Eutectic Phenomenon of LiNH2-KH Composite in MH-NH3 Hydrogen Storage System
Abstract
1. Introduction
2. Results and Discussion
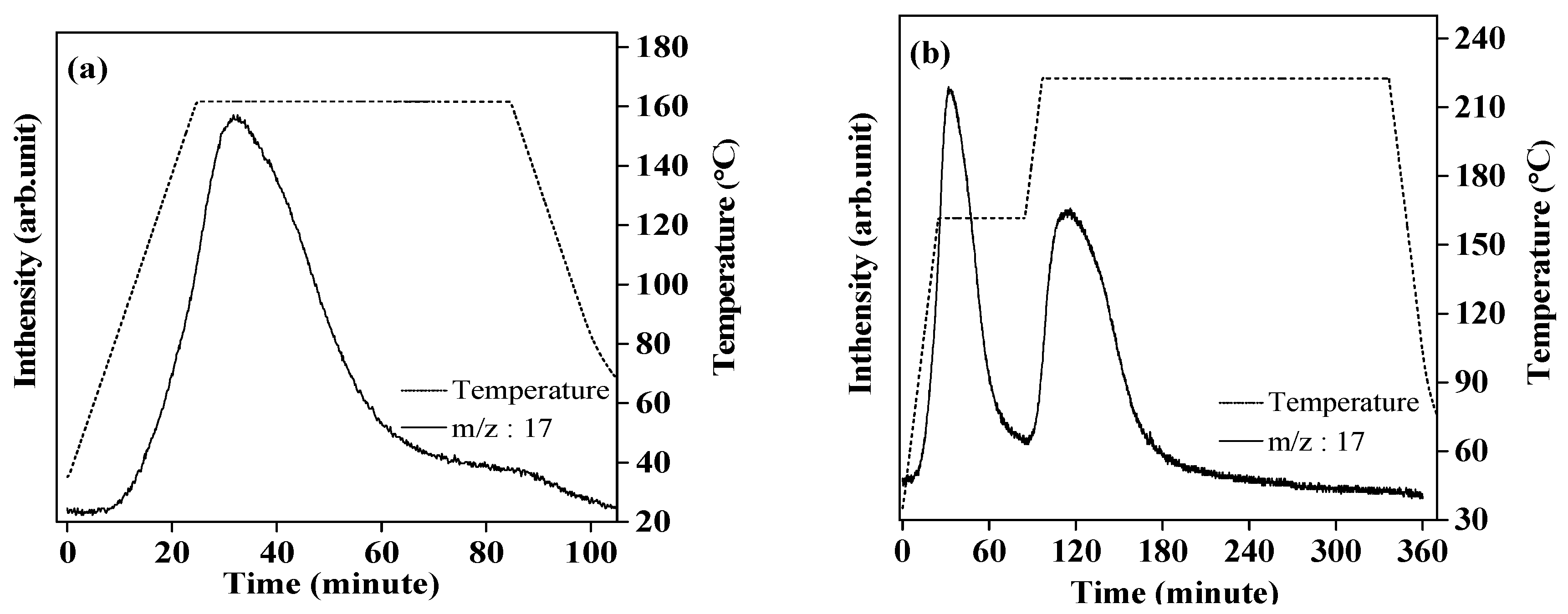
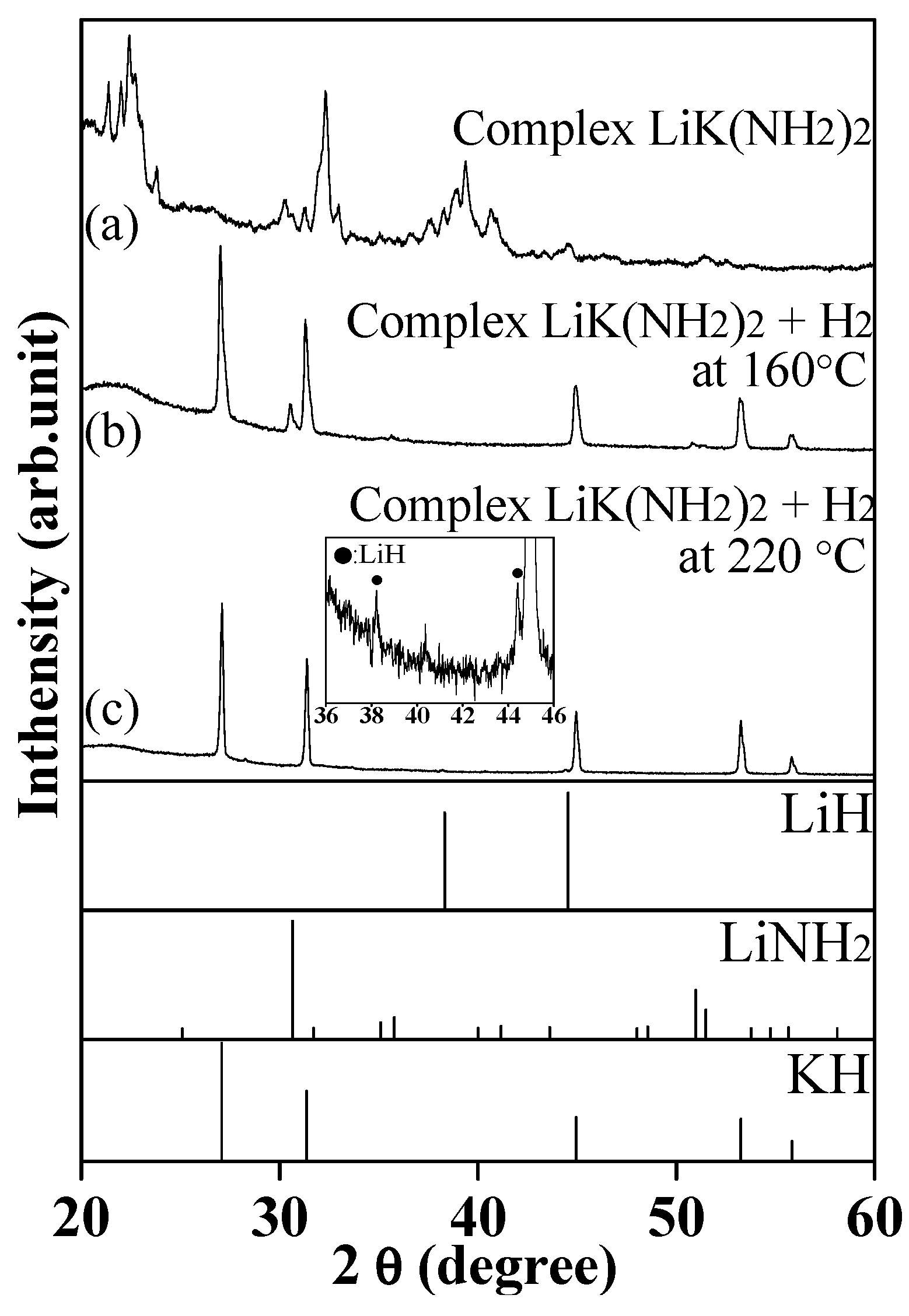
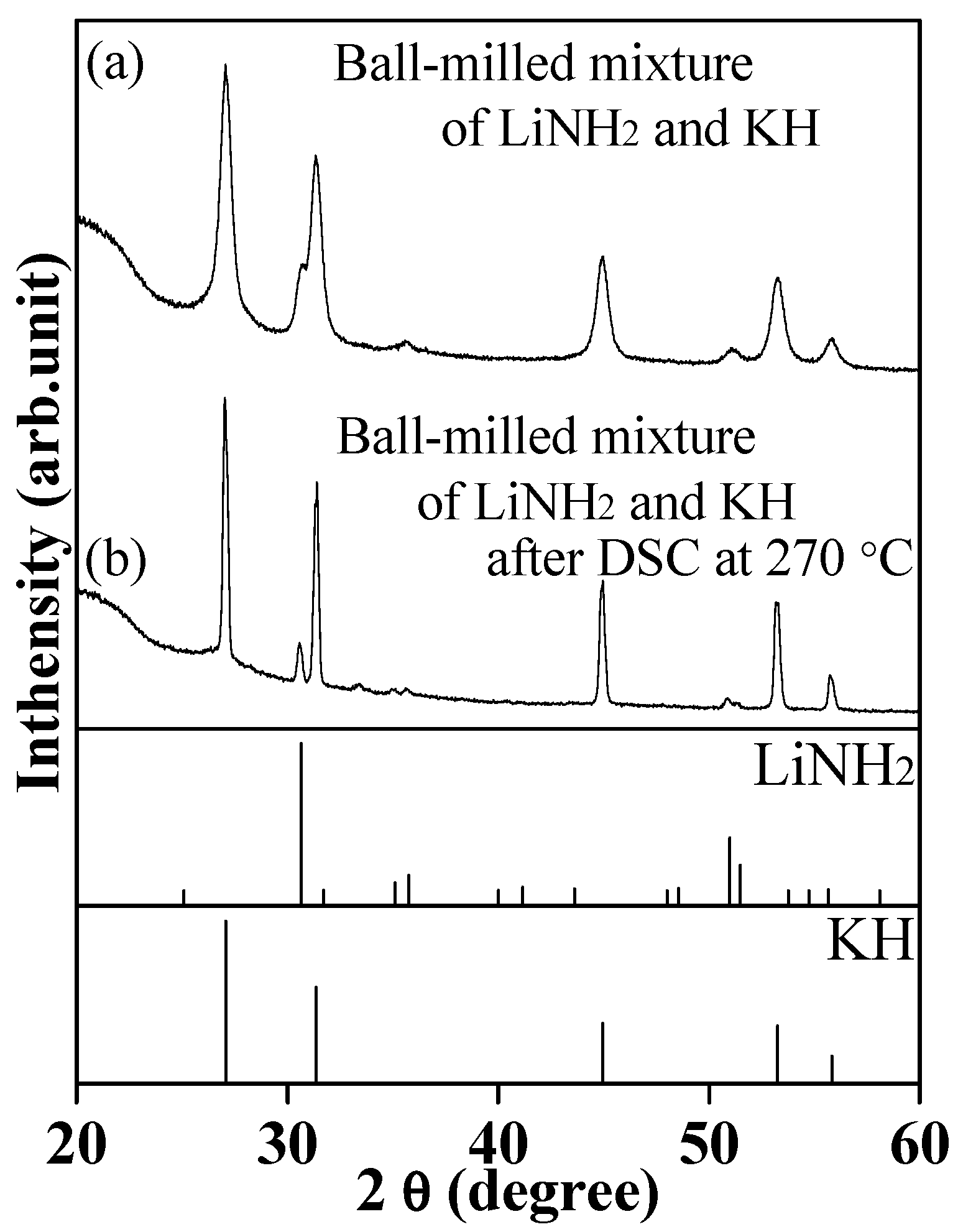
2.1. Investigation for Hydrogenation of Complex LiK(NH2)2 at Lower Temperature
2.2. Investigation for Hydrogenation of Complex LiK(NH2)2 at Higher Temperature
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hanley, E.S.; Deane, J.P.; Gallachoir, B.P.O. The role of hydrogen in low carbon energy futures—A review of existing perspectives. Renew. Sustain. Energy Rev. 2018, 82, 3027–3045. [Google Scholar] [CrossRef]
- Endo, N.; Suzuki, S.; Goshome, K.; Maeda, T. Operation of a bench-scale TiFe-based alloy tank under mild conditions for low-cost stationary hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 5246–5251. [Google Scholar] [CrossRef]
- Veras, T.D.; Mozer, T.S.; dos Santos, D.; Cesar, A.D. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C. Metal hydride hydrogen compressors: A review. Int. J. Hydrogen Energy 2014, 39, 5818–5851. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.C.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Sinigaglia, T.; Lewiski, F.; Martins, M.E.S.; Siluk, J.C.M. Production, storage, fuel stations of hydrogen and its utilization in automotive applications—A review. Int. J. Hydrogen Energy 2017, 42, 24597–24611. [Google Scholar] [CrossRef]
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.W.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.F.; Mao, J.F.; Huang, Z.G.; Liu, H.K.; et al. Hydrogen Storage Materials for Mobile and Stationary Applications: Current State of the Art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Hino, S.; Ichikawa, T.; Ogita, N.; Udagawa, M.; Fujii, H. Quantitative estimation of NH3 partial pressure in H2 desorbed from the Li-N-H system by Raman spectroscopy. Chem. Commun. 2005. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hanada, N.; Isobe, S.; Leng, H.Y.; Fujii, H. Hydrogen storage properties in Ti catalyzed Li-N-H system. J. Alloys Compd. 2005, 404, 435–438. [Google Scholar] [CrossRef]
- Isobe, S.; Ichikawa, T.; Hino, S.; Fujii, H. Hydrogen desorption mechanism in a Li-N-H system by means of the isotopic exchange technique. J. Phys. Chem. B 2005, 109, 14855–14858. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Leng, H.Y.; Isobe, S.; Hanada, N.; Fujii, H. Recent development on hydrogen storage properties in metal-N-H systems. J. Power Sources 2006, 159, 126–131. [Google Scholar] [CrossRef]
- Cao, H.J.; Wang, J.H.; Chua, Y.S.; Wang, H.; Wu, G.T.; Xiong, Z.T.; Qiu, J.S.; Chen, P. NH3 Mediated or Ion Migration Reaction: The Case Study on Halide-Amide System. J. Phys. Chem. C 2014, 118, 2344–2349. [Google Scholar] [CrossRef]
- Amica, G.; Larochette, P.A.; Gennari, F.C. Hydrogen storage properties of LiNH2-LiH system with MgH2, CaH2 and TiH2 added. Int. J. Hydrogen Energy 2015, 40, 9335–9346. [Google Scholar] [CrossRef]
- Lin, H.J.; Li, H.W.; Murakami, H.; Akiba, E. Remarkably improved hydrogen storage properties of LiNH2-LiH composite via the addition of CeF4. J. Alloys Compd. 2018, 735, 1017–1022. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.T.; Luo, J.Z.; Lin, J.Y.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Kawai, Y. IR characterizations of lithium imide and amide. J. Alloys Compd. 2005, 395, 236–239. [Google Scholar] [CrossRef]
- Osborn, W.; Markmaitree, T.; Shaw, L.L.; Hu, J.Z.; Kwak, J.; Yang, Z.G. Low temperature milling of the LiNH2 + LiH hydrogen storage system. Int. J. Hydrogen Energy 2009, 34, 4331–4339. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hanada, N.; Isobe, S.; Leng, H.Y.; Fujii, H. Mechanism of novel reaction from LiNH2 and LiH to Li2NH and H2 as a promising hydrogen storage system. J. Phys. Chem. B 2004, 108, 7887–7892. [Google Scholar] [CrossRef]
- Leng, H.Y.; Ichikawa, T.; Hino, S.; Fujii, H. Investigation of reaction between LiNH2 and H2. J. Alloys Compd. 2008, 463, 462–465. [Google Scholar] [CrossRef]
- Kojima, Y.; Tange, K.; Hino, S.; Isobe, S.; Tsubota, M.; Nakamura, K.; Nakatake, M.; Miyaoka, H.; Yamamoto, H.; Ichikawa, T. Molecular hydrogen carrier with activated nanohydride and ammonia. J. Mater. Res. 2009, 24, 2185–2190. [Google Scholar] [CrossRef]
- Yamamoto, H.; Miyaoka, H.; Hino, S.; Nakanishi, H.; Ichikawa, T.; Kojima, Y. Recyclable hydrogen storage system composed of ammonia and alkali metal hydride. Int. J. Hydrogen Energy 2009, 34, 9760–9764. [Google Scholar] [CrossRef]
- Dong, B.X.; Song, L.; Ge, J.; Teng, Y.L.; Zhang, S.Y. The ternary amide KLi3(NH2)4: An important intermediate in the potassium compound-added Li-N-H systems. RSC Adv. 2014, 4, 10702–10707. [Google Scholar] [CrossRef]
- Dong, B.X.; Teng, Y.L.; Ge, J.; Song, L.; Zhang, S.Y. The interesting and superior hydrogenation properties of potassium-doped LiNH2 and its ternary mixed-cationic amide. RSC Adv. 2013, 3, 16977–16980. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. Ultrafast reaction between LiH and NH3 during H2 storage in Li3N. J. Phys. Chem. A 2003, 107, 9737–9739. [Google Scholar] [CrossRef]
- Miyaoka, H.; Fujii, H.; Yamamoto, H.; Hino, S.; Nakanishi, H.; Ichikawa, T.; Kojima, Y. Improvement of reaction kinetics by metal chloride on ammonia and lithium hydride system. Int. J. Hydrogen Energy 2012, 37, 16025–16030. [Google Scholar] [CrossRef]
- Miyaoka, H.; Ichikawa, T.; Hino, S.; Kojima, Y. Compressed hydrogen production via reaction between liquid ammonia and alkali metal hydride. Int. J. Hydrogen Energy 2011, 36, 8217–8220. [Google Scholar] [CrossRef]
- Teng, Y.L.; Ichikawa, T.; Miyaoka, H.; Kojima, Y. Improvement of hydrogen desorption kinetics in the LiH-NH3 system by addition of KH. Chem. Commun. 2011, 47, 12227–12229. [Google Scholar] [CrossRef]
- Miyaoka, H.; Nakajima, K.; Yamaguchi, S.; Aoki, T.; Yamamoto, H.; Okuda, T.; Goshome, K.; Ichikawa, T.; Kojima, Y. Catalysis of Lithium Chloride and Alkali Metal Borohydrides on Hydrogen Generation of Ammonia and Lithium Hydride System. J. Phys. Chem. C 2015, 119, 19922–19927. [Google Scholar] [CrossRef]
- Goshome, K.; Miyaoka, H.; Yamamoto, H.; Ichikawa, T.; Kojima, Y. Hydrogen Ab/Desorption of LiH-KH Composite and Ammonia System. Mater. Trans. 2016, 57, 1215–1219. [Google Scholar] [CrossRef]
- Goshome, K.; Miyaoka, H.; Ichikawa, T.; Yamamoto, H.; Ichikawa, T.; Kojima, Y. Ammonia Synthesis via Non-Equilibrium Reaction of Lithium Nitride in Hydrogen Flow Condition. Mater. Trans. 2015, 56, 410–414. [Google Scholar] [CrossRef]
- Izuhara, T.; Takeshita, H.; Miyake, H. Thermodynamic Property Change in Li-N-H Hydrogen Storage System by Melting Lithium Amide. J. Jpn. Inst. Met. 2011, 75, 115–121. [Google Scholar] [CrossRef]
- Wu, C.; Bai, Y.; Yang, J.H.; Wu, F.; Long, F. Characterizations of composite NaNH2-NaBH4 hydrogen storage materials synthesized via ball milling. Int. J. Hydrogen Energy 2012, 37, 889–893. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, Z.; Wu, G.; Chen, P.; Murata, K.; Sakata, K. Hydrogen releasing reaction between Mg(NH2)2 and CaH2. J. Power Sources 2006, 159, 116–119. [Google Scholar] [CrossRef]
- Leng, H.Y.; Ichikawa, T.; Hino, S.; Hanada, N.; Isobe, S.; Fujii, H. Synthesis and decomposition reactions of metal amides in metal-N-H hydrogen storage system. J. Power Sources 2006, 156, 166–170. [Google Scholar] [CrossRef]
- Wu, G.; Xiong, Z.; Liu, T.; Liu, Y.; Hu, J.; Chen, P.; Feng, Y.; Wee, A.T.S. Synthesis and characterization of a new ternary imide-Li2Ca(NH)2. Inorg. Chem. 2007, 46, 517–521. [Google Scholar] [CrossRef]
- Tokoyoda, K.; Hino, S.; Ichikawa, T.; Okamoto, K.; Fujii, H. Hydrogen desorption/absorption properties of Li-Ca-N-H system. J. Alloys Compd. 2007, 439, 337–341. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, G.; Hu, J.; Chen, P. Ca-Na-N-H system for reversible hydrogen storage. J. Alloys Compd. 2007, 441, 152–156. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Paskevicius, M.; Buckley, C.E. Hydrogen Desorption from the NaNH2-MgH2 System. J. Phys. Chem. C 2011, 115, 8407–8413. [Google Scholar] [CrossRef]
- Wang, J.H.; Wu, G.T.; Chua, Y.S.; Guo, J.P.; Xiong, Z.T.; Zhang, Y.; Gao, M.X.; Pan, H.G.; Chen, P. Hydrogen Sorption from the Mg(NH2)2-KH System and Synthesis of an Amide-Imide Complex of KMg(NH)(NH2). ChemSusChem 2011, 4, 1622–1628. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T. Two-Peak Mystery of LiNH2 NaH Dehydrogenation Is Solved? A Study of the Analogous Sodium Amide/Lithium Hydride System. J. Phys. Chem. C 2016, 120, 27903–27909. [Google Scholar] [CrossRef]
- Nakamori, Y.; Kitahara, G.; Orimo, S. Synthesis and dehydriding studies of Mg-N-H systems. J. Power Sources 2004, 138, 309–312. [Google Scholar] [CrossRef]
- Aoki, M.; Noritake, T.; Kitahara, G.; Nakamori, Y.; Towata, S.; Orimo, S. Dehydriding reaction of Mg(NH2)2-LiH system under hydrogen pressure. J. Alloys Compd. 2007, 428, 307–311. [Google Scholar] [CrossRef]
- Leng, H.; Ichikawa, T.; Fujii, H. Hydrogen storage properties of Li-Mg-N-H systems with different ratios of LiH/Mg(NH2)2. J. Phys. Chem. B 2006, 110, 12964–12968. [Google Scholar] [CrossRef]
- Leng, H.Y.; Ichikawa, T.; Hino, S.; Hanada, N.; Isobe, S.; Fujii, H. New metal-N-H system composed of Mg(NH2)2 and LiH for hydrogen storage. J. Phys. Chem. B 2004, 108, 8763–8765. [Google Scholar] [CrossRef]
- Xiong, Z.T.; Hu, J.J.; Wu, G.T.; Chen, P.; Luo, W.F.; Gross, K.; Wang, J. Thermodynamic and kinetic investigations of the hydrogen storage in the Li-Mg-N-H system. J. Alloys Compd. 2005, 398, 235–239. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, Y.F.; Wu, G.T.; Xiong, Z.T.; Chua, Y.S.; Chen, P. Improvement of hydrogen storage properties of the Li-Mg-N-H system by addition of LiBH4. Chem. Mater. 2008, 20, 4398–4402. [Google Scholar] [CrossRef]
- Wang, J.H.; Liu, T.; Wu, G.T.; Li, W.; Liu, Y.F.; Araujo, C.M.; Scheicher, R.H.; Blomqvist, A.; Ahuja, R.; Xiong, Z.T.; et al. Potassium-Modified Mg(NH2)2/2 LiH System for Hydrogen Storage. Angew. Chem.-Int. Ed. 2009, 48, 5828–5832. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.G.; Shi, S.B.; Liu, Y.F.; Li, B.; Yang, Y.J.; Gao, M.X. Improved hydrogen storage kinetics of the Li-Mg-N-H system by addition of Mg(BH4)2. Dalton Trans. 2013, 42, 3802–3811. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Y.F.; Gao, M.X.; Pan, H.G. Understanding the role of K in the significantly improved hydrogen storage properties of a KOH-doped Li-Mg-N-H system. J. Mat. Chem. A 2013, 1, 5031–5036. [Google Scholar] [CrossRef]
- Miyaoka, H.; Wang, Y.M.; Hino, S.; Isobe, S.; Tokoyoda, K.; Ichikawa, T.; Kojima, Y. Kinetic Modification on Hydrogen Desorption of Lithium Hydride and Magnesium Amide System. Materials 2015, 8, 3896–3909. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.X.; Wang, L.; Ge, J.; Ping, C.; Teng, Y.L.; Li, Z.W. The effect of KH on enhancing the dehydrogenation properties of the Li-N-H system and its catalytic mechanism. Phys. Chem. Chem. Phys. 2018, 20, 11116–11122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: All the samples of the compounds are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goshome, K.; Jain, A.; Miyaoka, H.; Yamamoto, H.; Kojima, Y.; Ichikawa, T. Eutectic Phenomenon of LiNH2-KH Composite in MH-NH3 Hydrogen Storage System. Molecules 2019, 24, 1348. https://doi.org/10.3390/molecules24071348
Goshome K, Jain A, Miyaoka H, Yamamoto H, Kojima Y, Ichikawa T. Eutectic Phenomenon of LiNH2-KH Composite in MH-NH3 Hydrogen Storage System. Molecules. 2019; 24(7):1348. https://doi.org/10.3390/molecules24071348
Chicago/Turabian StyleGoshome, Kiyotaka, Ankur Jain, Hiroki Miyaoka, Hikaru Yamamoto, Yoshitsugu Kojima, and Takayuki Ichikawa. 2019. "Eutectic Phenomenon of LiNH2-KH Composite in MH-NH3 Hydrogen Storage System" Molecules 24, no. 7: 1348. https://doi.org/10.3390/molecules24071348
APA StyleGoshome, K., Jain, A., Miyaoka, H., Yamamoto, H., Kojima, Y., & Ichikawa, T. (2019). Eutectic Phenomenon of LiNH2-KH Composite in MH-NH3 Hydrogen Storage System. Molecules, 24(7), 1348. https://doi.org/10.3390/molecules24071348







