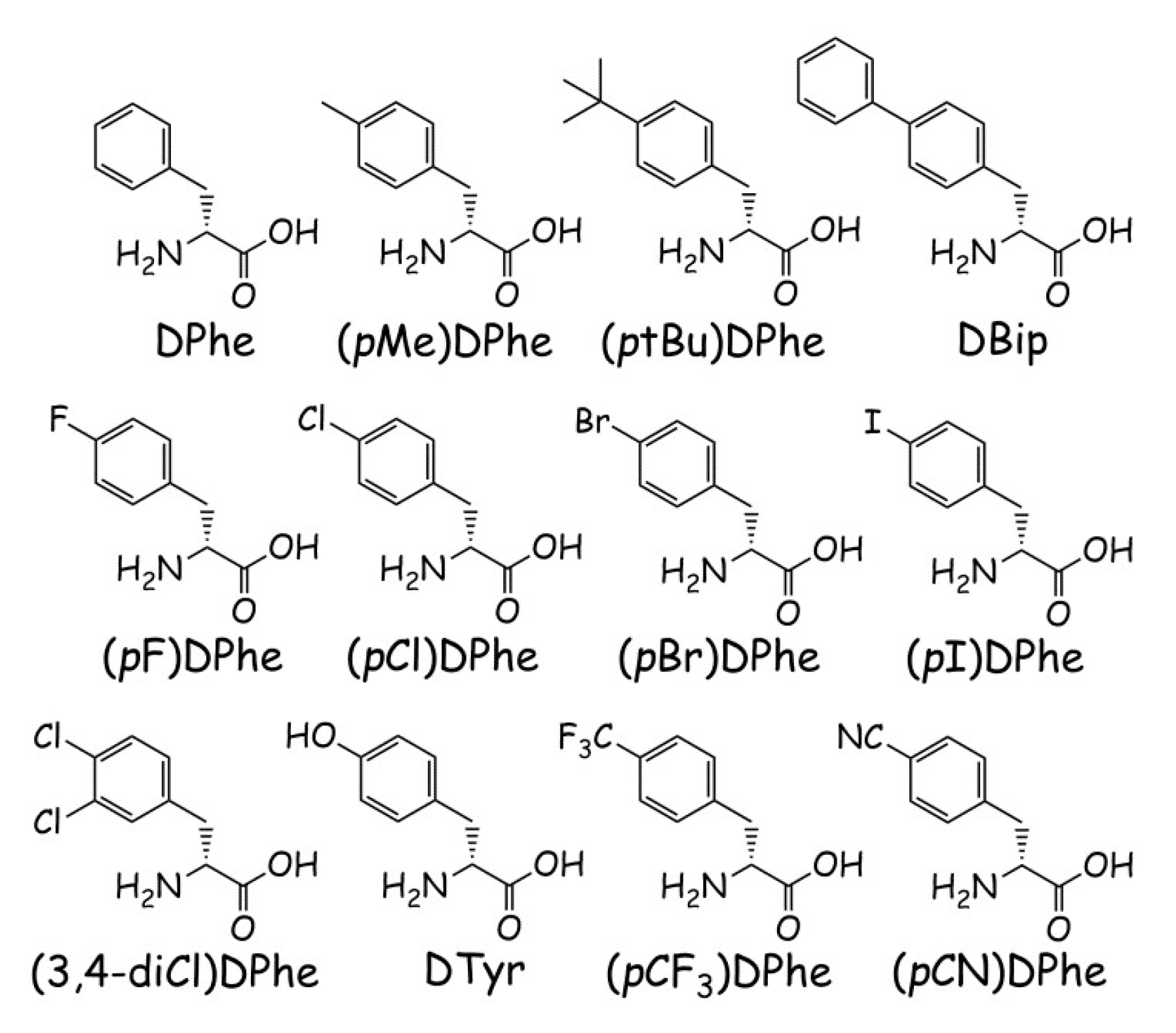
| Peptide | Sequence | Retention Time (min) | M (Calculated) | M + H (Observed) | Purity (%) | |
|---|---|---|---|---|---|---|
| System 1 | System 2 | |||||
| KNS2-153 | Ac-His-DPhe-Arg-Trp-NH2 | 10.1 | 15.6 | 685.3 | 686.4 | >98 |
| KNS2-22-4 | Ac-His-Arg-(pI)DPhe-Tic-NH2 | 14.9 | 23.6 | 784.2 | 785.3 | >97 |
| KNS2-22-3 | Ac-His-Arg-(pBr)DPhe-Tic-NH2 | 14.9 | 23.2 | 736.3, 738.3 b | 737.3, 739.3 b | >98 |
| KNS2-22-1 | Ac-His-Arg-(pCl)DPhe-Tic-NH2 | 14.6 | 22.7 | 692.3 | 693.5 | >97 |
| KNS2-22-2 | Ac-His-Arg-(pF)DPhe-Tic-NH2 | 13.5 | 20.8 | 676.3 | 677.5 | >95 |
| KNS3-10 | Ac-His-Arg-DPhe-Tic-NH2 | 12.8 | 20.1 | 658.3 | 659.5 | >99 |
| KNS2-23-4 | Ac-His-Arg-(3,4-diCl)DPhe-Tic-NH2 | 15.6 | 24.2 | 726.3 | 727.4 | >97 |
| KNS2-23-7 | Ac-His-Arg-(pMe)DPhe-Tic-NH2 | 14.3 | 22.3 | 672.4 | 673.5 | >97 |
| KNS2-23-6 | Ac-His-Arg-(pCF3)DPhe-Tic-NH2 | 15.0 | 23.4 | 726.7 | 727.5 | >98 |
| KNS2-23-3 | Ac-His-Arg-(ptBu)DPhe-Tic-NH2 | 17.5 | 26.5 | 714.4 | 715.4 | >95 |
| KNS2-23-1 | Ac-His-Arg-DBip-Tic-NH2 | 16.7 | 25.9 | 734.3 | 735.5 | >96 |
| KNS2-23-9 | Ac-His-Arg-DTyr-Tic-NH2 | 10.5 | 15.0 | 674.3 | 675.4 | >96 |
| KNS2-23-8 | Ac-His-Arg-(pCN)DPhe-Tic-NH2 | 11.5 | 17.2 | 683.3 | 684.3 | >97 |
| Peptide | Sequence | mMC1R EC50 (nM) |
|---|---|---|
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.009 ± 0.002 |
| KNS2-153 | Ac-His-DPhe-Arg-Trp-NH2 | 10 ± 3 |
| KNS2-22-4 | Ac-His-Arg-(pI)DPhe-Tic-NH2 | 0.7 ± 0.2 |
| KNS2-22-3 | Ac-His-Arg-(pBr)DPhe-Tic-NH2 | 0.7 ± 0.3 |
| KNS2-22-1 | Ac-His-Arg-(pCl)DPhe-Tic-NH2 | 0.8 ± 0.2 |
| KNS2-22-2 | Ac-His-Arg-(pF)DPhe-Tic-NH2 | 1.8 ± 0.7 |
| KNS3-10 | Ac-His-Arg-DPhe-Tic-NH2 | 4.6 ± 0.4 |
| KNS2-23-4 | Ac-His-Arg-(3,4-diCl)DPhe-Tic-NH2 | 5 ± 2 |
| KNS2-23-7 | Ac-His-Arg-(pMe)DPhe-Tic-NH2 | 1.0 ± 0.3 |
| KNS2-23-6 | Ac-His-Arg-(pCF3)DPhe-Tic-NH2 | 5 ± 1 |
| KNS2-23-3 | Ac-His-Arg-(ptBu)DPhe-Tic-NH2 | 9 ± 3 |
| KNS2-23-1 | Ac-His-Arg-DBip-Tic-NH2 | 0.6 ± 0.1 |
| KNS2-23-9 | Ac-His-Arg-DTyr-Tic-NH2 | 40 ± 10 |
| KNS-2-23-8 | Ac-His-Arg-(pCN)DPhe-Tic-NH2 | 27 ± 6 |
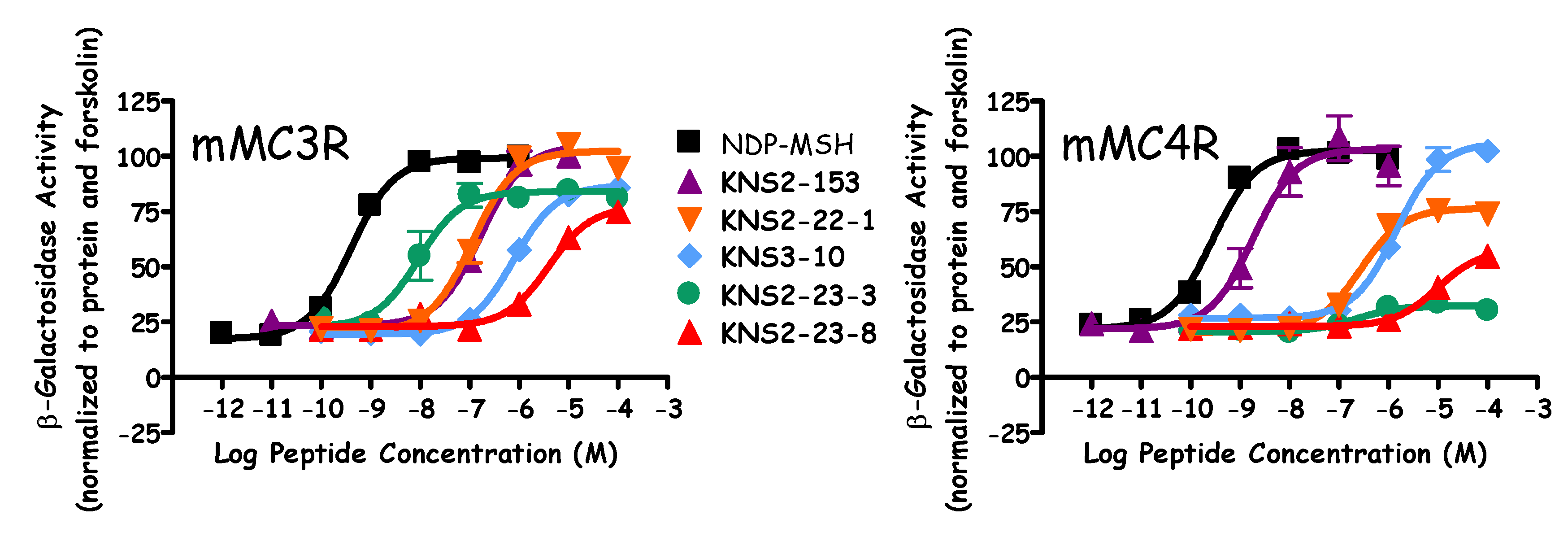
| Peptide | Sequence | mMC3R EC50 (nM) | mMC4R | mMC5R EC50 (nM) | |
|---|---|---|---|---|---|
| EC50 (nM) | pA2 | ||||
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.52 ± 0.05 | 0.32 ± 0.02 | - | 3.4 ± 0.7 |
| KNS2-153 | Ac-His-DPhe-Arg-Trp-NH2 | 190 ± 40 | 12 ± 3 | - | 5 ± 2 |
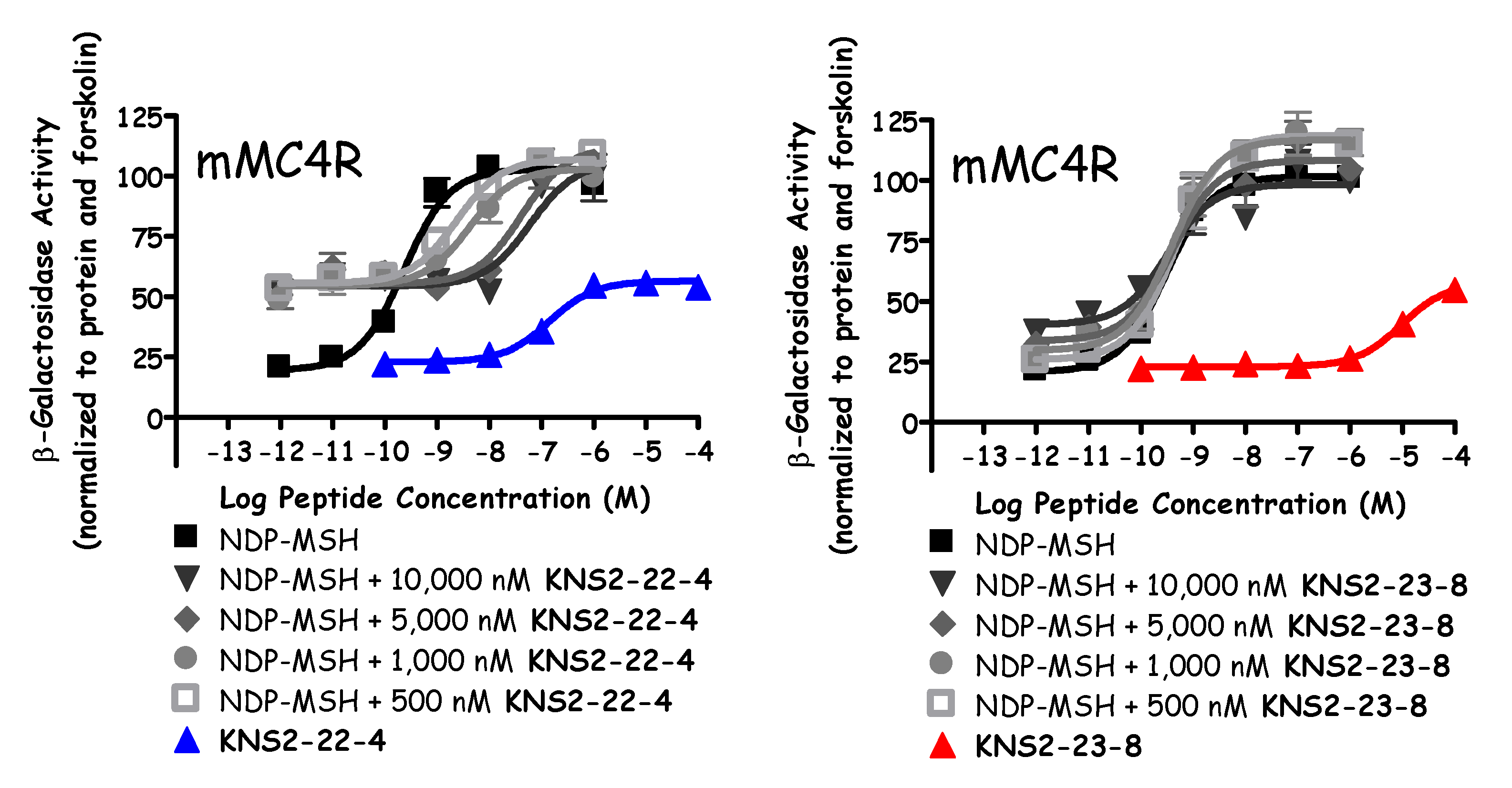
| KNS2-22-4 | Ac-His-Arg-(pI)DPhe-Tic-NH2 | 13 ± 2 | Partial Agonist 150 ± 40 (40% NDP) | 7.3 ± 0.8 | 5 ± 1 |
| KNS2-22-3 | Ac-His-Arg-(pBr)DPhe-Tic-NH2 | 90 ± 20 | Partial Agonist 290 ± 50 (55% NDP) | - | 9.7 ± 0.5 |
| KNS2-22-1 | Ac-His-Arg-(pCl)DPhe-Tic-NH2 | 120 ± 20 | Partial Agonist 280 ± 60 (70% NDP) | - | 18 ± 6 |
| KNS2-22-2 | Ac-His-Arg-(pF)DPhe-Tic-NH2 | 450 ± 70 | Partial Agonist 560 ± 60 (70% NDP) | - | 70 ± 40 |
| KNS3-10 | Ac-His-Arg-DPhe-Tic-NH2 | Partial Agonist 900 ± 200 (85% NDP) | 3000 ± 2000 | - | Partial Agonist 200 ± 30 (65% NDP) |
| KNS2-23-4 | Ac-His-Arg-(3,4-diCl)DPhe-Tic-NH2 | 400 ± 100 | >100,000 | 6.15 ± 0.05 | 70 ± 7 |
| KNS2-23-7 | Ac-His-Arg-(pMe)DPhe-Tic-NH2 | 110 ± 20 | Partial Agonist 700 ± 200 (50% NDP) | - | 17 ± 4 |
| KNS2-23-6 | Ac-His-Arg-(pCF3)DPhe-Tic-NH2 | 90 ± 30 | Partial Agonist 600 ± 300 (20% NDP) | 6.5 ± 0.2 | 13 ± 4 |
| KNS2-23-3 | Ac-His-Arg-(ptBu)DPhe-Tic-NH2 | Partial Agonist 13 ± 4 (85% NDP) | >100,000 | 6.8 ± 0.3 | 3.4 ± 0.3 |
| KNS2-23-1 | Ac-His-Arg-DBip-Tic-NH2 | 14 ± 2 | Partial Agonist 1400 ± 700 (45% NDP) | 5.9 ± 0.2 | 7.6 ± 0.7 |
| KNS2-23-9 | Ac-His-Arg-DTyr-Tic-NH2 | Partial Agonist 4200 ± 800 (85% NDP) | >100,000 | <5.5 | 1000 ± 500 |
| KNS2-23-8 | Ac-His-Arg-(pCN)DPhe-Tic-NH2 | Partial Agonist 4000 ± 1000 (75% NDP) | 40% @ 100 µM | <5.5 | 500 ± 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlasner, K.N.; Ericson, M.D.; Doering, S.R.; Freeman, K.T.; Weinrich, M.; Haskell-Luevano, C. Structure–Activity Relationships of the Tetrapeptide Ac-His-Arg-(pI)DPhe-Tic-NH2 at the Mouse Melanocortin Receptors: Modification at the (pI)DPhe Position Leads to mMC3R Versus mMC4R Selective Ligands. Molecules 2019, 24, 1463. https://doi.org/10.3390/molecules24081463
Schlasner KN, Ericson MD, Doering SR, Freeman KT, Weinrich M, Haskell-Luevano C. Structure–Activity Relationships of the Tetrapeptide Ac-His-Arg-(pI)DPhe-Tic-NH2 at the Mouse Melanocortin Receptors: Modification at the (pI)DPhe Position Leads to mMC3R Versus mMC4R Selective Ligands. Molecules. 2019; 24(8):1463. https://doi.org/10.3390/molecules24081463
Chicago/Turabian StyleSchlasner, Katherine N., Mark D. Ericson, Skye R. Doering, Katie T. Freeman, Mary Weinrich, and Carrie Haskell-Luevano. 2019. "Structure–Activity Relationships of the Tetrapeptide Ac-His-Arg-(pI)DPhe-Tic-NH2 at the Mouse Melanocortin Receptors: Modification at the (pI)DPhe Position Leads to mMC3R Versus mMC4R Selective Ligands" Molecules 24, no. 8: 1463. https://doi.org/10.3390/molecules24081463
APA StyleSchlasner, K. N., Ericson, M. D., Doering, S. R., Freeman, K. T., Weinrich, M., & Haskell-Luevano, C. (2019). Structure–Activity Relationships of the Tetrapeptide Ac-His-Arg-(pI)DPhe-Tic-NH2 at the Mouse Melanocortin Receptors: Modification at the (pI)DPhe Position Leads to mMC3R Versus mMC4R Selective Ligands. Molecules, 24(8), 1463. https://doi.org/10.3390/molecules24081463