Polysaccharide-Rich Fractions from Rosa rugosa Thunb.—Composition and Chemopreventive Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Content and Composition of Rose Polysaccharide-Rich Fractions
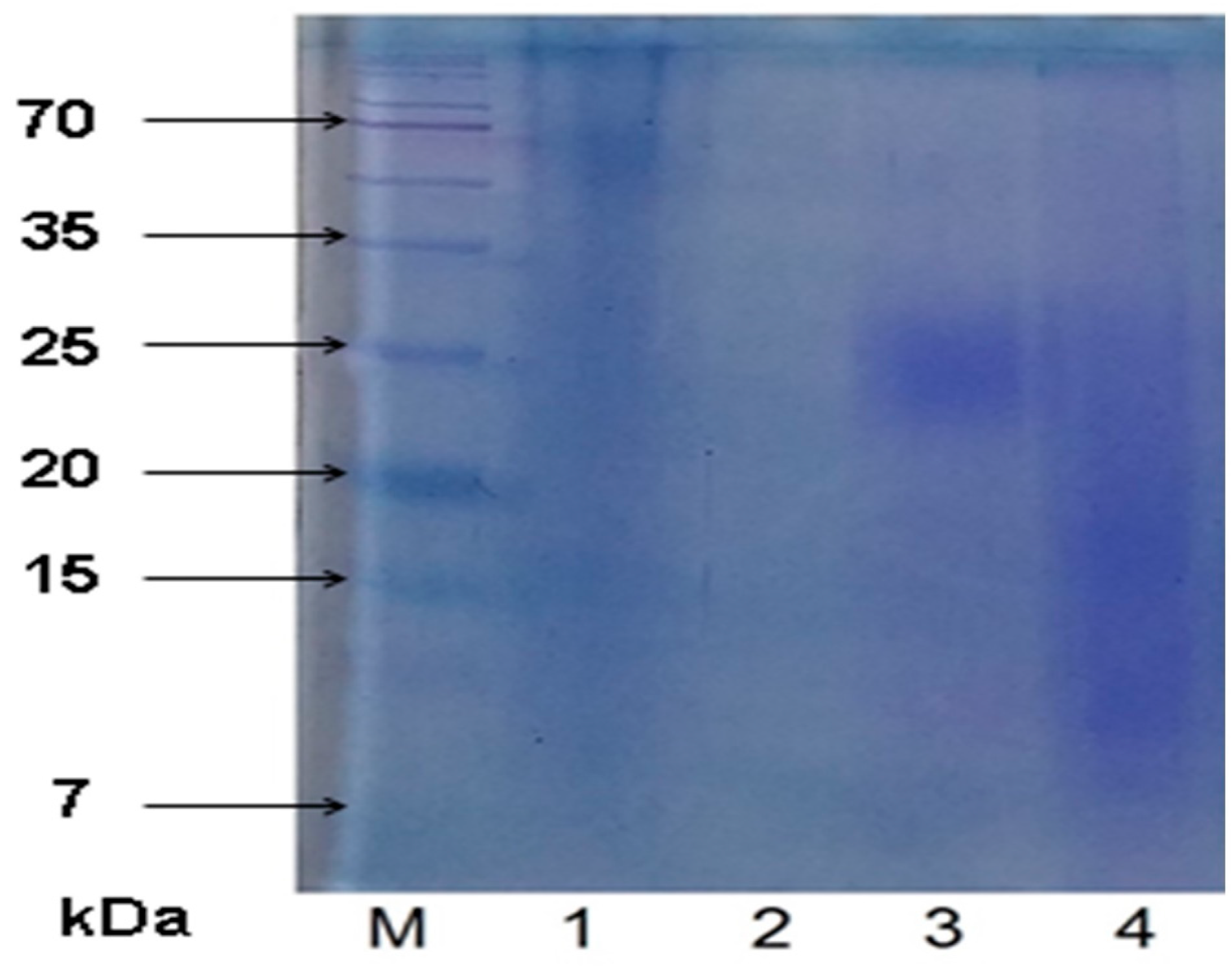
2.2. SDS-PAGE Results
2.3. Biological Activity of CPLs
2.3.1. Anti-inflammatory Activity
2.3.2. Anti-Hyaluronidase Potential
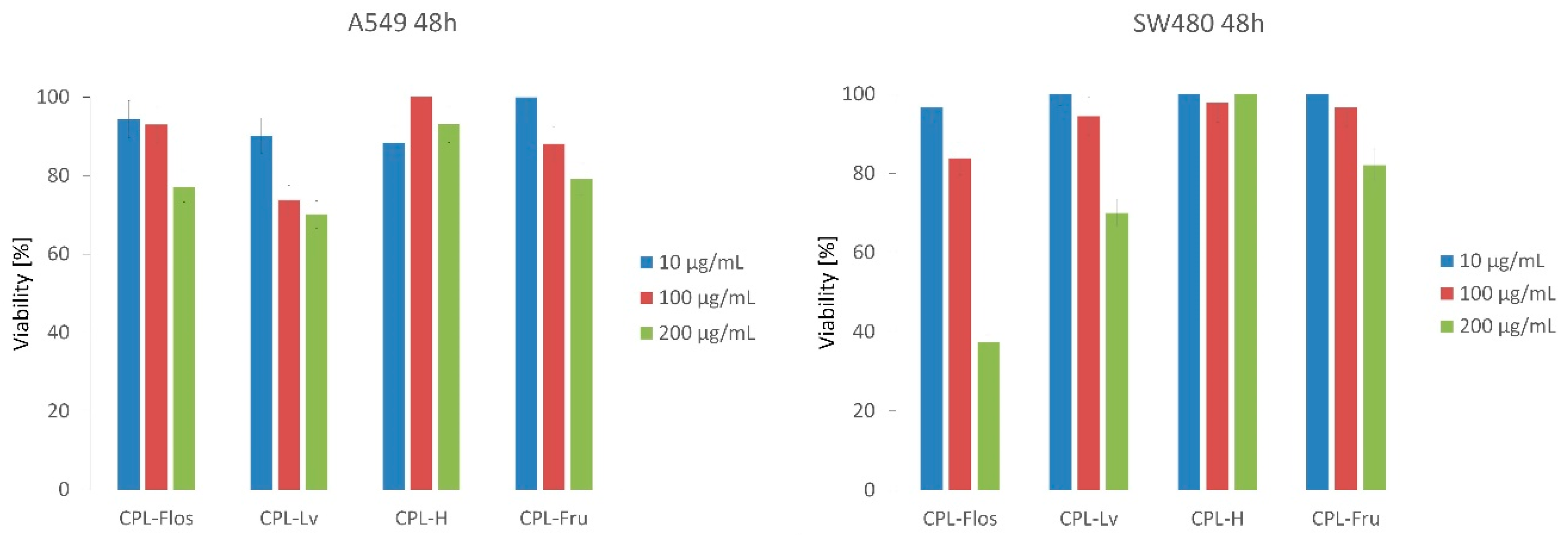
2.3.3. Antiproliferative Activity
2.3.4. Antioxidant Activity
3. Materials and Methods
3.1. Plant Material and Preparation of Extracts
3.2. Chemicals
3.3. Extraction of Crude Polysaccharides
3.4. Total Sugar Content
3.5. Determination of the Content of Total Glucans as Well as α- and β-Glucans
3.6. Total Phenolic Content (TPC)
3.7. Content of Proteins
3.8. SDS-PAGE
3.9. Anti-Inflammatory Activity (COX)
3.10. Anti-hyaluronidase Activity
3.11. Antiproliferative Activity
3.11.1. Cell Culture
3.11.2. Antiproliferative Activity Assay
3.12. DPPH• Radical Scavenging Activity Assay
3.13. ABTS•+ Assay
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, H.; Xu, X. Polysaccharide, a potential anti-cancer drug with high efficacy and safety. Adv. Oncol. Res. Treat. 2016, 1, 1–2. [Google Scholar]
- Slavov, A.; Denev, P.; Panchev, I.; Shikov, V.; Nenov, N.; Yantcheva, N.; Vasileva, I. Combined recovery of polysaccharides and polyphenols from Rosa damascena wastes. Ind. Crops Prod. 2017, 100, 85–94. [Google Scholar] [CrossRef]
- Nowacka-Jechalke, N.; Nowak, R.; Juda, M.; Malm, A.; Lemieszek, M.; Rzeski, W.; Kaczyński, Z. New biological activity of the polysaccharide fraction from Cantharellus cibarius and its structural characterization. Food Chem. 2018, 268, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Venturini, B.A.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Chemical composition and biological properties of Portuguese wild mushrooms: A comprehensive study. J. Agric. Food Chem. 2008, 56, 3856–3862. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, antiradical potential and phenolic compounds of thirty-one polish mushrooms. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mills, G.L.; Nair, M.G. Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J. Agric. Food Chem. 2002, 50, 7581–7585. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.R.M.; Boller, C.; Zibetti, R.G.M.; de Souza, D.; Pedroso, L.L.; Soccol, C.R. Anti-inflammatory and angiogenic activity of polysaccharide extract obtained from Tibetan kefir. Microvasc. Res. 2016, 108, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Urzua, U.; Termini, L.; Aguayo, F. Oxidative stress in female cancers. Oncotarget 2018, 9, 23824–23842. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Nowak, R.; Olech, M.; Pecio, L.; Oleszek, W.; Los, R.; Malm, A.; Rzymowska, J. Cytotoxic, antioxidant, antimicrobial properties and chemical composition of rose petals. J. Sci. Food Agric. 2014, 94, 560–567. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Nowacka, N.; Pecio, Ł.; Oleszek, W.; Los, R.; Malm, A.; Rzymowska, J. Evaluation of rose roots, a post-harvest plantation residue as a source of phytochemicals with radical scavenging, cytotoxic, and antimicrobial activity. Ind. Crops Prod. 2015, 69, 129–136. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Los, R.; Rzymowska, J.; Malm, A.; Chrusciel, K. Biological activity and composition of teas and tinctures prepared from Rosa rugosa Thunb. Cent. Eur. J. Biol. 2012, 7, 172–182. [Google Scholar] [CrossRef]
- Ng, T.B.; Pi, Z.F.; Yue, H.; Zhao, L.; Fu, M.; Li, L.; Hou, J.; Shi, L.S.; Chen, R.R.; Jiang, Y.; et al. A polysaccharopeptide complex and a condensed tannin with antioxidant activity from dried rose (Rosa rugosa) flowers. J. Pharm. Pharmacol. 2006, 58, 529–534. [Google Scholar] [CrossRef]
- Wang, B.; Diao, Q.; Zhang, Z.; Liu, Y.; Gao, Q.; Zhou, Y.; Li, S. Antitumor activity of bee pollen polysaccharides from Rosa rugosa. Mol. Med. Rep. 2013, 7, 1555–1558. [Google Scholar] [CrossRef]
- Dourado, F.; Vasco, P.; Gama, F.M.; Coimbra, M.A.; Mota, M. Characterisation of Rosa Mosqueta seeds: Cell wall polysaccharide composition and light microscopy observations. J. Sci. Food Agric. 2000, 80, 1859–1865. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, G.; Guan, X.; Wu, H.; Song, K.; Cheng, K.; Sun, X. Characterization of a polysaccharide from Rosa davurica and inhibitory activity against neutrophil migration. Int. J. Biol. Macromol. 2016, 89, 111–117. [Google Scholar] [CrossRef]
- Chen, G.; Kan, J. Ultrasound-assisted extraction, characterization, and antioxidant activity in vitro and in vivo of polysaccharides from Chestnut rose (Rosa roxburghii tratt) fruit. J. Food Sci. Technol. 2018, 55, 1083–1092. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Vetvicka, V.; Gover, O.; Karpovsky, M.; Hayby, H.; Danay, O.; Ezov, N.; Hadar, Y.; Schwartz, B. Immune-modulating activities of glucans extracted from Pleurotus ostreatus and Pleurotus eryngii. J. Funct. Foods 2019, 54, 81–91. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Steinhart, H. Phenolic compounds as cross-links of plant derived polysaccharides. Czech. J. Food Sci. 2004, 22, 39–42. [Google Scholar] [CrossRef]
- Wefers, D.; Gmeiner, B.M.; Tyl, C.E.; Bunzel, M. Characterization of diferuloylated pectic polysaccharides from quinoa (Chenopodium quinoa WILLD.). Phytochemistry 2015, 116, 320–328. [Google Scholar] [CrossRef]
- Souza, R.O.S.; Assreuy, A.M.S.; Madeira, J.C.; Chagas, F.D.S.; Parreiras, L.A.; Santos, G.R.C.; Mourão, P.A.S.; Pereira, M.G. Purified polysaccharides of Geoffroea spinosa barks have anticoagulant and antithrombotic activities devoid of hemorrhagic risks. Carbohydr. Polym. 2015, 124, 208–215. [Google Scholar] [CrossRef]
- Li, X.; Gong, H.; Yang, S.; Yang, L.; Fan, Y.; Zhou, Y. Pectic bee pollen polysaccharide from Rosa rugosa alleviates diet-induced hepatic steatosis and insulin resistance via induction of AMPK/mTOR-Mediated autophagy. Molecules 2017, 22, 699. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.J.; Liu, J.; Liu, L.K.; Zhang, E.S.; Li, W.L. Inhibition of metastasis and invasion of ovarian cancer cells by crude polysaccharides from Rosa Roxburghii Tratt in vitro. Asian Pac. J. Cancer Prev. 2014, 15, 10351–10354. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R. Influence of different extraction procedures on the antiradical activity and phenolic profile of Rosa rugosa petals. Acta Pol. Pharm. Drug Res. 2012, 69, 501–507. [Google Scholar]
- Ng, T.B.; He, J.S.; Niu, S.M.; Zhao, L.; Pi, Z.F.; Shao, W.; Liu, F. A gallic acid derivative and polysaccharides with antioxidative activity from rose (Rosa rugosa) flowers. J. Pharm. Pharmacol. 2004, 56, 537–545. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Nowak, R.; Załuski, D.; Kapusta, I.; Amarowicz, R.; Oleszek, W. Hyaluronidase, acetylcholinesterase inhibiting potential, antioxidant activity, and LC-ESI-MS/MS analysis of polyphenolics of rose (Rosa rugosa Thunb.) teas and tinctures. Int. J. Food Prop. 2017, 20, 16–25. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
Sample Availability: Samples of the crude polysaccharides are not available. |
| Plant Part | Water soluble Sugars (g/100 g d.w.) | Total Glucans (g/100 g d.w.) | α-Glucans (g/100 g d.w.) | β-Glucans (g/100 g d.w.) |
|---|---|---|---|---|
| Petals | 0.80 ± 0.00 c | 6.67 ± 0.12 b | 3.91 ± 0.07 c,* | 2.76 ± 0.05 c |
| Leaves | 0.42 ± 0.00 b | 2.85 ± 0.04 a | 0.94 ± 0.01 b | 1.91 ± 0.03 b |
| Hips | 15.37 ± 0.02 d | 12.26 ± 0.30 c | 5.92 ± 0.11 d | 6.35 ± 0.19 d |
| Achenes | 0.21 ± 0.00 a | 2.21 ± 0.02 a | 0.70 ± 0.00 a | 1.52 ± 0.02 a |
| Sample | Yield (g/100 g d.w.) | Sugar Content (% of CPL) | Protein Content (% of CPL) | Phenolic Content (GaE) |
|---|---|---|---|---|
| CPL-Flos | 3.81 ± 0.12 b | 20.90 ± 0.00 b | 0.87 ± 0.03 c | 0.22 ± 0.001 c |
| CPL-Lv | 3.20 ± 0.11 b | 13.10 ± 0.01 a | 0.49 ± 0.01 b | 0.11 ± 0.00 b |
| CPL-H | 19.10 ± 0.24 c | 80.49 ± 0.11 d | 0.23 ± 0.00 a | 0.11 ± 0.00 b |
| CPL-Fru | 0.81 ± 0.03 a | 25.40 ± 0.02 c | 1.08 ± 0.04 d | 0.02 ± 0.00 a |
| Sample | Extract Concentration (mg/mL) | COX-1 Inhibition (%) Average ± SD | COX-2 Inhibition (%) Average ± SD |
|---|---|---|---|
| CPL-Flos | 0.25 | 14.78 ± 0.21 | 35.12 ± 1.25 |
| CPL-Flos | 0.5 | 35.21 ± 0.89 | 49.67 ± 2.42 |
| CPL-Flos | 0.75 | 54.36 ± 1.87 | 48.71 ± 0.40 |
| CPL-Flos | 1 | 78.42 ± 2.22 | 67.38 ± 4.81 |
| CPL-Flos | 2 | 92.13 ± 1.64 | 77.50 ± 1.66 |
| CPL-Flos | 5 | 93.5 ± 2.04 | 82.06 ± 3.68 |
| CPL-Lv | 0.25 | 5.05 ± 0.13 | 55.31 ± 2.03 |
| CPL-Lv | 0.5 | 15.12 ± 0.86 | 68.24 ± 2.15 |
| CPL-Lv | 0.75 | 19.05 ± 0.39 | 71.86 ± 0.45 |
| CPL-Lv | 1 | 27.34 ± 1.15 | 79.58 ± 0.53 |
| CPL-Lv | 2 | 33.02 ± 1.44 | 76.43 ± 3.15 |
| CPL-Lv | 5 | 38.6 ± 1.60 | 81.10 ± 3.07 |
| CPL-H | 0.25 | 5.26 ± 0.27 | 6.08 ± 0.12 |
| CPL-H | 0.5 | 12.79 ± 0.84 | 11.76 ± 0.43 |
| CPL-H | 0.75 | 22.67 ± 0.89 | 13.46 ± 0.61 |
| CPL-H | 1 | 38.32 ± 1.31 | 18.88 ± 1.01 |
| CPL-H | 2 | 66.46 ± 1.63 | 45.0 ± 2.16 |
| CPL-H | 5 | 100 ± 0.0 | 94.34 ± 2.54 |
| CPL-Fru | 0.5 | n.a. | n.a. |
| CPL-Fru | 0.75 | n.a. | n.a. |
| CPL-Fru | 1 | n.a. | n.a. |
| CPL-Fru | 2 | n.a. | n.a. |
| CPL-Fru | 5 | n.a. | n.a. |
| Acetylsalicylic acid | 1 mM | 40.55 ± 1.43 | 98.21 ± 3.12 |
| Sample | IC50 (mg/mL) |
| CPL-Flos | 0.40 ± 0.01 a |
| CPL-Lv | 0.45 ± 0.02 a |
| CPL-H | 6.50 ± 0.14 c |
| CPL-Fru | 1.14 ± 0.01 b |
| Standard | IC50 (mg/mL) |
| Methyl indole-3-carboxylate | 0.49 ± 0.00 |
| Escin | 8.14 ± 0.09 |
| Naringenin | 1.28 ± 0.03 |
| Sample | DPPH Scavenging Activity | TEAC | ||
|---|---|---|---|---|
| EC50 (mg of Dry Extract /mg DPPH•) | TE | AscE | (mmol Trolox/g Dry Extract) | |
| CPL-Flos | 0.25 ± 0.01 a | 2.86 | 2.41 | 1.53 ± 0.03 d |
| CPL-Lv | 0.24 ± 0.01 a | 2.70 | 2.27 | 0.69 ± 0.02 c,* |
| CPL-H | 1.14 ± 0.02 b | 12.76 | 10.73 | 0.56 ± 0.03 b,* |
| CPL-Fru | 6.88 ± 0.13 c | 76.54 | 64.38 | 0.14 ± 0.00 a,* |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olech, M.; Nowacka-Jechalke, N.; Masłyk, M.; Martyna, A.; Pietrzak, W.; Kubiński, K.; Załuski, D.; Nowak, R. Polysaccharide-Rich Fractions from Rosa rugosa Thunb.—Composition and Chemopreventive Potential. Molecules 2019, 24, 1354. https://doi.org/10.3390/molecules24071354
Olech M, Nowacka-Jechalke N, Masłyk M, Martyna A, Pietrzak W, Kubiński K, Załuski D, Nowak R. Polysaccharide-Rich Fractions from Rosa rugosa Thunb.—Composition and Chemopreventive Potential. Molecules. 2019; 24(7):1354. https://doi.org/10.3390/molecules24071354
Chicago/Turabian StyleOlech, Marta, Natalia Nowacka-Jechalke, Maciej Masłyk, Aleksandra Martyna, Wioleta Pietrzak, Konrad Kubiński, Daniel Załuski, and Renata Nowak. 2019. "Polysaccharide-Rich Fractions from Rosa rugosa Thunb.—Composition and Chemopreventive Potential" Molecules 24, no. 7: 1354. https://doi.org/10.3390/molecules24071354
APA StyleOlech, M., Nowacka-Jechalke, N., Masłyk, M., Martyna, A., Pietrzak, W., Kubiński, K., Załuski, D., & Nowak, R. (2019). Polysaccharide-Rich Fractions from Rosa rugosa Thunb.—Composition and Chemopreventive Potential. Molecules, 24(7), 1354. https://doi.org/10.3390/molecules24071354