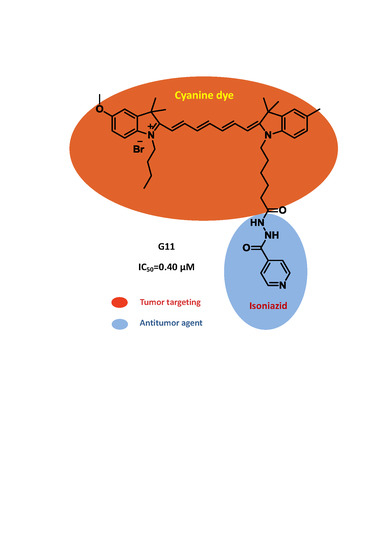
Design, Synthesis, and Evaluation of Monoamine Oxidase A Inhibitors–Indocyanine Dyes Conjugates as Targeted Antitumor Agents
Abstract
1. Introduction
2. Results and Discussion
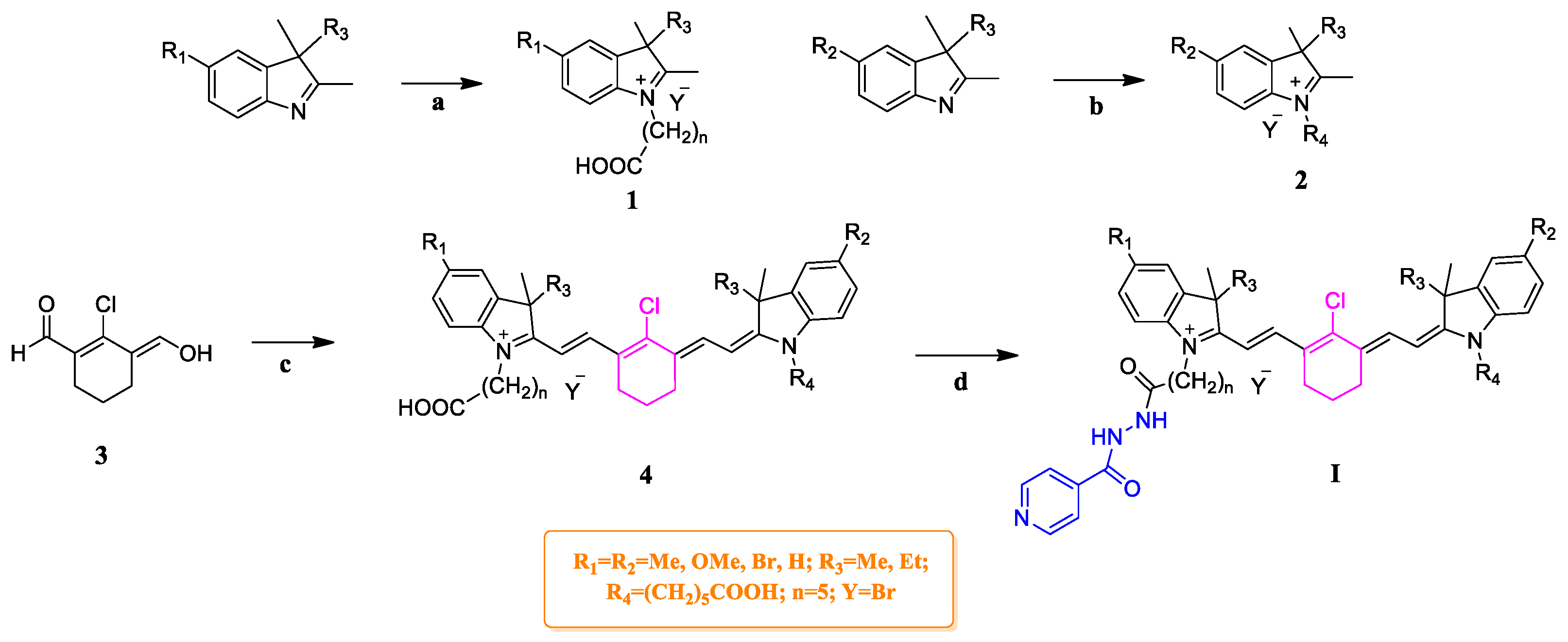
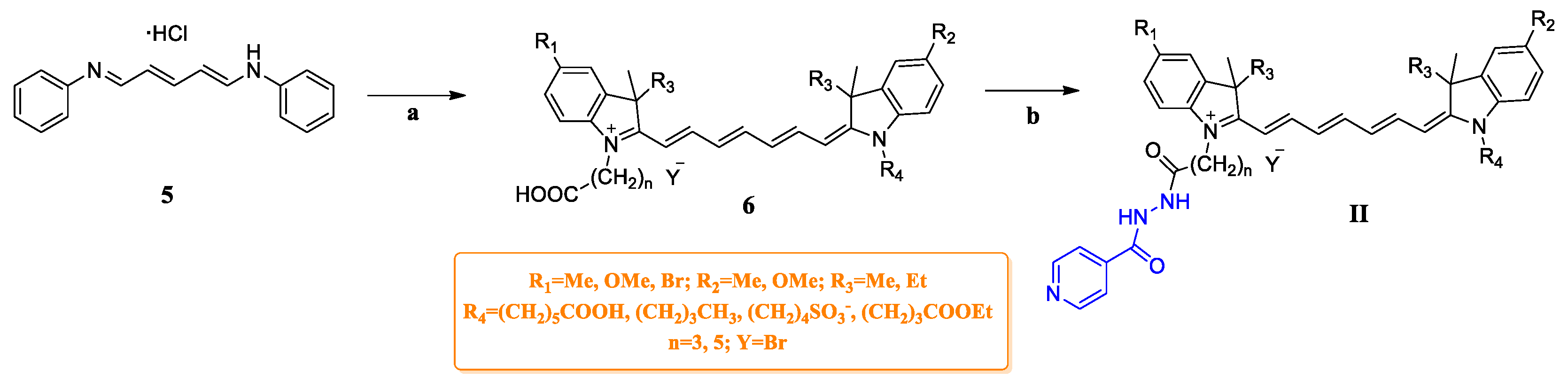
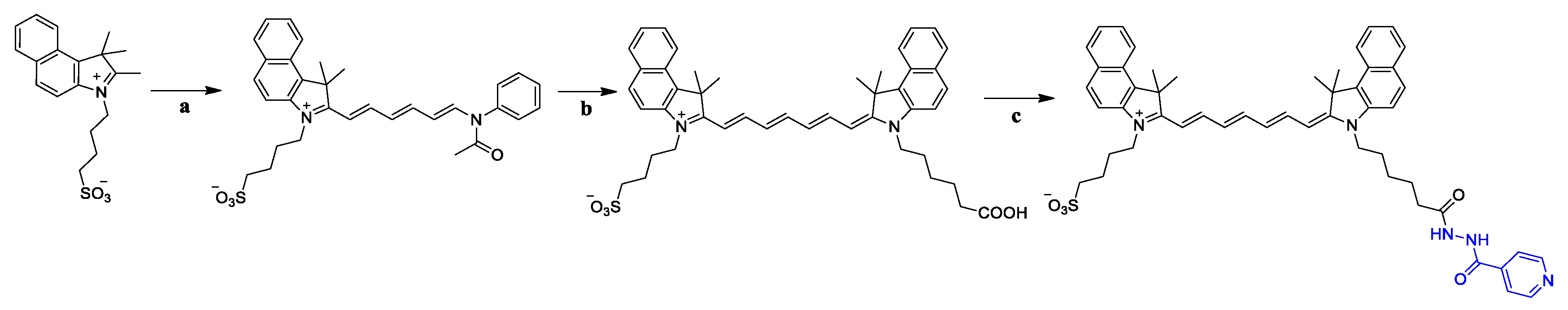
2.1. Chemistry
2.2. Cytotoxicity against PC-3
2.3. MAOA Inhibitory Activity of G10, G11, and G12
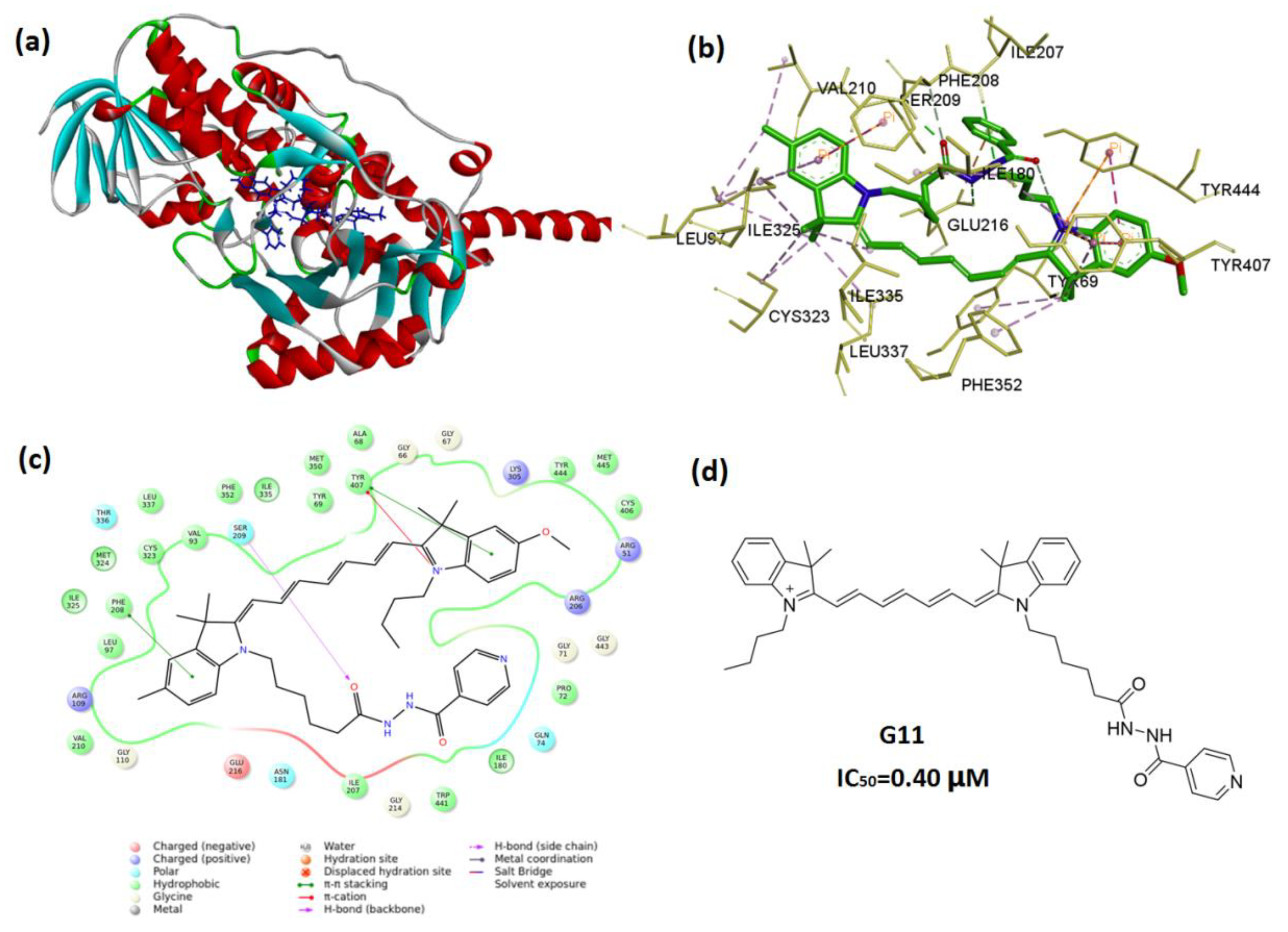
2.4. In Silico Molecular Docking
3. Materials and Methods
3.1. Materials
3.2. Molecular Docking
3.3. Cytotoxicity Evaluation
3.4. MAOA Inhibition Activity Assay
3.5. General Procedure for the Synthesis of Compounds G1–G3
3.6. General Procedure for the Synthesis of Compounds G4–G6
3.7. General Procedure for the Synthesis of Compounds G7–G13
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aggarwal, R.; Ryan, C.J. Castration-resistant prostate cancer: Targeted therapies and individualized treatment. Oncologist 2011, 16, 264–275. [Google Scholar] [CrossRef]
- Heidegger, I.; Massoner, P.; Eder, I.E.; Pircher, A.; Pichler, R.; Aigner, F.; Bektic, J.; Horninger, W.; Klocker, H. Novel therapeutic approaches for the treatment of castration-resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 2013, 138, 248–256. [Google Scholar] [CrossRef]
- Kgatle, M.M.; Kalla, A.A.; Islam, M.M.; Sathekge, M.; Moorad, R. Prostate cancer: Epigenetic alterations, risk factors, and therapy. Prostate Cancer. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stavridi, F.; Karapanagiotou, E.M.; Syrigos, K.N. Targeted therapeutic approaches for hormone-refractory prostate cancer. Cancer Treat. Rev. 2010, 36, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Mou, Q.; Ma, Y.; Zhu, X.; Yan, D. Inhibition of excessive monoamine oxidase A/B activity protects against stress-induced neuronal death in huntington disease. J. Control. Release 2016, 230, 34–44. [Google Scholar]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine oxidase: From genes to behavior. Annu Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef]
- Wu, H.F.; Chen, K.J.; Shih, C. Site-directed mutagenesis of monoamine oxidase A and B: Role of cysteines. Mol. Pharmacol. 1993, 43, 888–893. [Google Scholar] [PubMed]
- Sandi, C.; Haller, J. Stress and the social brain: Behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015, 16, 290–304. [Google Scholar] [CrossRef]
- Sun, W.; Choi, J.; Cha, Y.; Koo, J. Evaluation of the Expression of Amine Oxidase Proteins in Breast Cancer. Int. J. Mol. Sci. 2017, 18, 2775. [Google Scholar] [CrossRef]
- Liu, F.; Hu, L.; Ma, Y.; Huang, B.; Xiu, Z.; Zhang, P.; Zhou, K.; Tang, X. Increased expression of monoamine oxidase A is associated with epithelial to mesenchymal transition and clinicopathological features in non-small cell lung cancer. Oncol. Lett. 2018, 15, 3245–3251. [Google Scholar] [CrossRef]
- Li, P.C.; Siddiqi, I.N.; Mottok, A.; Loo, E.Y.; Wu, C.H.; Cozen, W.; Steidl, C.; Shih, J.C. Monoamine oxidase A is highly expressed in classical Hodgkin lymphoma. J. Pathol. 2017, 243, 220–229. [Google Scholar] [CrossRef]
- Schwartz, T.L. A neuroscientific update on monoamine oxidase and its inhibitor. CNS Spectrums 2013, 18, 25–33. [Google Scholar] [CrossRef]
- True, L.; Coleman, I.; Hawley, S.; Huang, C.Y.; Gifford, D.; Coleman, R.; Beer, T.M.; Gelmann, E.; Datta, M.; Mostaghel, E.; et al. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc. Natl. Acad. Sci. USA 2006, 103, 10991–10996. [Google Scholar] [CrossRef]
- Peehl, D.M.; Coram, M.; Khine, H.; Reese, S.; Nolley, R.; Zhao, H. The significance of monoamine oxidase-A expression in high grade prostate cancer. J. Urol. 2008, 180, 2206–2211. [Google Scholar] [CrossRef]
- Liao, C.P.; Lin, T.P.; Li, P.C.; Geary, L.A.; Chen, K.; Vaikari, V.P.; Wu, J.B.; Lin, C.H.; Gross, M.E.; Shih, J.C. Loss of MAOA in epithelia inhibits adenocarcinoma development, cell proliferation and cancer stem cells in prostate. Oncogene 2018, 37, 5175–5190. [Google Scholar] [CrossRef]
- Zhao, H.; Flamand, V.; Peehl, D.M. Anti-oncogenic and pro-differentiation effects of clorgyline, a monoamine oxidase A inhibitor, on high grade prostate cancer cells. BMC Med. Genom. 2009, 2, 55. [Google Scholar] [CrossRef]
- Xu, S.; Adisetiyo, H.; Tamura, S.; Grande, F.; Garofalo, A.; Roy-Burman, P.; Neamati, N. Dual inhibition of survivin and MAOA synergistically impairs growth of PTEN-negative prostate cancer. Br. J. Cancer 2015, 113, 242–251. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, T.P.; Gallagher, J.D.; Kushal, S.; Chung, L.W.; Zhau, H.E.; Olenyuk, B.Z.; Shih, J.C. Monoamine oxidase A inhibitor-near-infrared dye conjugate reduces prostate tumor growth. J. Am. Chem. Soc. 2015, 137, 2366–2374. [Google Scholar] [CrossRef]
- Flamand, V.; Zhao, H.; Peehl, D.M. Targeting monoamine oxidase A in advanced prostate cancer. J. Cancer Res. Clin. 2010, 136, 1761–1771. [Google Scholar] [CrossRef]
- Alan, S.; Waggoner, P.P. Cyanine Dye as Labeling Reagents for Detection of Biological and othEr Materials by Luminescence Methods. U.S. Patent 5627027, 6 May 1997. [Google Scholar]
- Chen, X.; Peng, X.; Cui, A.; Wang, B.; Wang, L.; Zhang, R. Photostabilities of novel heptamethine 3H-indolenine cyanine dyes with different N-substituents. J. Photochem. Photobiol. A 2006, 181, 79–85. [Google Scholar] [CrossRef]
- Song, F.; Peng, X.; Lu, E.; Zhang, R.; Chen, X.; Song, B. Syntheses, spectral properties and photostabilities of novel water-soluble near-infrared cyanine dyes. J. Photochem. Photobiol. A 2004, 168, 53–57. [Google Scholar] [CrossRef]
- Khemthongcharoen, N.; Jolivot, R.; Rattanavarin, S.; Piyawattanametha, W. Advances in imaging probes and optical microendoscopic imaging techniques for early in vivo cancer assessment. Adv. Drug Deliv. Rev. 2014, 74, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wu, J.B.; Chu, G.; Li, Q.; Wang, R.; Zhang, C.; Zhang, Y.; Kim, H.L.; Wang, J.; Zhau, H.E.; et al. Heptamethine carbocyanine dye-mediated near-infrared imaging of canine and human cancers through the HIF-1α/OATPs signaling axis. Oncotarget 2014, 5, 10114–10126. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Tan, X.; Qi, Q.; Guo, Q.; Ran, X.; Zhang, L.; Zhang, E.; Liang, Y.; Weng, L.; Zheng, H.; et al. A multifunctional heptamethine near-infrared dye for cancer theranosis. Biomaterials 2013, 34, 2244–2251. [Google Scholar] [CrossRef]
- Henary, M.; Pannu, V.; Owens, E.A.; Aneja, R. Near infrared active heptacyanine dyes with unique cancer-imaging and cytotoxic properties. Bioorg. Med. Chem. Lett. 2012, 22, 1242–1246. [Google Scholar] [CrossRef]
- Yang, X.; Shi, C.; Tong, R.; Qian, W.; Zhau, H.E.; Wang, R.; Zhu, G.; Cheng, J.; Yang, V.W.; Cheng, T.; et al. Near IR Heptamethine Cyanine Dye-Mediated Cancer Imaging. Clin Cancer Res. 2010, 16, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Shao, C.; Li, X.; Shi, C.; Li, Q.; Hu, P.; Chen, Y.T.; Dou, X.; Sahu, D.; Li, W.; et al. Near-infrared fluorescence imaging of cancer mediated by tumor hypoxia and HIF1alpha/OATPs signaling axis. Biomaterials 2014, 35, 8175–8185. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Tan, X.; Luo, S.; Wang, D.; Su, Y.; Cheng, T.; Shi, C. A NIR heptamethine dye with intrinsic cancer targeting, imaging and photosensitizing properties. Biomaterials 2012, 33, 2230–2239. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Sevick-Muraca, E.M. Translation of near-infrared fluorescence imaging technologies: Emerging clinical applications. Annu. Rev. Med. 2012, 63, 217–231. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, D.; Yang, Z.; Yang, J.; Zhang, R.; Yang, X.; Wang, M.; Wang, Y. Repurposing antitubercular agent isoniazid for treatment of prostate cancer. Biomater. Sci. 2018, 7, 296–306. [Google Scholar] [CrossRef]
- Lv, Q.; Yang, X.; Wang, M.; Yang, J.; Qin, Z.; Kan, Q.; Zhang, H.; Wang, Y.; Wang, D.; He, Z. Mitochondria-targeted prostate cancer therapy using a near-infrared fluorescence dye-monoamine oxidase A inhibitor conjugate. J. Control. Release 2018, 279, 234–242. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Zhang, E.; Luo, S.; Tan, X.; Shi, C. Preferential accumulation of the near infrared heptamethine dye IR-780 in the mitochondria of drug-resistant lung cancer cells. Biomaterials 2014, 35, 4116–4124. [Google Scholar] [CrossRef]
- Oliveira, P.F.M.; Guidetti, B.; Chamayou, A.; Andre-Barres, C.; Madacki, J.; Kordulakova, J.; Mori, G.; Orena, B.S.; Chiarelli, L.R.; Pasca, M.R.; et al. Mechanochemical synthesis and biological evaluation of novel isoniazid derivatives with potent antibumercular activity. Molecules 2017, 22, 1457. [Google Scholar] [CrossRef]
- Pahontu, E.; Ilies, D.C.; Shova, S.; Oprean, C.; Paunescu, V.; Olaru, O.T.; Radulescu, F.S.; Gulea, A.; Rosu, T.; Draganescu, D. Synthesis, characterization, antimicrobial and antiproliferative activity evaluation of Cu(II), Co(II), Zn(II), Ni(II) and Pt(II) complexes with isoniazid-derived compound. Molecules 2017, 22, 650. [Google Scholar] [CrossRef]
- Vila-Vicosa, D.; Victor, B.I.; Ramos, J.; Machado, D.; Viveiros, M.; Switala, J.; Loewen, P.C.; Leitao, R.; Martins, F.; Machuqueiro, M. Insights on the mechanism of action of INH-C10 as an antitubercular prodrug. Mol. Pharm. 2017, 14, 4597–4605. [Google Scholar] [CrossRef]
- Wu, J.B.; Shao, C.; Li, X.; Li, Q.; Hu, P.; Shi, C.; Li, Y.; Chen, Y.T.; Yin, F.; Liao, C.P.; et al. Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J. Clin. Invest. 2014, 124, 2891–2908. [Google Scholar] [CrossRef]
- Colibus, L.D.; Li, M.; Binda, C.; Lustig, A.; Edmondson, D.E.; Mattevi, A. Three-dimensional structure of human monoamine oxidase A (MAOA): Relation to the structures of rat MAOA and human MAOB. Proc. Natl. Acad. Sci. USA 2005, 102, 12684–12689. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds G1–G13 are available from the authors. |
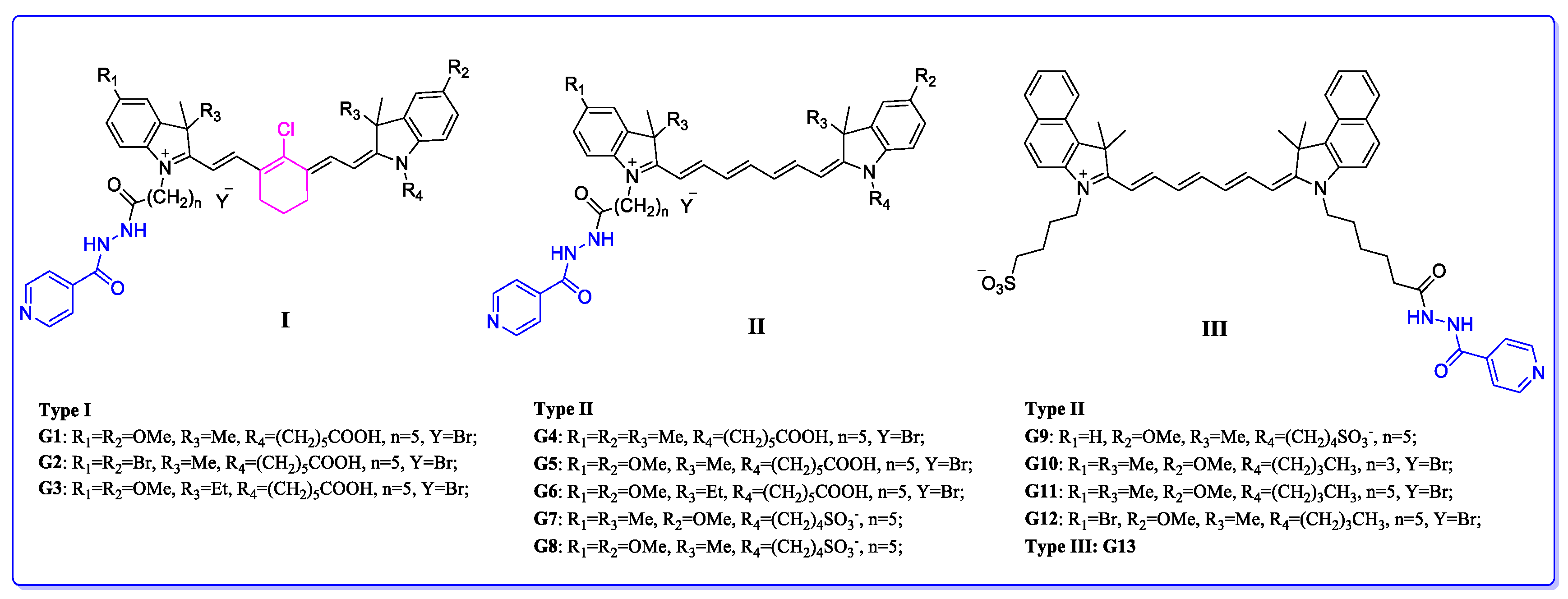
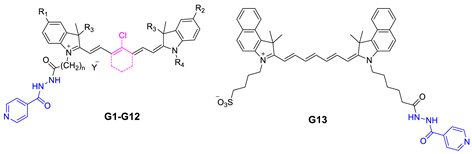
| Compound | R1 | R2 | R3 | R4 | n | Y | IC50 (μM) |
|---|---|---|---|---|---|---|---|
| G1 | OMe | OMe | Me | (CH2)5COOH | 5 | Br | 18.41 |
| G2 | Br | Br | Me | (CH2)5COOH | 5 | Br | 75.00 |
| G3 | OMe | OMe | Et | (CH2)5COOH | 5 | Br | 13.29 |
| G4 | Me | Me | Me | (CH2)5COOH | 5 | Br | 46.66 |
| G5 | OMe | OMe | Me | (CH2)5COOH | 5 | Br | 5.06 |
| G6 | OMe | OMe | Et | (CH2)5COOH | 5 | Br | 37.07 |
| G7 | Me | OMe | Me | (CH2)4SO3− | 5 | - | 28.59 |
| G8 | OMe | OMe | Me | (CH2)4SO3− | 5 | - | 101.80 |
| G9 | H | OMe | Me | (CH2)4SO3− | 5 | - | 90.53 |
| G10 | Me | OMe | Me | (CH2)3CH3 | 3 | Br | 0.85 |
| G11 | Me | OMe | Me | (CH2)3CH3 | 5 | Br | 0.40 |
| G12 | Br | OMe | Me | (CH2)3CH3 | 5 | Br | 1.55 |
| G13 | - | - | - | - | - | - | 10.54 |
| INH | - | - | - | - | - | - | >200 |
| DOX | - | - | - | - | - | - | 0.21 |
| Compound | G10 | G11 | G12 | Isoniazid | Clorgyline |
|---|---|---|---|---|---|
| IC50 (nM) | 221.1 | 469.5 | 660.6 | >1000 | 1.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.-G.; Mou, Y.-H.; Wang, Y.-J.; Wang, J.; Li, Y.-Y.; Kong, R.-H.; Ding, M.; Wang, D.; Guo, C. Design, Synthesis, and Evaluation of Monoamine Oxidase A Inhibitors–Indocyanine Dyes Conjugates as Targeted Antitumor Agents. Molecules 2019, 24, 1400. https://doi.org/10.3390/molecules24071400
Yang X-G, Mou Y-H, Wang Y-J, Wang J, Li Y-Y, Kong R-H, Ding M, Wang D, Guo C. Design, Synthesis, and Evaluation of Monoamine Oxidase A Inhibitors–Indocyanine Dyes Conjugates as Targeted Antitumor Agents. Molecules. 2019; 24(7):1400. https://doi.org/10.3390/molecules24071400
Chicago/Turabian StyleYang, Xiao-Guang, Yan-Hua Mou, Yong-Jun Wang, Jian Wang, Yan-Yu Li, Rui-Heng Kong, Meng Ding, Dun Wang, and Chun Guo. 2019. "Design, Synthesis, and Evaluation of Monoamine Oxidase A Inhibitors–Indocyanine Dyes Conjugates as Targeted Antitumor Agents" Molecules 24, no. 7: 1400. https://doi.org/10.3390/molecules24071400
APA StyleYang, X.-G., Mou, Y.-H., Wang, Y.-J., Wang, J., Li, Y.-Y., Kong, R.-H., Ding, M., Wang, D., & Guo, C. (2019). Design, Synthesis, and Evaluation of Monoamine Oxidase A Inhibitors–Indocyanine Dyes Conjugates as Targeted Antitumor Agents. Molecules, 24(7), 1400. https://doi.org/10.3390/molecules24071400