Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Treatments
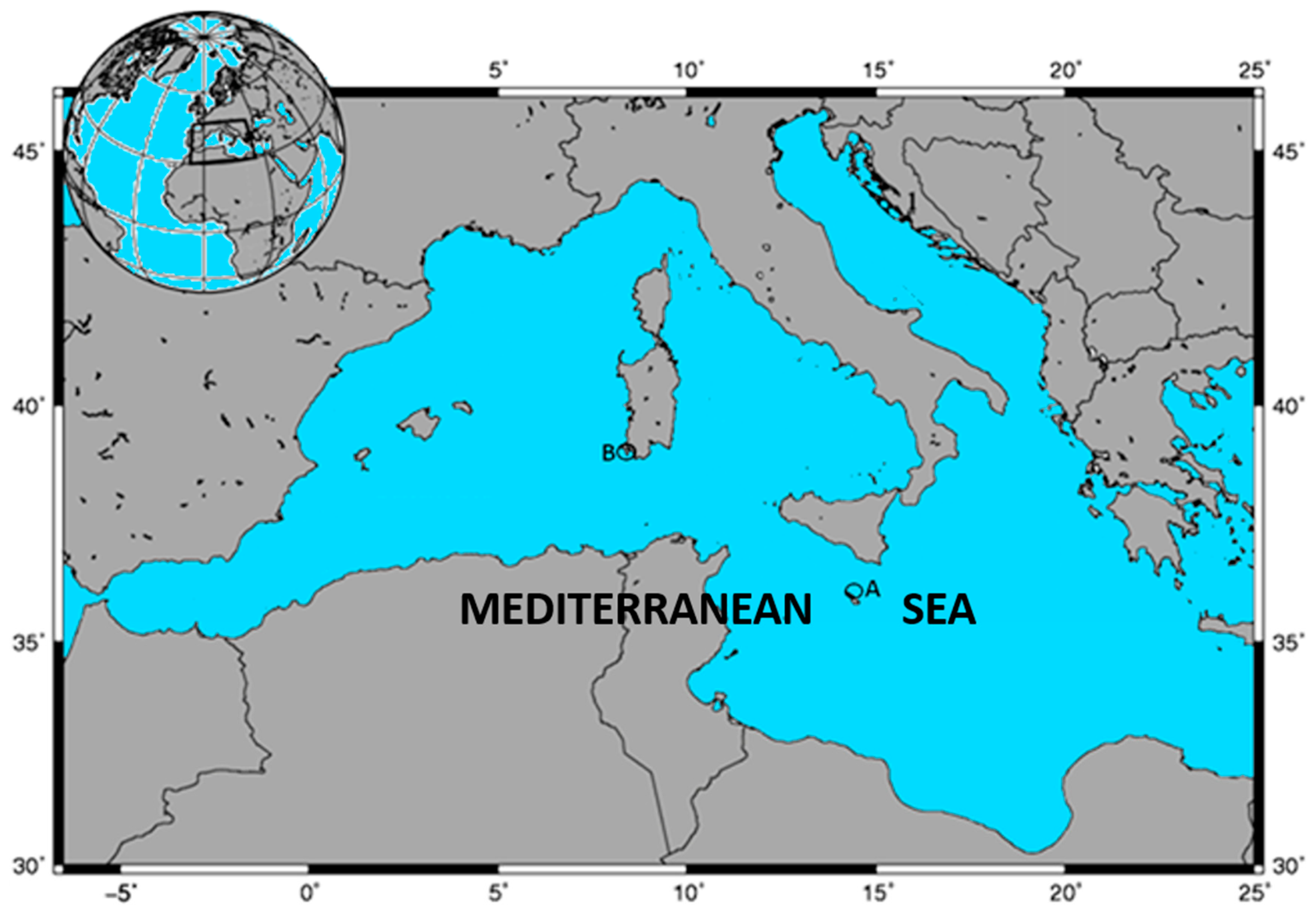
2.1.1. Sampling
2.1.2. Samples Treatment
2.1.3. Mercury and Selenium Analysis
2.1.4. Lipid Determination
2.2. Laboratory and Apparatus
2.3. Mercury and Selenium Indices
2.4. Statistical Analysis
3. Results
3.1. Accuracy
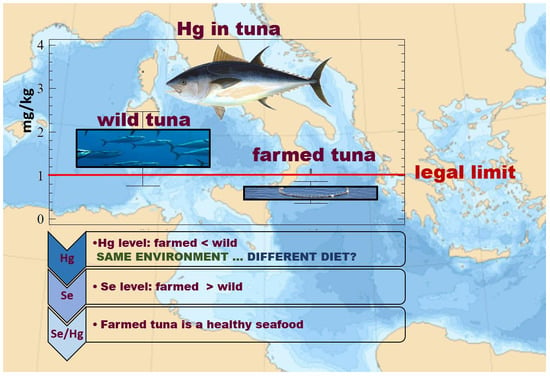
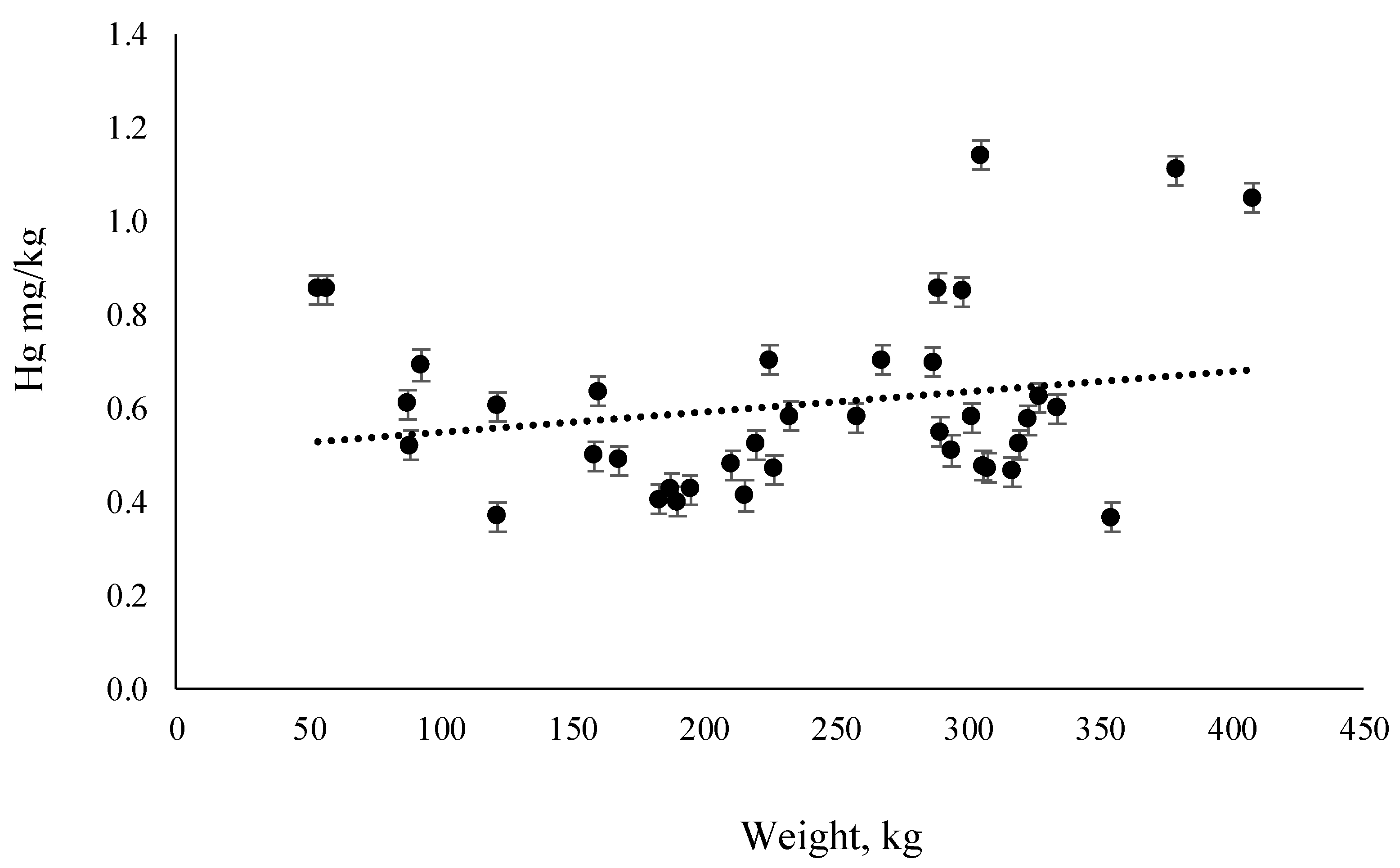
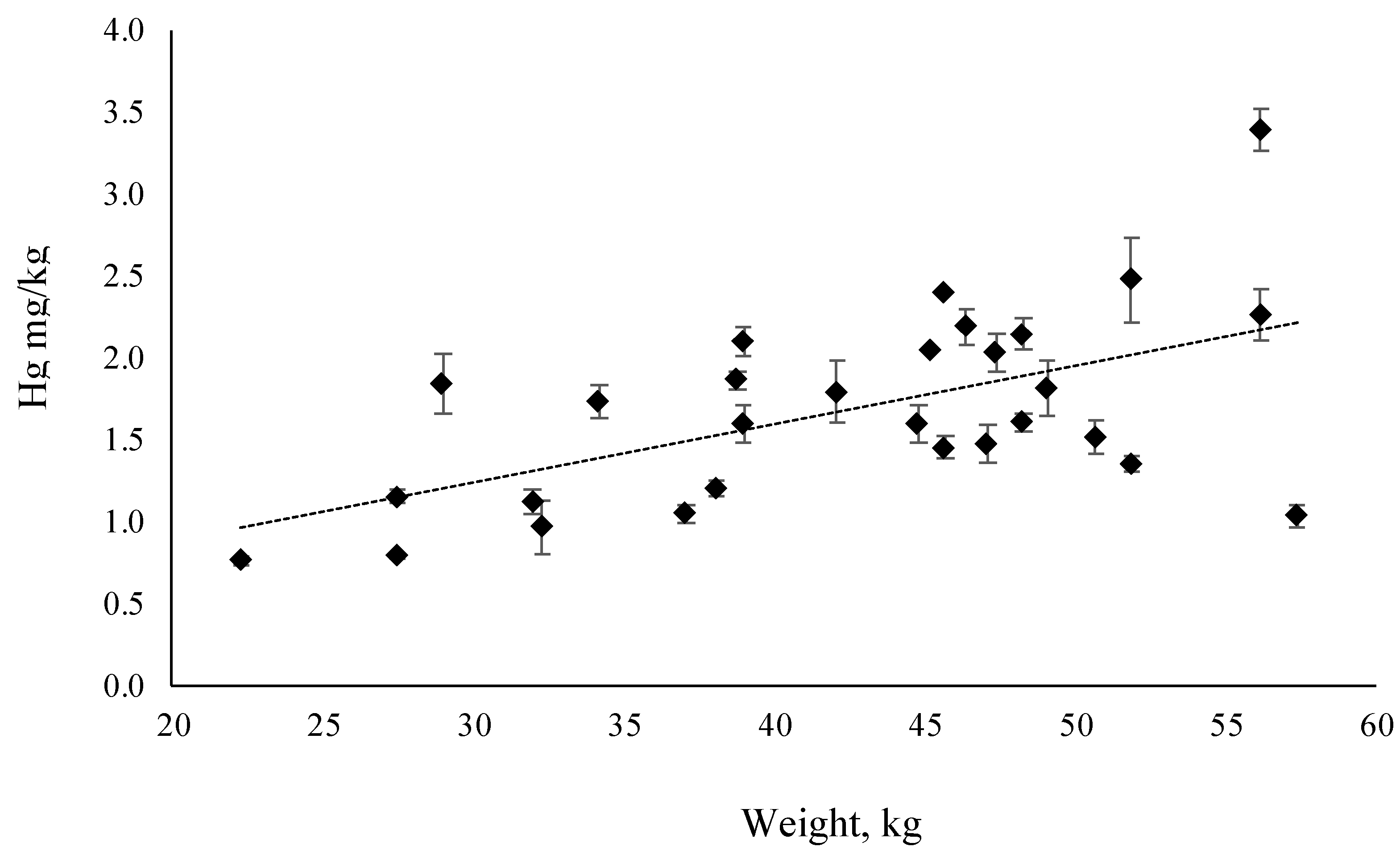
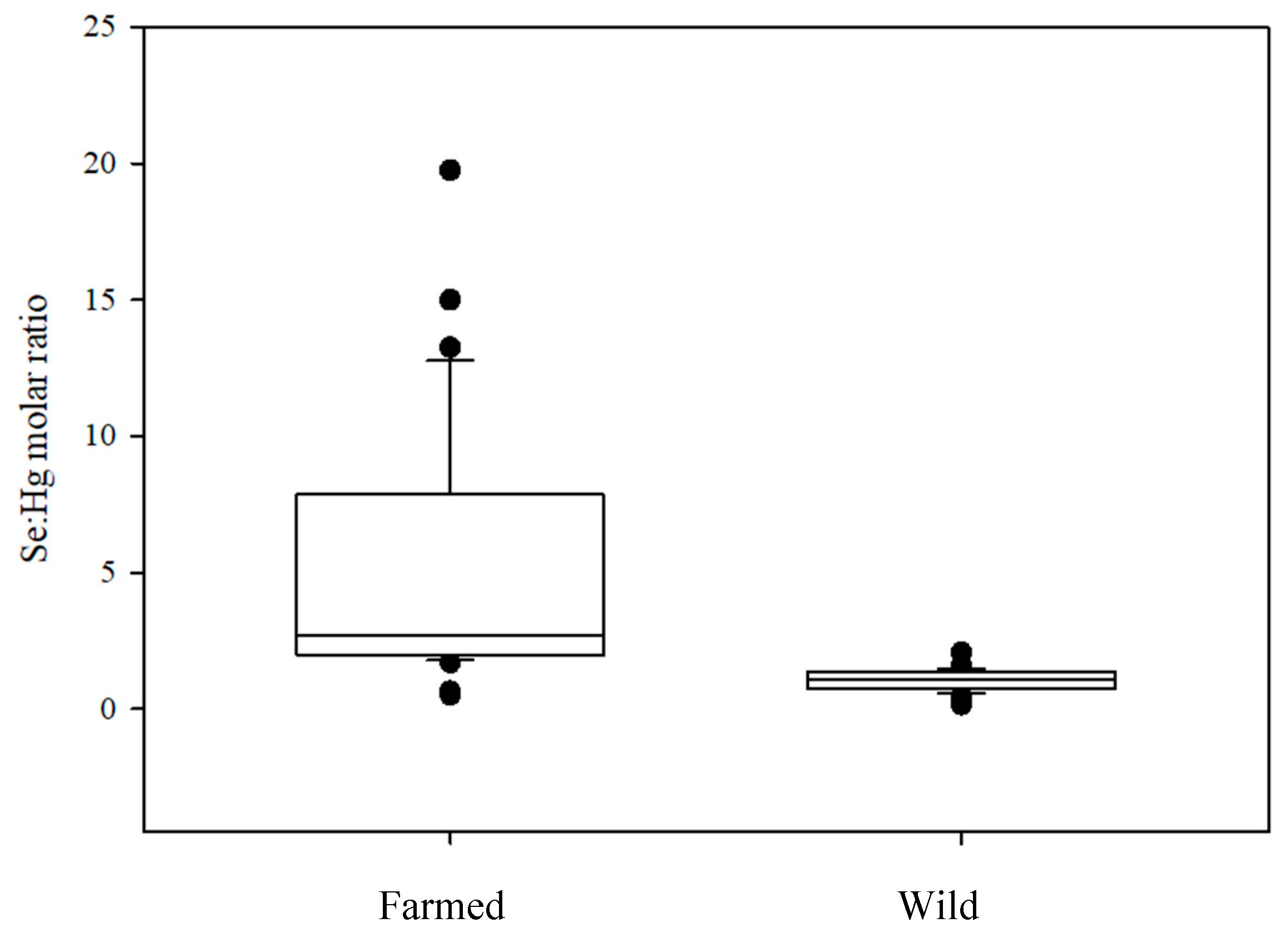
3.2. Mercury and Selenium Content
3.3. Lipid Content in Farmed and Wild Specimens
4. Discussion
4.1. Mercury in Farmed and Wild Atlantic BlueFin Tuna (ABFT)
4.2. Selenium in Farmed and Wild Atlantic BlueFin Tuna (ABFT)
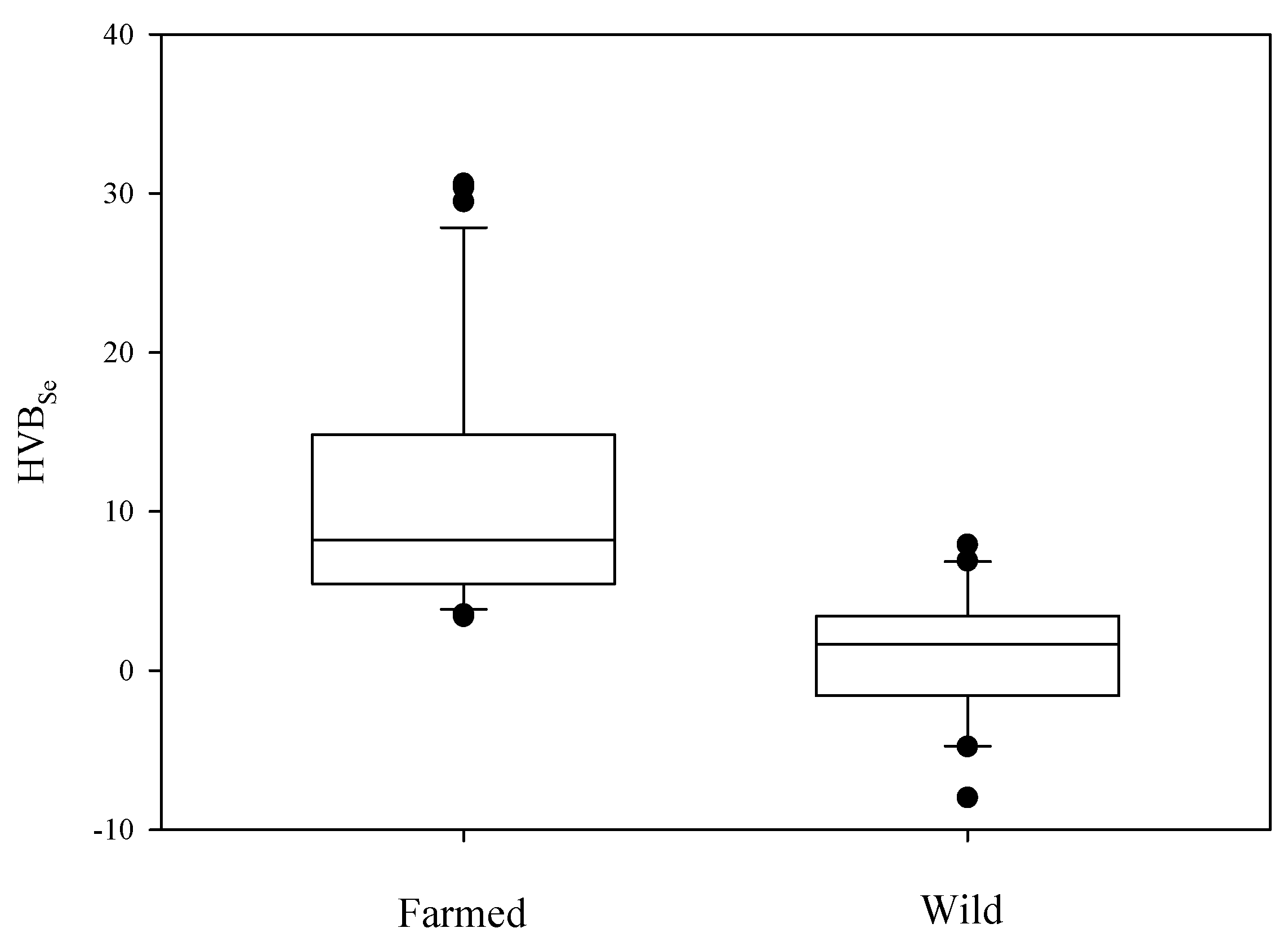
4.3. Risk Assessment: Selenium vs. Mercury Indices
4.4. Comparison with Law Limit
4.5. Comparison with Literature Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajar, R.; Četina, M.; Horvat, M.; Žagar, D. Mass Balance of Mercury in the Mediterranean Sea. Mar. Chem. 2007, 107, 89–102. [Google Scholar] [CrossRef]
- Hintelmann, H. Organomercurials. Their Formation and Pathways in the Environment. Met. Ions Life Sci. 2010, 7, 365–401. [Google Scholar] [CrossRef] [PubMed]
- Scheuhammer, A.M.; Meyer, M.W.; Sandheinrich, M.B.; Murray, M.W. Effects of Environmental Methylmercury on the Health of Wild Birds, Mammals, and Fish. Ambio 2007, 36, 12–18. [Google Scholar] [CrossRef]
- Depew, D.C.; Basu, N.; Burgess, N.M.; Campbell, L.M.; Devlin, E.W.; Drevnick, P.E.; Hammerschmidt, C.R.; Murphy, C.A.; Sandheinrich, M.B.; Wiener, J.G. Toxicity of Dietary Methylmercury to Fish: Derivation of Ecologically Meaningful Threshold Concentrations. Environ. Toxicol. Chem. 2012, 31, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L.; Díez, S.; Soto, D.X.; Catalan, J.; Bayona, J.M. Assessment of Mercury and Methylmercury Pollution with Zebra Mussel (Dreissena polymorpha) in the Ebro River (NE Spain) Impacted by Industrial Hazardous Dumps. Sci. Total Environ. 2008, 407, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Pereira, H.M.V.S.; Soares, A.M.V.M. Effects of Mercury on Growth, Emergence, and Behavior of Chironomus riparius Meigen (Diptera: Chironomidae). Arch. Environ. Contam. Toxicol. 2010, 59, 216–224. [Google Scholar] [CrossRef]
- Faria, M.; López, M.A.; Díez, S.; Barata, C. Are Native Naiads More Tolerant to Pollution than Exotic Freshwater Bivalve Species? An Hypothesis Tested Using Physiological Responses of Three Species Transplanted to Mercury Contaminated Sites in the Ebro River (NE, Spain). Chemosphere 2010, 81, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.; Solé, M.; Fernández-Gómez, C.; Díez, S. New Insights into Mercury Bioaccumulation in Deep-Sea Organisms from the NW Mediterranean and Their Human Health Implications. Sci. Total Environ. 2013, 442, 329–335. [Google Scholar] [CrossRef]
- Cabañero, A.I.; Madrid, Y.; Cámara, C. Mercury–Selenium Species Ratio in Representative Fish Samples and Their Bioaccessibility by an In Vitro Digestion Method. Biol. Trace Elem. Res. 2007, 119, 195–211. [Google Scholar] [CrossRef]
- Cossa, D.; Coquery, M. The Mediterranean Mercury Anomaly, a Geochemical or a BiologocalIssue. In The Mediterranean Sea. Handbook of Environmental Chemistry Vol 5K; Saliot, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 177–208. ISBN 978-3-540-25018-0. [Google Scholar]
- Entwisle, J.; Malinovsky, D.; Dunn, P.J.H.; Goenaga-Infante, H. Hg Isotope Ratio Measurements of Methylmercury in Fish Tissues Using HPLC with off Line Cold Vapour Generation MC-ICPMS. J. Anal. At. Spectrom. 2018, 33, 1645–1654. [Google Scholar] [CrossRef]
- Liu, Y.; Buchanan, S.; Anderson, H.A.; Xiao, Z.; Persky, V.; Turyk, M.E. Association of Methylmercury Intake from Seafood Consumption and Blood Mercury Level among the Asian and Non-Asian Populations in the United States. Environ. Res. 2018, 160, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Covaci, E.; Senila, M.; Ponta, M.; Darvasi, E.; Frentiu, M.; Frentiu, T. Mercury Speciation in Seafood Using Non-Chromatographic Chemical Vapor Generation Capacitively Coupled Plasma Microtorch Optical Emission Spectrometry Method—Evaluation of Methylmercury Exposure. Food Control 2017, 82, 266–273. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). Joint Fao/Who Food Standards Programme. Report of the Twenty-Third Session of the Codex Committee on Food Additives and Contaminats, The Hague, The Netherlands, 4–9 March 1991; Available online: www.fao.org/input/download/report/27/Al0112Ae.pdf (accessed on 27 March 2019).
- COMMISSION REGULATION (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881&from=IT (accessed on 27 March 2019).
- de Paiva, E.L.; Alves, J.C.; Milani, R.F.; Boer, B.S.; Quintaes, K.D.; Morgano, M.A. Sushi Commercialized in Brazil: Organic Hg Levels and Exposure Intake Evaluation. Food Control 2016, 69, 115–123. [Google Scholar] [CrossRef]
- Aquafarm, Fish Consumption in Italy Is Still Growing, But Dependence from Imports Increases. Pordenone. 9 January 2018. Available online: http://www.aquafarm.show/en/press-releases/#edizione-2018 (accessed on 27 March 2019).
- Morrissey, M.T.; Rasmussen, R.; Okada, T. Mercury Content in Pacific Troll-Caught Albacore Tuna (Thunnus alalunga). J. Aquat. Food Prod. Technol. 2004, 13, 41–52. [Google Scholar] [CrossRef]
- Nakao, M.; Seoka, M.; Tsukamasa, Y.; Kawasaki, K.I.; Ando, M. Possibility for Decreasing of Mercury Content in Bluefin Tuna Thunnus orientalis by Fish Culture. Fish. Sci. 2007, 73, 724–731. [Google Scholar] [CrossRef]
- Kawakami, H.; Amakura, Y.; Tsutsumi, T.; Sasaki, K.; Iketsu, A.; Inasaki, M.; Kubota, E.; Toyoda, M. Correlation of Fat Content and Dioxins, Total Mercury and Methyl Mercury Levels in Tuna. Shokuhin eiseigaku zasshi. J. Food Hyg. Soc. Jpn. 2010, 51, 258–263. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Ralston, C.R.; Blackwell, J.L.; Raymond, L.J. Dietary and Tissue Selenium in Relation to Methylmercury Toxicity. Neurotoxicology 2008, 29, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Sandheinrich, M. Effects of Dietary Methylmercury on Reproduction of Fish. Environ. Sci. Technol. 2002, 877–883. [Google Scholar] [CrossRef]
- Dang, F.; Wang, W.X. Antagonistic Interaction of Mercury and Selenium in a Marine Fish Is Dependent on Their Chemical Species. Environ. Sci. Technol. 2011, 45, 3116–3122. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Ralston, C.R.; Raymond, L.J. Selenium Health Benefit Values: Updated Criteria for Mercury Risk Assessments. Biol. Trace Elem. Res. 2016, 171, 262–269. [Google Scholar] [CrossRef]
- Balshaw, S.; Edwards, J.W.; Ross, K.E.; Daughtry, B.J. Mercury Distribution in the Muscular Tissue of Farmed Southern Bluefin Tuna (Thunnus maccoyii) Is Inversely Related to the Lipid Content of Tissues. Food Chem. 2008, 111, 616–621. [Google Scholar] [CrossRef]
- Balshaw, S.; Edwards, J.W.; Ross, K.E.; Ellis, D.; Padula, D.J.; Daughtry, B.J. Empirical Models to Identify Mechanisms Driving Reductions in Tissue Mercury Concentration during Culture of Farmed Southern Bluefin Tuna Thunnnus maccoyii. Mar. Pollut. Bull. 2008, 56, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council of the European Union. Regulation (EU) No 508/2014 of the European Parliament and of the Council of 15 May 2014 on the European Maritime and Fisheries Fund and Repealing Council Regulations N° 2328/2003, N° 861/2006 and N° 791/2007 and Regulation N° 1255/2011 of the European Pa. Off. J. Eur. Union 2014, L149, 1–66. [Google Scholar]
- Api, M.; Bonfanti, E.; Lombardo, F.; Pignalosa, P.; Hardiman, G.; Carnevali, O. Effects of Age on Growth in Atlantic Bluefin Tuna (Thunnus thynnus). Gen. Comp. Endocrinol. 2018, 265, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, C.; Annibaldi, A.; Illuminati, S.; Antonucci, M.; Api, M.; Scarponi, G.; Lombardo, F.; Pignalosa, P.; Carnevali, O. Characterization of the Fatty Acid Composition in Cultivated Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle by Gas Chromatography-Mass Spectrometry. Anal. Lett. 2018, 51, 2981–2993. [Google Scholar] [CrossRef]
- Ramalhosa, M.J.; Paíga, P.; Morais, S.; Rui Alves, M.; Delerue-Matos, C.; Oliveira, M.B.P.P. Lipid Content of Frozen Fish: Comparison of Different Extraction Methods and Variability during Freezing Storage. Food Chem. 2012, 131, 328–336. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Scarponi, G. Recent Temporal Variations of Trace Metal Content in an Italian White Wine. Food Chem. 2014, 159, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, C.; Illuminati, S.; Annibaldi, A.; Finale, C.; Rossetti, M.; Scarponi, G. Physicochemical Properties of Honey from Marche, Central Italy: Classification of Unifloral and Multifloral Honeys by Multivariate Analysis. Nat. Prod. Commun. 2014, 9, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Nthunya, L.N.; Maifadi, S.; Mamba, B.B.; Verliefde, A.R.; Mhlanga, S.D. Spectroscopic Determination of Water Salinity in Brackish Surface Water in Nandoni Dam, at Vhembe District, Limpopo Province, South Africa. Water 2018, 10, 990. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Masheane, M.L.; Malinga, S.P.; Nxumalo, E.E.; Mamba, B.B.; Mhlanga, S.D. Determination of toxic metals in drinking water sources in the Chief Albert Luthuli Local Municipality in Mpumalanga, South Africa. Phys. Chem. Earth Parts A/B/C 2017, 100, 94–100. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Dickey, D.A.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill: New York, NY, USA, 1997; ISBN 0070610282. [Google Scholar]
- Vizzini, S.; Tramati, C.; Mazzola, A. Comparison of Stable Isotope Composition and Inorganic and Organic Contaminant Levels in Wild and Farmed Bluefin Tuna, Thunnus thynnus, in the Mediterranean Sea. Chemosphere 2010, 78, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Anual, Z.F.; Maher, W.; Krikowa, F.; Hakim, L.; Ahmad, N.I.; Foster, S. Mercury and Risk Assessment from Consumption of Crustaceans, Cephalopods and Fish from West Peninsular Malaysia. Microchem. J. 2018, 140, 214–221. [Google Scholar] [CrossRef]
- Baldi, F.; Renzoni, A.; Berhnard, M. Mercury concentration in pelagic fishes (anchovy, mackerel and sardine) from the Italian coast and Strait of Gibraltar. Journées d’Études Pollutions Antalya CIESM 1978, 4, 251–254. [Google Scholar]
- Renzoni, A.; Zino, F.; Franchi, E. Mercury Levels Along the Food Chain. Environ Res. 1998, 72, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Jardine, L.B.; Burt, M.D.B.; Arp, P.A.; Diamond, A.W. Mercury Comparisons between Farmed and Wild Atlantic Salmon (Salmo salar L.) and Atlantic Cod (Gadus morhua L.). Aquac. Res. 2009, 40, 1148–1159. [Google Scholar] [CrossRef]
- Cuvin-Aralar, M.L.A.; Furness, R.W. Mercury and Selenium Interaction: A Review. Ecotoxicol. Environ. Saf. 1991, 21, 348–364. [Google Scholar] [CrossRef]
- Khan, M.A.K.; Wang, F. Mercury-Selenium Compounds and Their Toxicological Significance: Toward a Molecular Understanding of the Mercury-Selenium Antagonism. Environ. Toxicol. Chem. 2009, 28, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yamashita, M.; Iida, H. Selenium Content in Seafood in Japan. Nutrients 2013, 5, 388–395. [Google Scholar] [CrossRef]
- Kroepfl, N.; Jensen, K.B.; Francesconi, K.A.; Kuehnelt, D. Human Excretory Products of Selenium Are Natural Constituents of Marine Fish Muscle. Anal. Bioanal. Chem. 2015, 407, 7713–7719. [Google Scholar] [CrossRef]
- Bryszewska, M.A.; Måge, A. Determination of Selenium and Its Compounds in Marine Organisms. J. Trace Elem. Med. Biol. 2015, 29, 91–98. [Google Scholar] [CrossRef]
- Bergés-Tiznado, M.E.; Márquez-Farías, J.F.; Torres-Rojas, Y.; Galván-Magaña, F.; Páez-Osuna, F. Mercury and selenium in tissues and stomach contents of the migratory sailfish, Istiophorus platypterus, from the Eastern Pacific: Concentration, biomagnification, and dietary intake. Mar. Poll. Bull. 2015, 101, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Grasso, A.; Fiore, M.; Cristaldi, A.; Zuccarello, P.; Signorelli, S.S.; Conti, G.O.; Ferrante, M. Trace Elements in Seafood from the Mediterranean Sea: An Exposure Risk Assessment. Food Chem. Toxicol. 2018, 115, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.J.; Ralston, N.V.C. Selenium and Mercury in Pelagic Fish in the Central North Pacific near Hawaii. Biol. Trace Elem. Res. 2007, 119, 242–254. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health & Human Services the Office of Dietary Supplements (ODS) of the National Institutes of Health (NIH). Selenium Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/ (accessed on 18 December 2018).
- Srebocan, E.; Pompe-Gotal, J.; Prevendar-Crnic, A.; Ofner, E. Mercury concentrations in captive Atlantic bluefin tuna (Thunnus thynnus) farmed in the Adriatic Sea. Vet. Med. 2007, 52, 175–177. [Google Scholar] [CrossRef]
- Milatou, N.; Dassenakis, M.; Megalofonou, P. Do Fattening Process and Biological Parameters Affect the Accumulation of Metals in Atlantic Bluefin Tuna? Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 1129–1139. [Google Scholar] [CrossRef]
- Ferrara, R.; Maserti, B. Mercury in the Mediterranean Basin. Mar. Pollut. Bull. 1986, 17, 533–534. [Google Scholar] [CrossRef]
- Ugarte, A.; Abrego, Z.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Evaluation of the Bioaccumulation of Trace Elements in Tuna Species by Correlation Analysis between Their Concentrations in Muscle and First Dorsal Spine Using Microwave-Assisted Digestion and ICP-MS. Int. J. Environ. Anal. Chem. 2012, 92, 1761–1775. [Google Scholar] [CrossRef]
- Olmedo, P.; Hernández, A.F.; Pla, A.; Femia, P.; Navas-Acien, A.; Gil, F. Determination of Essential Elements (Copper, Manganese, Selenium and Zinc) in Fish and Shellfish Samples. Risk and Nutritional Assessment and Mercury-Selenium Balance. Food Chem. Toxicol. 2013, 62, 299–307. [Google Scholar] [CrossRef]
- Cabañero, A.I.; Carvalho, C.; Madrid, Y.; Batoréu, C.; Cámara, C. Quantification and Speciation of Mercury and Selenium in Fish Samples of High Consumption in Spain and Portugal. Biol. Trace Elem. Res. 2005, 103, 17–36. [Google Scholar] [CrossRef]
- Burger, J.; Jeitner, C.; Gochfeld, M. Locational Differences in Mercury and Selenium Levels in 19 Species of Saltwater Fish from New Jersey. J. Toxicol. Environ. Heal. Part A Curr. Issues 2011, 74, 863–874. [Google Scholar] [CrossRef]
- Kehrig, H.A.; Seixas, T.G.; Di Beneditto, A.P.M.; Malm, O. Selenium and Mercury in Widely Consumed Seafood from South Atlantic Ocean. Ecotoxicol. Environ. Saf. 2013, 93, 156–162. [Google Scholar] [CrossRef]
- Polak-Juszczak, L. Selenium and Mercury Molar Ratios in Commercial Fish from the Baltic Sea: Additional Risk Assessment Criterion for Mercury Exposure. Food Control 2015, 50, 881–888. [Google Scholar] [CrossRef]
- Licata, P.; Trombetta, D.; Cristani, M.; Naccari, C.; Martino, D.; Calò, M.; Naccari, F. Heavy Metals in Liver and Muscle of Bluefin Tuna (Thunnus thynnus) Caught in the Straits of Messina (Sicily, Italy). Environ. Monit. Assess. 2005, 107, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Storelli, A.; Marcotrigiano, G.O. Accumulation of Mercury, Cadmium, Lead and Arsenic in Swordfish and Bluefin Tuna from the Mediterranean Sea: A Comparative Study. Mar. Pollut. Bull. 2005, 50, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, F.; Medina, J.; Nsuategui, J.; Conesa, M. Heavy Metal Concentrations in Some Marine Organisms from the Mediterranean Sea (Castellon, Spain): Metal Accumulation in Different Tissues. Sci. Mar. 1990, 54, 113–130. [Google Scholar]
- Burger, J.; Gochfeld, M. Selenium and Mercury Molar Ratios in Saltwater Fish from New Jersey: Individual and Species Variability Complicate Use in Human Health Fish Consumption Advisories. Environ. Res. 2012, 114, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Potortì, A.G.; Lo Turco, V.; Bua, D.; Licata, P.; Cicero, N.; Dugo, G. Trace Elements in Thunnus Thynnus from Mediterranean Sea and Benefit–risk Assessment for Consumers. Food Addit. Contam. Part B Surveill. 2015, 8, 175–181. [Google Scholar] [CrossRef]
- Storelli, M.M.; Marcotrigiano, G.O. Total Mercury Levels in Muscle Tissue of Swordfish (Xiphias gladius) and Bluefin Tuna (Thunnus Thynnus) from the Mediterranean Sea (Italy). J. Food Prot. 2001, 64, 1058–1061. [Google Scholar] [CrossRef]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Marcotrigiano, G.O. Total and Methylmercury Residues in Cartilaginous Fish from Mediterranean Sea. Mar. Pollut. Bull. 2002, 44, 1354–1358. [Google Scholar] [CrossRef]
- Besada, V.; González, J.J.; Schultze, F. Concentraciones de Mercurio, Cadmio, Plomo, Arsénico, Cobre y Zinc En Atún Blanco, Rabil y Patudo Procedentes Del Oca̧no Atlántico. Ciencias Mar. 2006, 32, 439–445. [Google Scholar] [CrossRef]
- Bodin, N.; Lesperance, D.; Albert, R.; Hollanda, S.; Michaud, P.; Degroote, M.; Churlaud, C.; Bustamante, P. Trace Elements in Oceanic Pelagic Communities in the Western Indian Ocean. Chemosphere 2017, 174, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Mercury Accumulation in Yellowfin Tuna (Thunnus albacares) with Regards to Muscle Type, Muscle Position and Fish Size. Food Chem. 2016, 190, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Selenium/Mercury Molar Ratios in Freshwater, Marine, and Commercial Fish from the USA: Variation, Risk, and Health Management. Rev. Environ. Health 2013, 28, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Ordiano-Flores, A.; Rosíles-Martínez, R.; Galván-Magaña, F. Biomagnification of Mercury and Its Antagonistic Interaction with Selenium in Yellowfin Tuna Thunnus albacares in the Trophic Web of Baja California Sur, Mexico. Ecotoxicol. Environ. Saf. 2012, 86, 182–187. [Google Scholar] [CrossRef] [PubMed]
| Sample | Weight, kg | Length, cm | |
|---|---|---|---|
| Male | 2 | 290 | 243 |
| 4 | 327 | 256 | |
| 5 | 211 | 233 | |
| 8 | 258 | 247 | |
| 13 | 289 | 244 | |
| 14 | 216 | 222 | |
| 15 | 93 | 177 | |
| 24 | 122 | 186 | |
| 30 | 408 | 270 | |
| 31 | 298 | 247 | |
| 34 | 294 | 247 | |
| 35 | 317 | 252 | |
| 36 | 379 | 269 | |
| 37 | 308 | 253 | |
| 38 | 287 | 246 | |
| 40 | 306 | 255 | |
| 41 | 355 | 260 | |
| 42 | 334 | 255 | |
| Female | 1 | 305 | 244 |
| 3 | 268 | 252 | |
| 7 | 168 | 217 | |
| 9 | 225 | 228 | |
| 10 | 190 | 223 | |
| 11 | 188 | 218 | |
| 12 | 160 | 210 | |
| 17 | 323 | 255 | |
| 18 | 183 | 220 | |
| 19 | 220 | 225 | |
| 20 | 54 | 154 | |
| 21 | 57 | 149 | |
| 22 | 89 | 169 | |
| 23 | 88 | 180 | |
| 25 | 233 | 227 | |
| 27 | 159 | 204 | |
| 28 | 195 | 228 | |
| 29 | 227 | 229 | |
| 33 | 320 | 247 | |
| 39 | 302 | 250 |
| Sample | Weight, kg | Length, cm | |
|---|---|---|---|
| Male | 89 | 32.3 | 122 |
| 90 | 42.1 | 129 | |
| 91 | 57.4 | 152 | |
| 92 | 46.4 | 138 | |
| 93 | 51.9 | 142 | |
| 95 | 29 | 111 | |
| 96 | 44.8 | 136 | |
| 97 | 34.2 | 125 | |
| 204 | 48.3 | 135 | |
| 206 | 38.1 | 121 | |
| 208 | 22.3 | 110 | |
| 210 | 56.2 | 143 | |
| 212 | 45.7 | 128 | |
| 213 | 47.1 | 128 | |
| 215 | 38.8 | 122 | |
| 357 | 27.5 | 114 | |
| 361 | 50.7 | 140 | |
| 365 | 27.5 | 117 | |
| Female | 88 | 37.1 | 124 |
| 94 | 49.1 | 118 | |
| 203 | 51.9 | 146 | |
| 209 | 39 | 126 | |
| 216 | 47.4 | 133 | |
| 354 | 39 | 130126 | |
| 355 | 35.9 | 125 | |
| 356 | 30.9 | 121 | |
| 360 | 45.7 | 140 | |
| 362 | 45.2 | 135 | |
| 363 | 56.2 | 147 | |
| 364 | 48.3 | 138 | |
| 366 | 32 | 125 |
| Sampling Area | Length (cm) or Weight (kg) | Hg Tot. (mg/kg ww) M ± DS (Min-Max) | Se tot. (mg/kg ww) M ± DS (Min-Max) | Se:Hg Molar Ratio | HBVSe M ± DS (Min-Max) | References |
|---|---|---|---|---|---|---|
| T. thynnus: | ||||||
| Malta (farmed) | 238 ± 93 (W) | 0.61 ± 0.20 | 1.07 ± 0.86 | 5.48 | (−7.69–46.88) | This study |
| Sardinia | 45 ± 26 (W) | 1.68 ± 0.58 | 0.64 ± 0.31 | 1.32 | (−59.39–10.7) | This study |
| Ionian Sea (farmed) | 8–540 (W) | (0.28-1.28) | [51] | |||
| Australia | 16.2–41.6 (W) | 0.32 ± 0.03 | [37] | |||
| Mediterranea Sea (farmed) | - | 0.54–0.67 | [50] | |||
| Japan | - | 0.33–0.61 | [20] | |||
| Strait of Messina | 50–190 (W) | (2.45–4.21) | [59] | |||
| Ionian Sea | 2.85–4.36 (W) | (0.13–0.35) | [60] | |||
| Castellon (ES) | 0.7–1.085 (W) | (0.081–0.306) | [61] | |||
| Adriatic Sea (farmed) | 100–300 (W) | (0.490–1.809) | [51] | |||
| Canary Islands (ES) | - | (0.298–0.779) | [54] | |||
| New Jersey (USA) | - | 0.52 ± 0.034 | 0.43 ± 0.038 | 2.07 | 4.15 | [62] |
| Mediterranean Sea | 130–290 (W) | (0.246–0.714) | (0.27–1.21) | [63] | ||
| Tyrrenian Sea | 0.33–158 (W) | (0.07–4.26) | [64] | |||
| Atlantic Ocean | >50 (W) | (0.24–0.90) | (0.58–2.3) | [53] | ||
| Mediterranean Sea | 5.3–83 (W) | (0.16–2.59) | [65] | |||
| T. alalunga: | ||||||
| Atlantic Ocean | - | (0.118–0.564) | [66] | |||
| Indian Ocean | 5.3–83 (W) | 0.478 ± 0.14 | [67] | |||
| Central Pacific Ocean | 22.6 ± 3.8 (L) | 0.50 ± 0.24 | 0.88 ± 0.19 | 5.26 | 10.4 | [16,48] |
| Mediterranea Sea | 4.0–8.7 (W) | 1.17 ± 0.23 | [65] | |||
| T. albacares: | ||||||
| South Africa (Atlantic Ocean) | 29–50.8 (W) | 0.80 ± 0.25 | [68] | |||
| Atlantic Ocean | - | (0.166–0.531) | [66] | |||
| New Jersey (USA) | - | 0.20 ± 0.025 | 0.47 ± 0.027 | 6.11 | [62] | |
| New Jersey (USA) | - | 0.43 ± 0.05 | 0.62 ± 0.03 | 3.6 | [69] | |
| Indian Ocean | 113 ± 13 (L) | 0.38 ± 0.17 | [67] | |||
| Pacific Ocean | 41.1 ± 16.7 (L) | 0.30 ± 0.18 | 1.25 ± 0.27 | 14.12 | 15.6 | [24,48] |
| Mexico | 60.5–94.2 (L) | 10.29 | 6.55 | [70] | ||
| Atlantic Ocean | - | (0.22–1.3) | (0.87–1.8) | [53] | ||
| T. obesus: | ||||||
| Atlantic Ocean | - | (0.344–1.29) | [66] | |||
| Indian Ocean | 87 ± 46 (L) | 0.339 ± 0.29 | [69] | |||
| Pacific Ocean | 41.2 ± 20.4 (L) | 0.60 ± 0.25 | 0.99 ± 0.28 | 5.17 | 10.0 | [24,48] |
| Thunnusspp.: | ||||||
| Portugal market | - | 0.31 ± 0.01 | 0.92 ± 0.01 | 8 | [9,55] | |
| Portugal market | - | 0.37 ± 0.02 | 0.72 ± 0.01 | 4.9 | [9] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle. Molecules 2019, 24, 1273. https://doi.org/10.3390/molecules24071273
Annibaldi A, Truzzi C, Carnevali O, Pignalosa P, Api M, Scarponi G, Illuminati S. Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle. Molecules. 2019; 24(7):1273. https://doi.org/10.3390/molecules24071273
Chicago/Turabian StyleAnnibaldi, Anna, Cristina Truzzi, Oliana Carnevali, Paolo Pignalosa, Martina Api, Giuseppe Scarponi, and Silvia Illuminati. 2019. "Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle" Molecules 24, no. 7: 1273. https://doi.org/10.3390/molecules24071273
APA StyleAnnibaldi, A., Truzzi, C., Carnevali, O., Pignalosa, P., Api, M., Scarponi, G., & Illuminati, S. (2019). Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle. Molecules, 24(7), 1273. https://doi.org/10.3390/molecules24071273