Tests on Material Compatibility of Phase Change Materials and Selected Plastics
Abstract
1. Introduction
- Organic PCMs represented by paraffins and non-paraffins
- Inorganic PCMs describes as salt hydrates or metallics
- Eutectics characterized as a mixture of two or more components, for example, organic-organic, organic-inorganic and inorganic-inorganic eutectics
- Strength and flexibility
- Corrosion resistance
- Thermal stability in desired temperature range of use
- Protection of the environment from harmful interaction with PCMs
- Sufficient surface for heat transfer
- Structural stability and easy handling
- Availability
- Non toxicity
- Contamination of fluids and perforation in vessels and pipes;
- Reduction of container wall thickness leads to loss of mechanical strength and structural failure of breakdown;
- Mechanical damage to major components and added complexity of equipment;
- Loss of technically important surface properties of component;
- Reduced value of goods due to deterioration of appearance.
2. Results
2.1. Organic PCM results
2.2. Inorganic PCM Results
2.3. Overall Results
3. Discussion
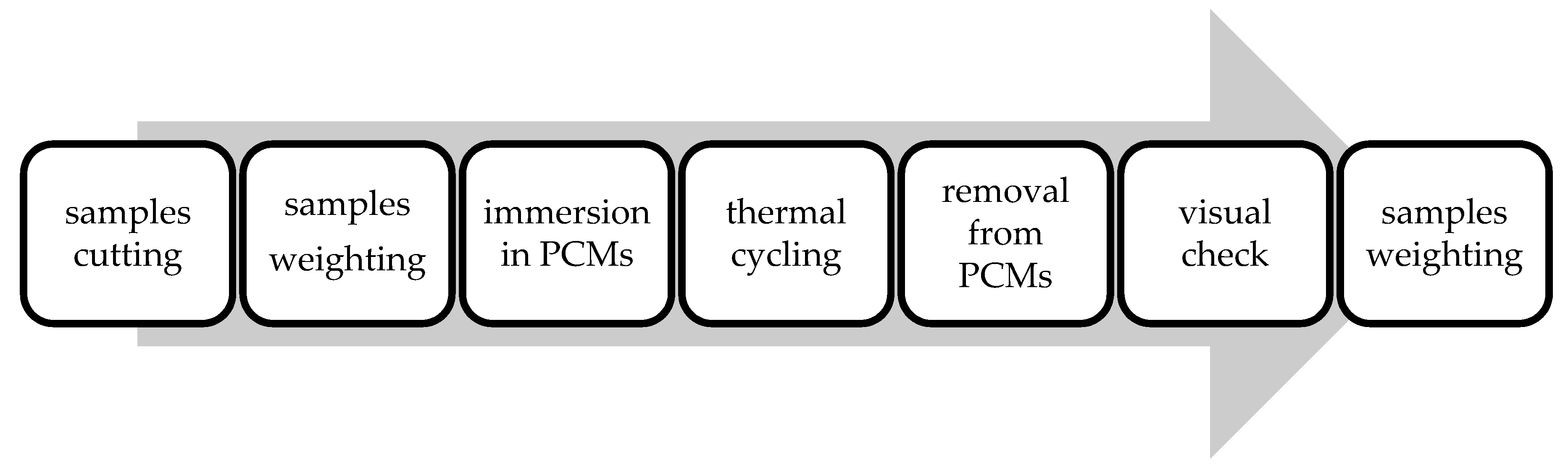
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Schnieders, J.; Feist, W.; Rongenc, L. Passive Houses for different climate zones. Energy Build. 2015, 105, 71–87. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renewable Sustainable Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Salukhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renewable Sustainable Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Milián, Y.E.; Gutiérrez, A.; Grágeda, M.; Ushak, S. A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renewable Sustainable Energy Rev. 2017, 73, 983–999. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.-J. A review on phase change materials integrated in building walls. Renewable Sustainable Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Regin, F.A.; Solanki, S.C.; Saini, J.S. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renewable Sustainable Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Ushak, S.; Marín, P.; Galazutdinova, Y.; Cabeza, L.F.; Farid, M.M.; Grágeda. Compatibility of materials for macroencapsulation of inorganic phase change materials: Experimental corrosion study. Appl. Therm. Eng. 2016, 107, 410–419. [Google Scholar] [CrossRef]
- Ferrer, G.; Solé, A.; Barreneche, C.; Martonell, I.; Cabeza, L.F. Corrosion of metal containers for use in PCM energy storage. Renewable Energy 2015, 76, 465–469. [Google Scholar] [CrossRef]
- Moreno, P.; Miró, L.; Solé, A.; Barreneche, C.; Solé, C.; Martonell, I.; Cabeza, L.F. Corrosion of metal and metal alloy containers in contact with phase change materials (PCM) for potential heating and cooling applications. Appl. Energy 2014, 125, 238–245. [Google Scholar] [CrossRef]
- Krishna, D.J.; Shinde, A. Step by Step Methodology for the Assessment of Metal Corrosion Rate with PCMs Suitable for Low Temperature Heat Storage Applications. Mater. Today: Proc. 2017, 4, 10039–10042. [Google Scholar] [CrossRef]
- Sarı, A.; Kaygusuz, K. Some fatty acids used for latent heat storage: Thermal stability and corrosion of metals with respect to thermal cycling. Renewable Energy 2003, 28, 939–948. [Google Scholar] [CrossRef]
- Browne, M.; Boyd, E.; McCormack, S.J. Investigation of the corrosive properties of phase change materials in contact with metals and plastic. Renewable Energy 2017, 108, 555–568. [Google Scholar] [CrossRef]
- Calogero, A.; Capra, G. Industrial test optimisation: Compatibility between plastics and chemical solutions. Polym. Test. 2001, 20, 749–752. [Google Scholar] [CrossRef]
- Durbin, T.D.; Karavalakis, G.; Norbeck, J.M.; Park, C.S.; Castillo, J.; Rheem, Y.; Bumiller, K.; Yang, J.; Van, V.; Hunter, K. Material compatibility evaluation for elastomers, plastics, and metals exposed to ethanol and butanol blends. Fuel 2016, 163, 248–259. [Google Scholar] [CrossRef]
- Oró, E.; Miró, L.; Barreneche, C.; Martonell, I.; Farid, M.M.; Cabeza, L.F. Corrosion of metal and polymer containers for use in PCM cold storage. Appl. Energy 2013, 109, 449–453. [Google Scholar]
- Lázaro, A.; Zalba, B.; Bobi, M.; Castellón, C.; Cabeza, L.F. Experimental study on phase change materials and plastics compatibility. Environ. Energy Eng. 2006, 52, 804–808. [Google Scholar] [CrossRef]
- Vasu, A.; Hagos, F.Y.; Noor, M.M.; Mamat, R.; Azmi, W.H.; Abdullah, A.A.; Ibrahim, T.K. Corrosion effect of phase change materials in solar thermal energy storage application. Renewable Sustainable Energy Rev. 2017, 76, 19–33. [Google Scholar] [CrossRef]
- Castellón, C.; Martorell, I.; Cabeza, L.F.; Fernández, A.I.; Manich, A.M. Compatibility of plastic with phase change materials (PCM). Int. J. Energy Res. 2011, 35, 765–771. [Google Scholar] [CrossRef]
- Kass, M.D.; Janke, C.J.; Connatser, R.M.; Lewis, S.A.; Keiser, J.R.; Gaston, K. Compatibility Assessment of Fuel System Infrastructure Plastics with Bio-oil and Diesel Fuel. Energy Fuels 2018, 32, 542–553. [Google Scholar] [CrossRef]
- ISO 175:2010 Plastics—Methods of test for the determination of the effect of immersion in liquid chemicals. Available online: https://www.iso.org/standard/55483.html (accessed on 15 February 2019).
Sample Availability: Samples of the compounds are available from the authors. |

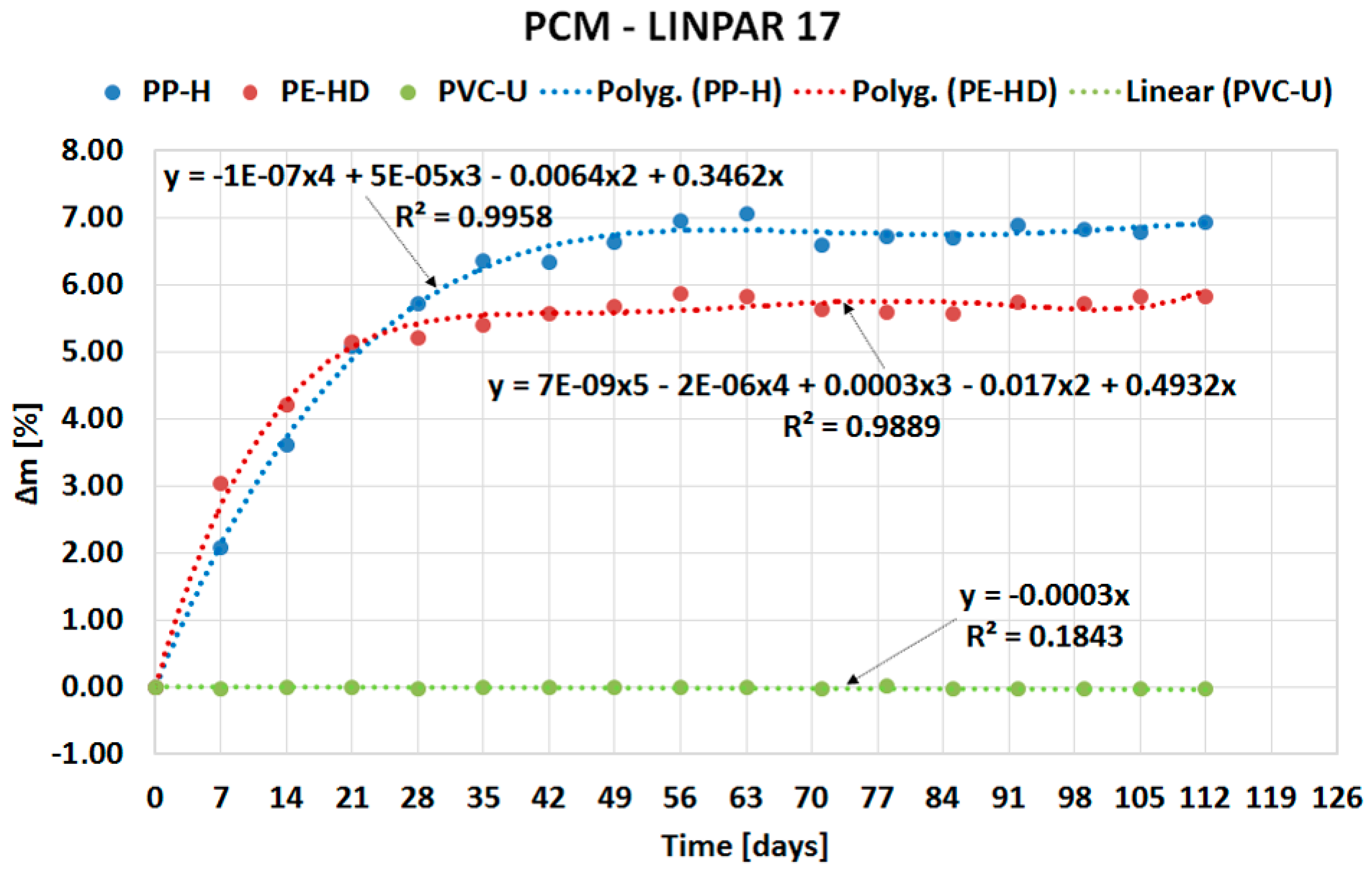
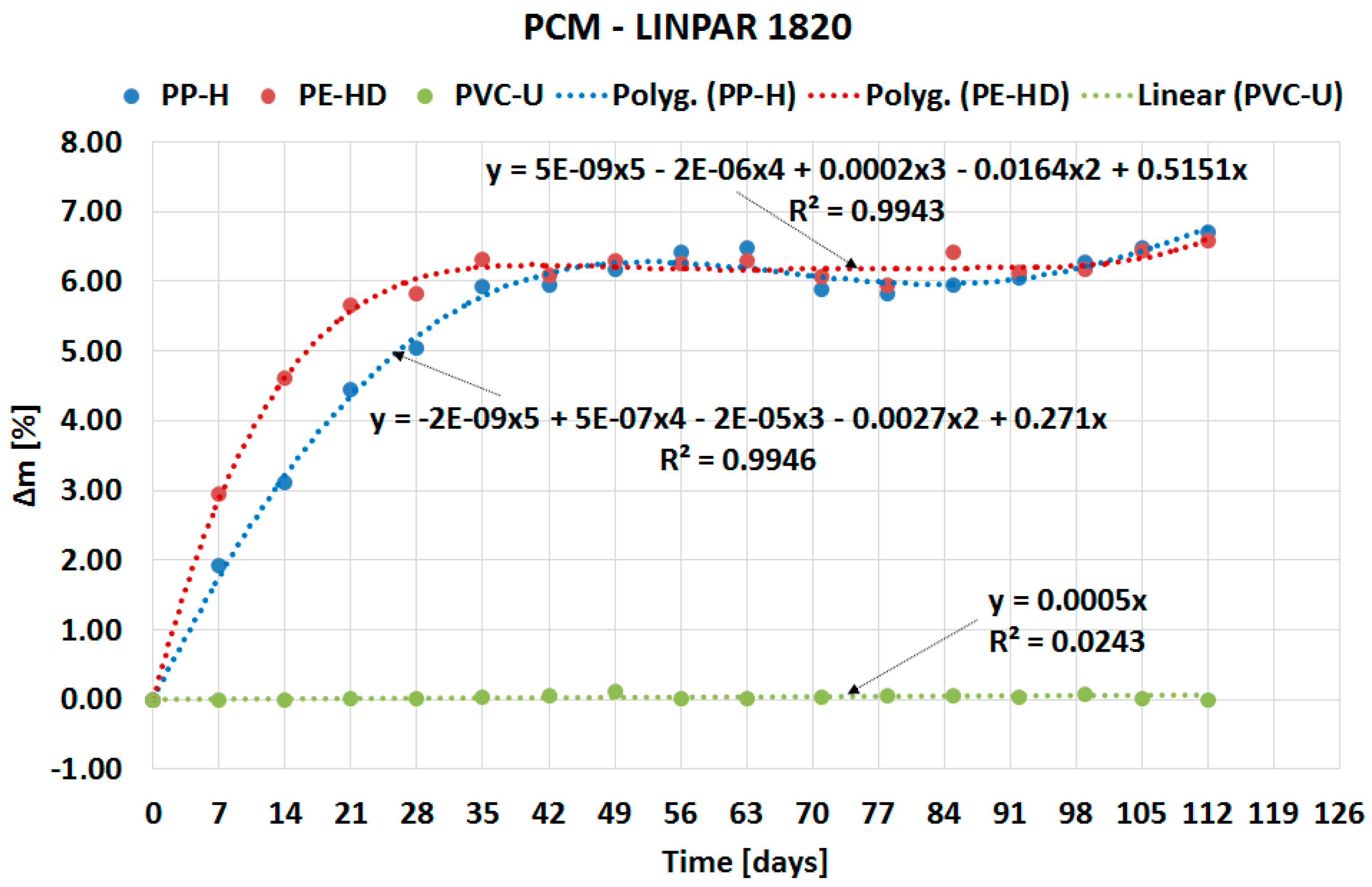
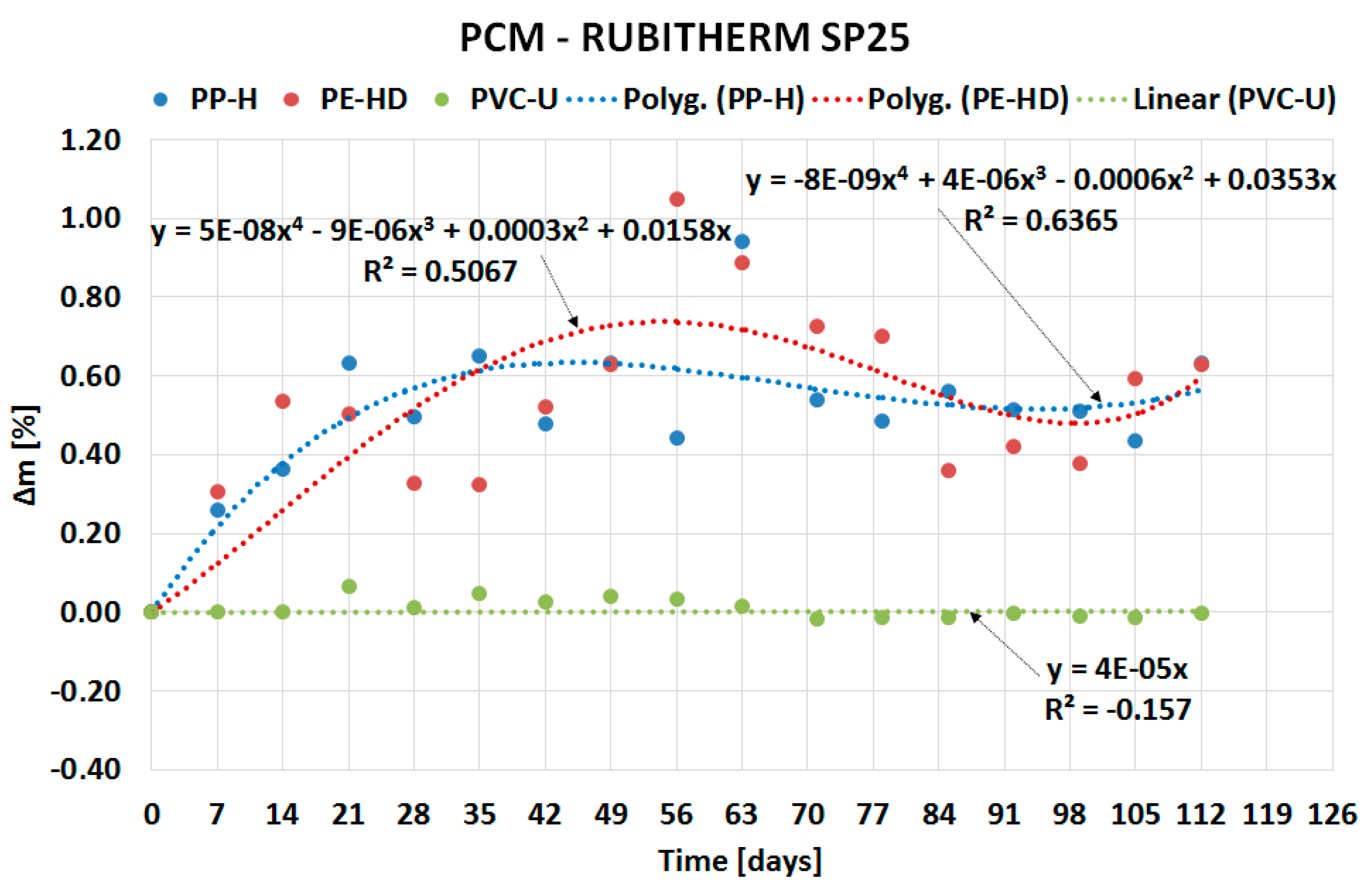
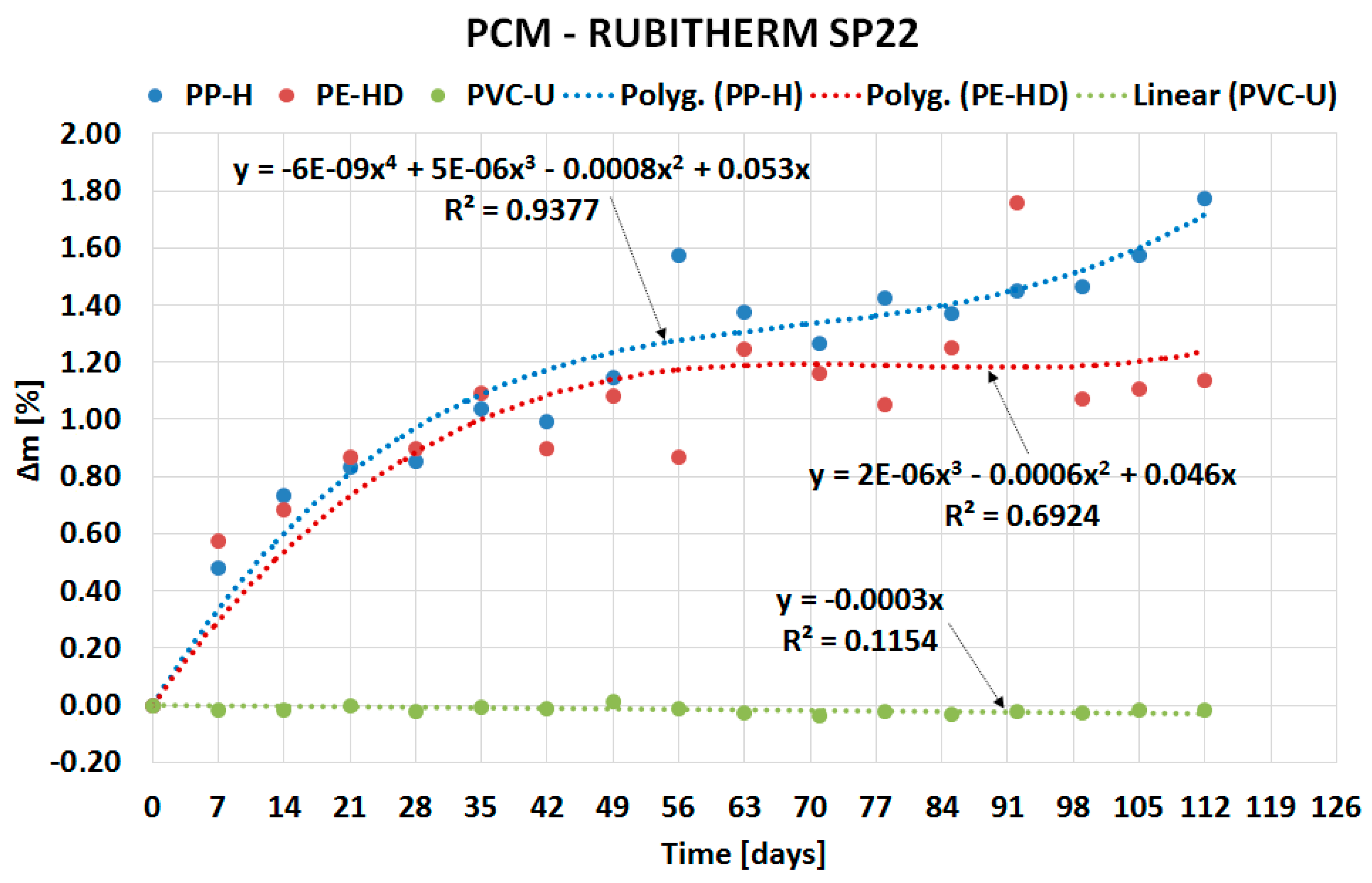
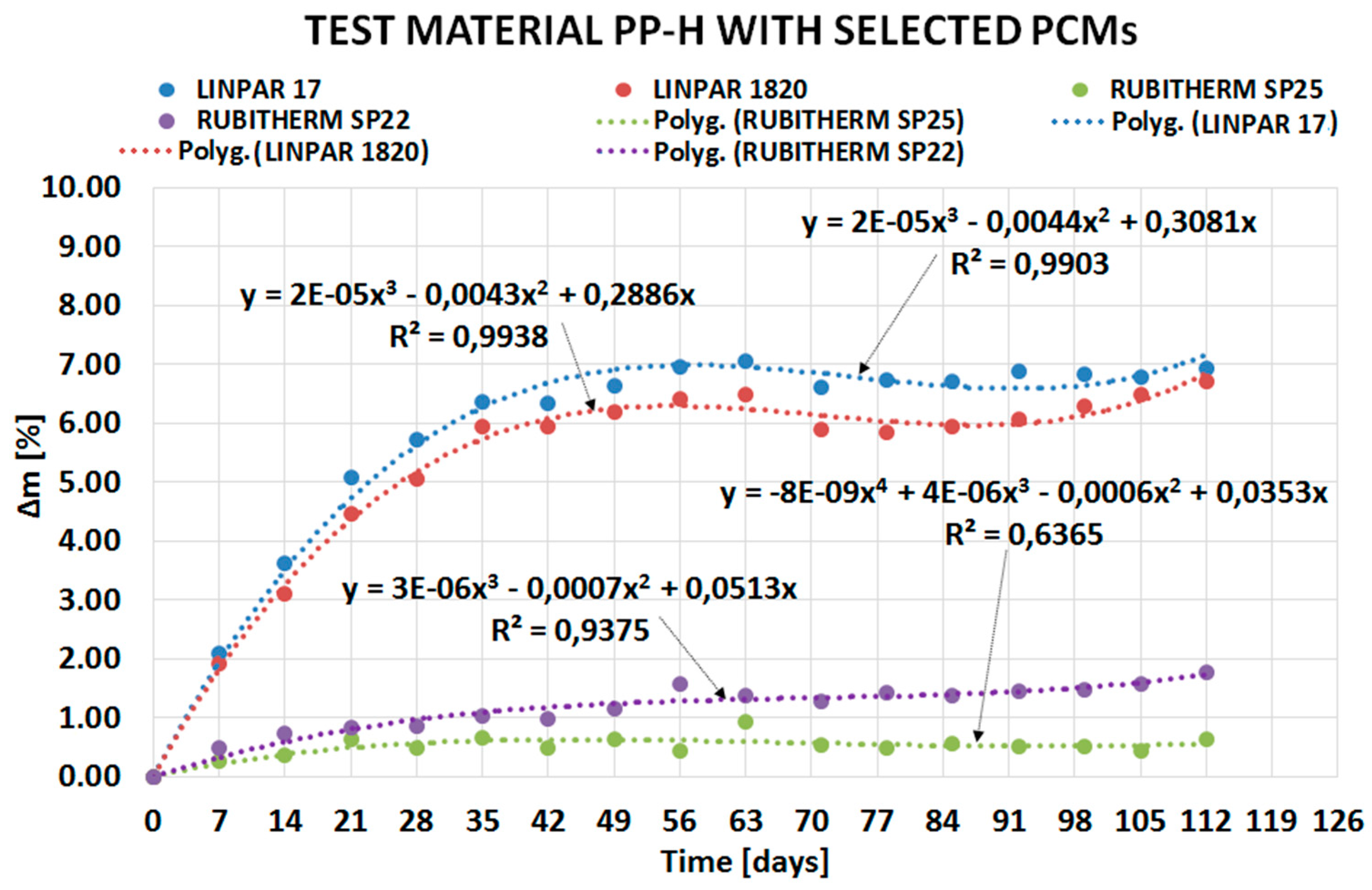

| Material (Plastics) | Time | Percentage Change in Mass Δm [%] | |||
|---|---|---|---|---|---|
| Linpar 17 | Linpar 1820 | SP25 | SP22 | ||
| PP-H | 4 weeks | 5.7175 | 5.0474 | 0.4968 | 0.8547 |
| 8 weeks | 6.9493 | 6.4214 | 0.4414 | 1.5721 | |
| 12 weeks | 6.6993 | 5.9488 | 0.5609 | 1.3682 | |
| 16 weeks | 6.9399 | 6.7153 | 0.6326 | 1.7722 | |
| PE-HD | 4 weeks | 5.2100 | 5.8225 | 0.3294 | 0.8979 |
| 8 weeks | 5.8614 | 6.2467 | 1.0484 | 0.8704 | |
| 12 weeks | 5.5740 | 6.4250 | 0.3588 | 1.2533 | |
| 16 weeks | 5.8169 | 6.5783 | 0.6281 | 1.1381 | |
| PVC-U | 4 weeks | −0.0285 | 0.0201 | 0.0107 | −0.0199 |
| 8 weeks | −0.0137 | 0.0244 | 0.0333 | −0.0106 | |
| 12 weeks | −0.0310 | 0.0624 | −0.0141 | −0.0312 | |
| 16 weeks | −0.0256 | 0.0000 | −0.0031 | −0.0172 | |
| Type | Product Name | Manufacturer | Latent Heat [J∙g−1] | Onset Temperature [°C] | Peak Temperature [°C] |
|---|---|---|---|---|---|
| inorganic | SP22 | Rubitherm | 145 | 14 | 25 |
| inorganic | SP25 | Rubitherm | 122 | 18 | 28 |
| organic | Linpar 17 | Sasol | 152 | 21 | 22 |
| organic | Linpar 1820 | Sasol | 141 | 24 | 27 |
| Material | Thermal Conductivity [W/(m·K)] | Modulus of Elasticity [MPa] | Density [g/cm3] | Melting Point [°C] |
|---|---|---|---|---|
| PP-H | 0.22 | 1350 | 0.92 | 90 |
| PVC-U | 0.20 | 3000 | 1.43 | 80 |
| PE-HD | 0.43 | 1000 | 0.95 | 75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrý, M.; Bantová, S.; Struhala, K. Tests on Material Compatibility of Phase Change Materials and Selected Plastics. Molecules 2019, 24, 1398. https://doi.org/10.3390/molecules24071398
Ostrý M, Bantová S, Struhala K. Tests on Material Compatibility of Phase Change Materials and Selected Plastics. Molecules. 2019; 24(7):1398. https://doi.org/10.3390/molecules24071398
Chicago/Turabian StyleOstrý, Milan, Sylva Bantová, and Karel Struhala. 2019. "Tests on Material Compatibility of Phase Change Materials and Selected Plastics" Molecules 24, no. 7: 1398. https://doi.org/10.3390/molecules24071398
APA StyleOstrý, M., Bantová, S., & Struhala, K. (2019). Tests on Material Compatibility of Phase Change Materials and Selected Plastics. Molecules, 24(7), 1398. https://doi.org/10.3390/molecules24071398





