In Vitro Enzymatic Digestibility of Glutaraldehyde-Crosslinked Chitosan Nanoparticles in Lysozyme Solution and Their Applicability in Pulmonary Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
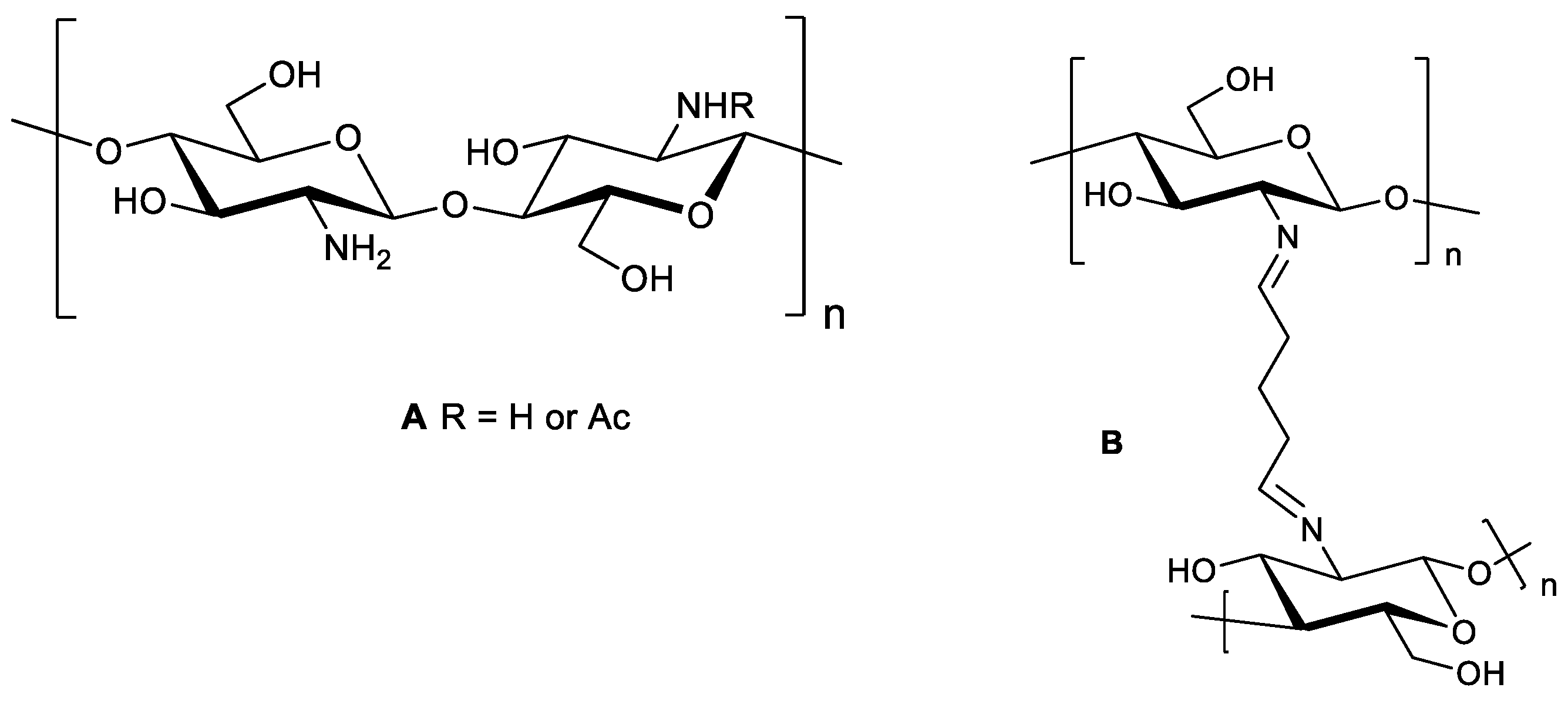
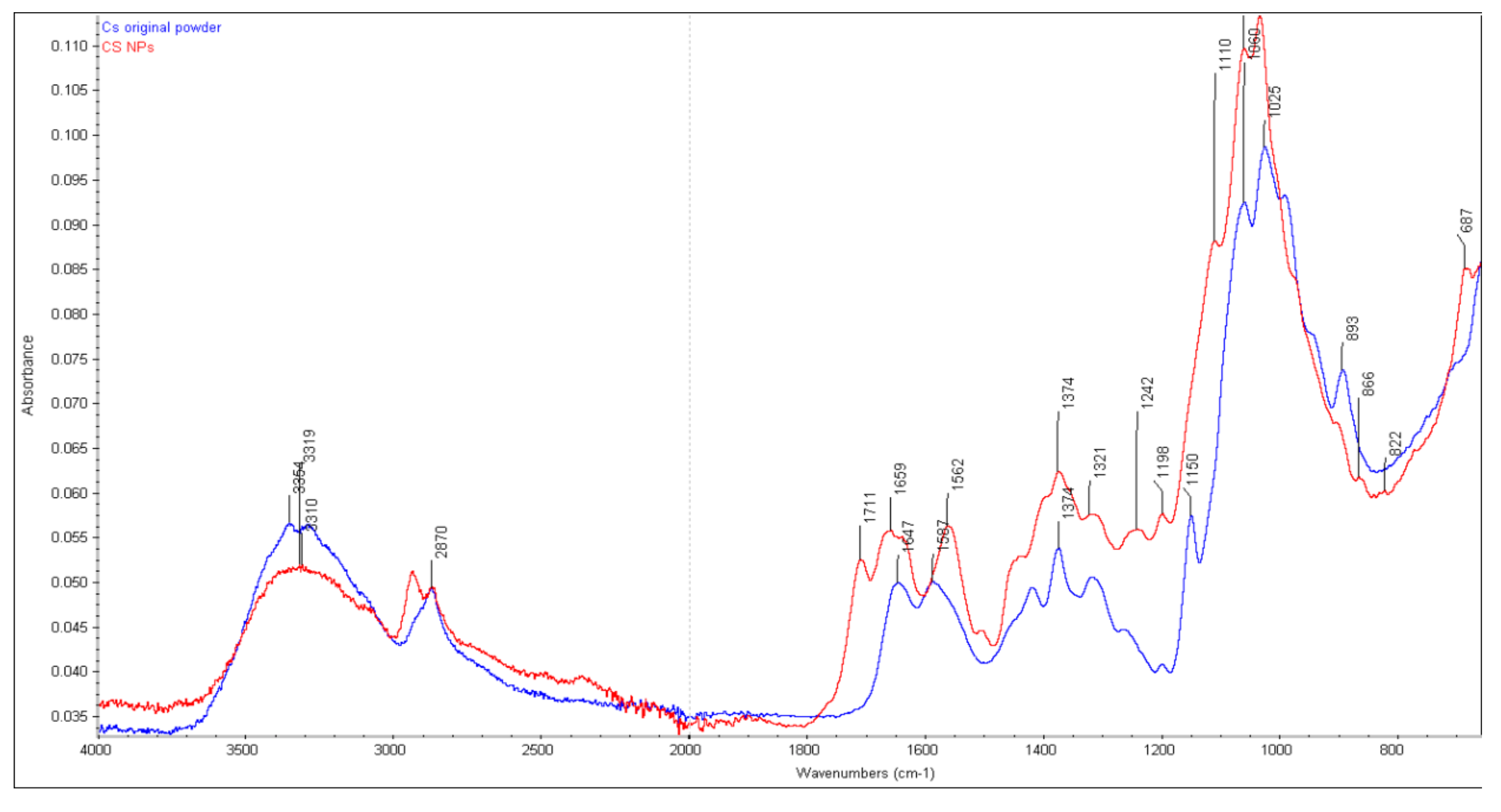
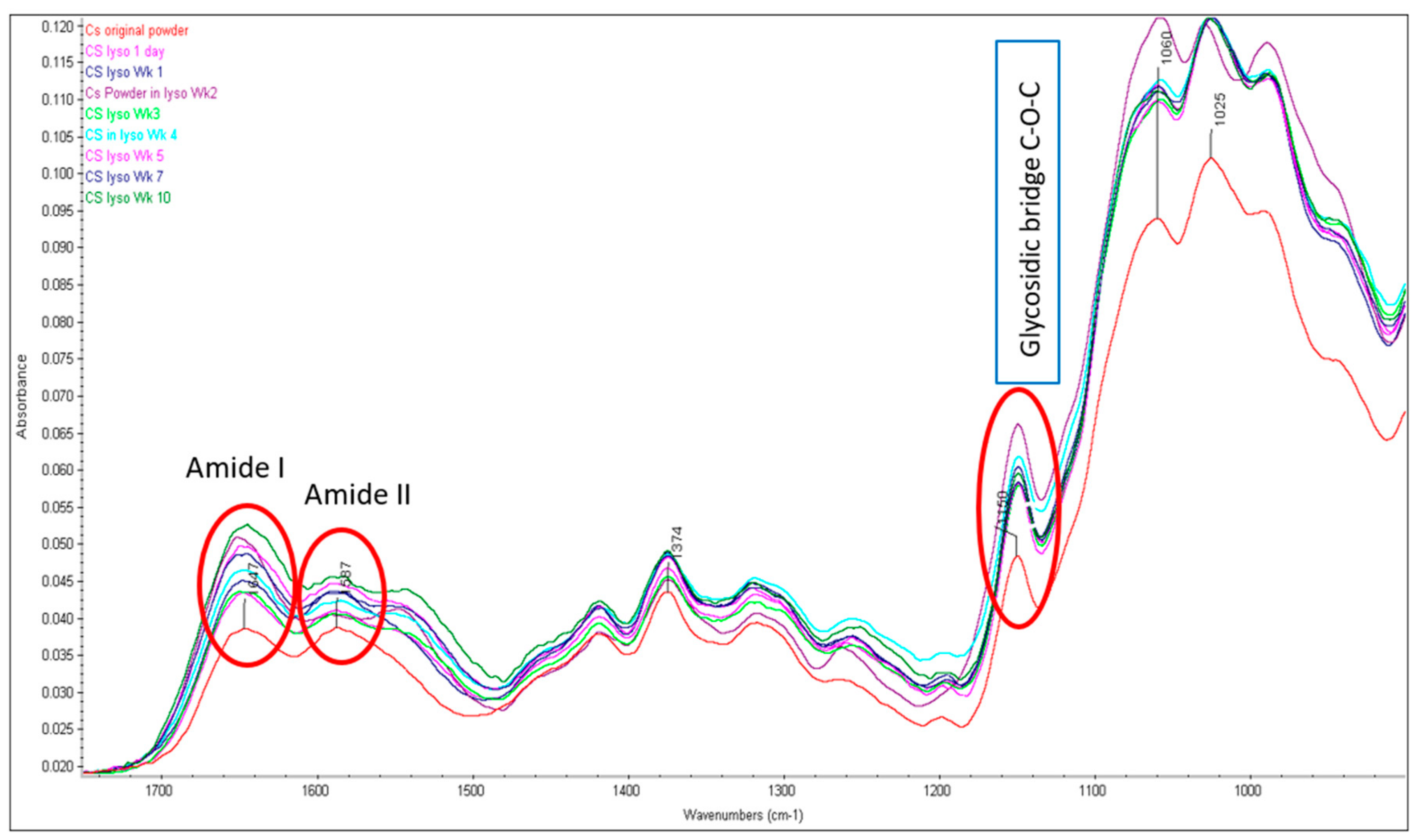
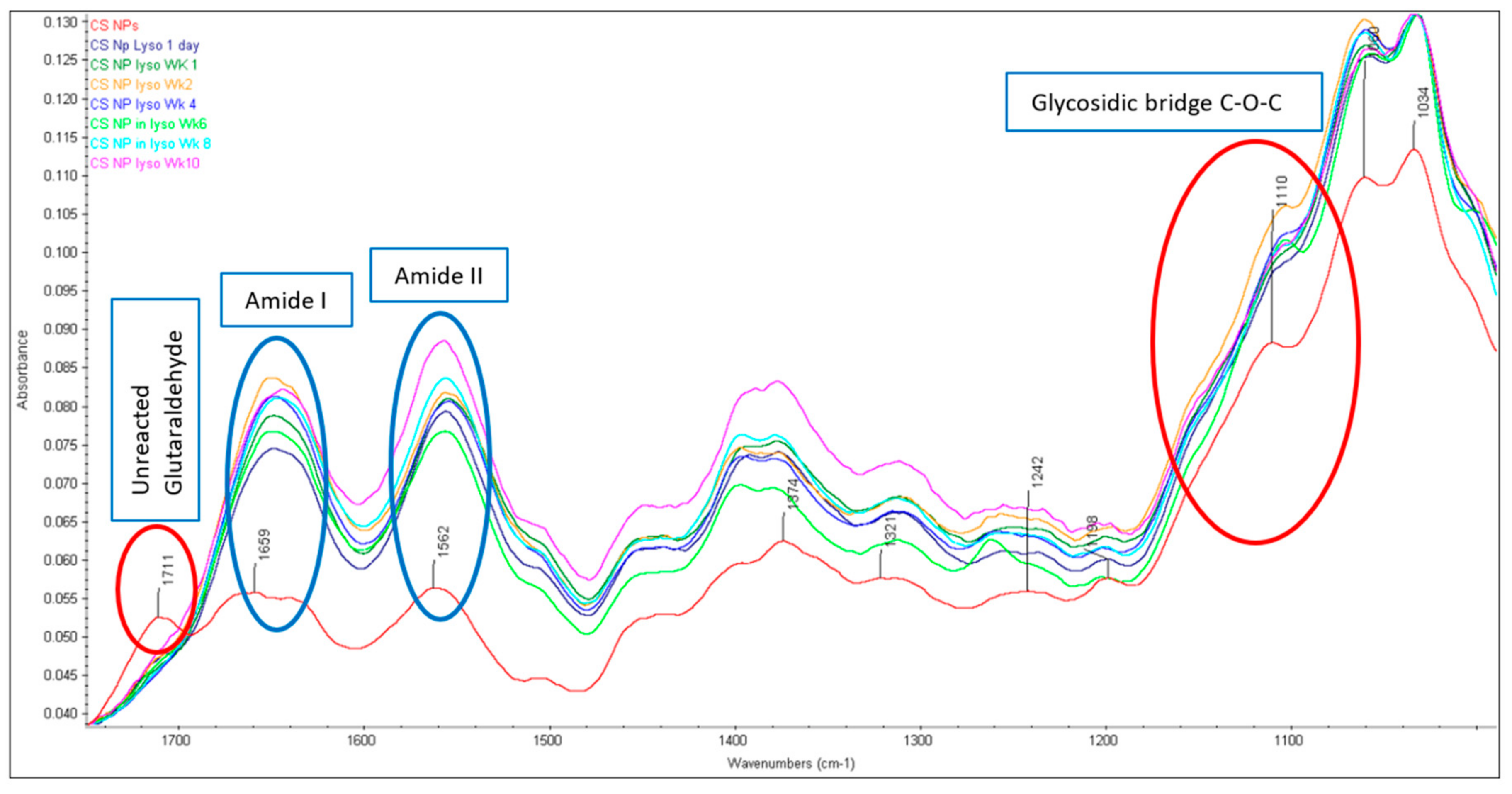
2.1. Structural Integrity Studies of CS by ATR-FTIR Analysis
2.1.1. CS Original Powder
2.1.2. Chitosan NPs
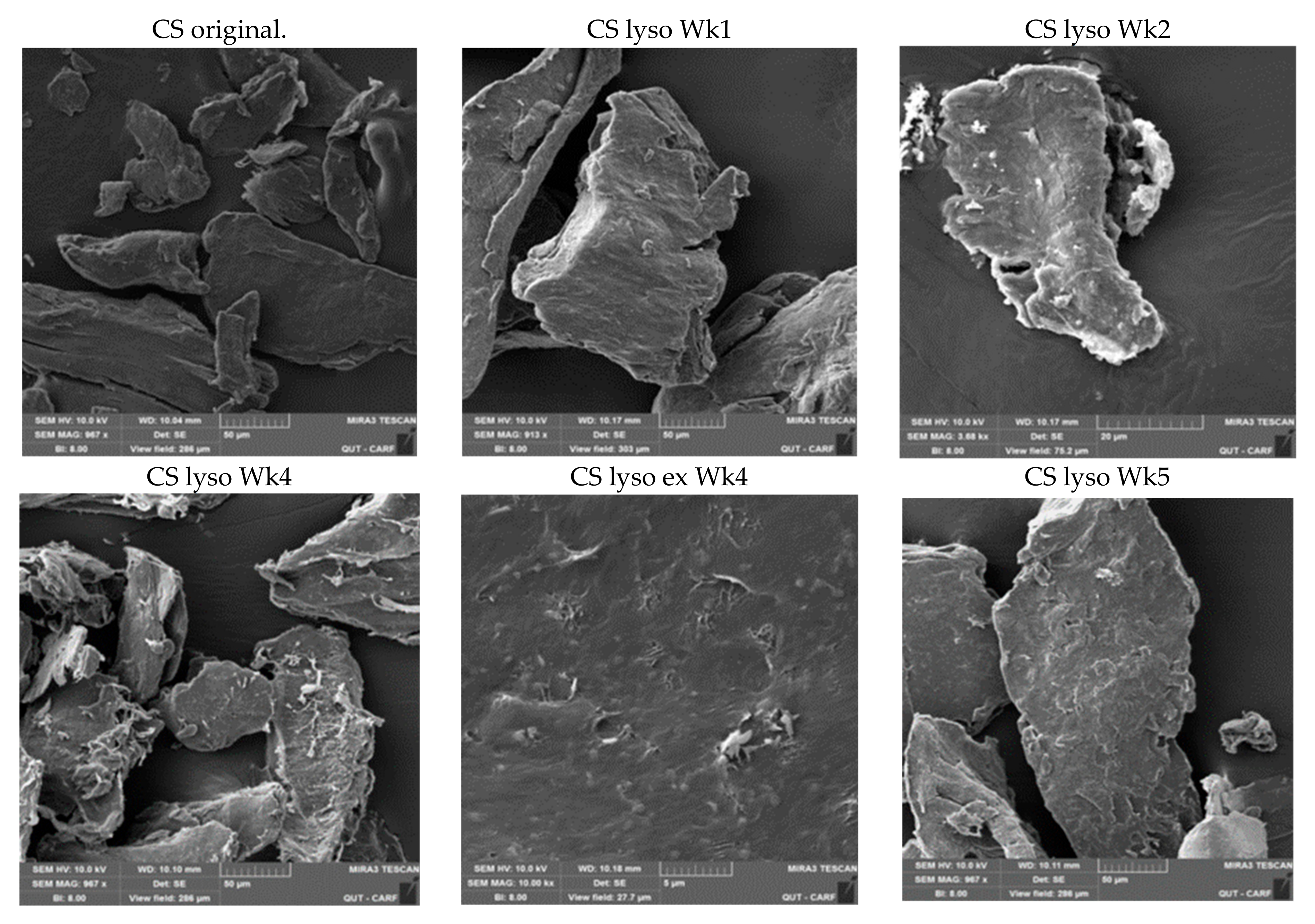
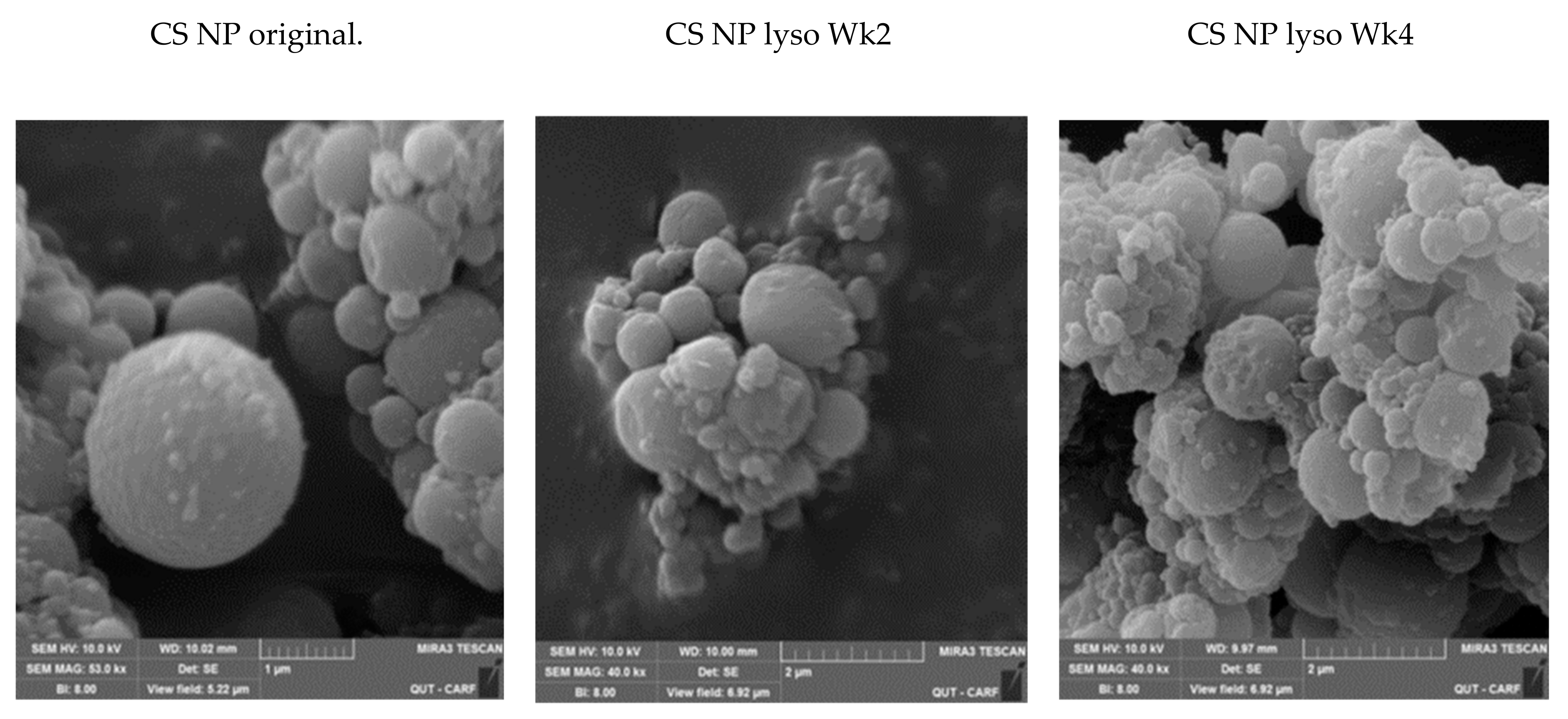
2.2. SEM Studies
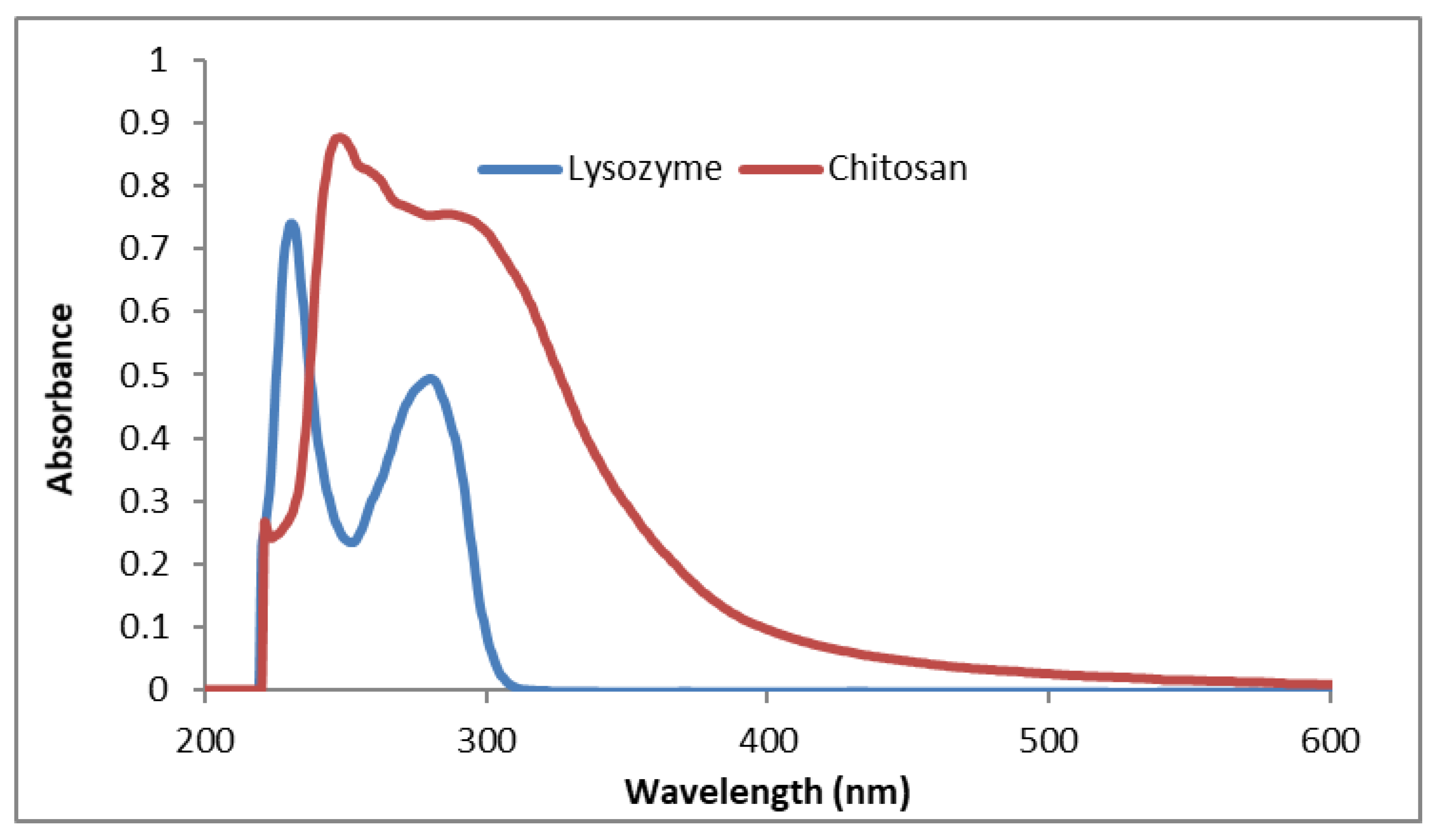
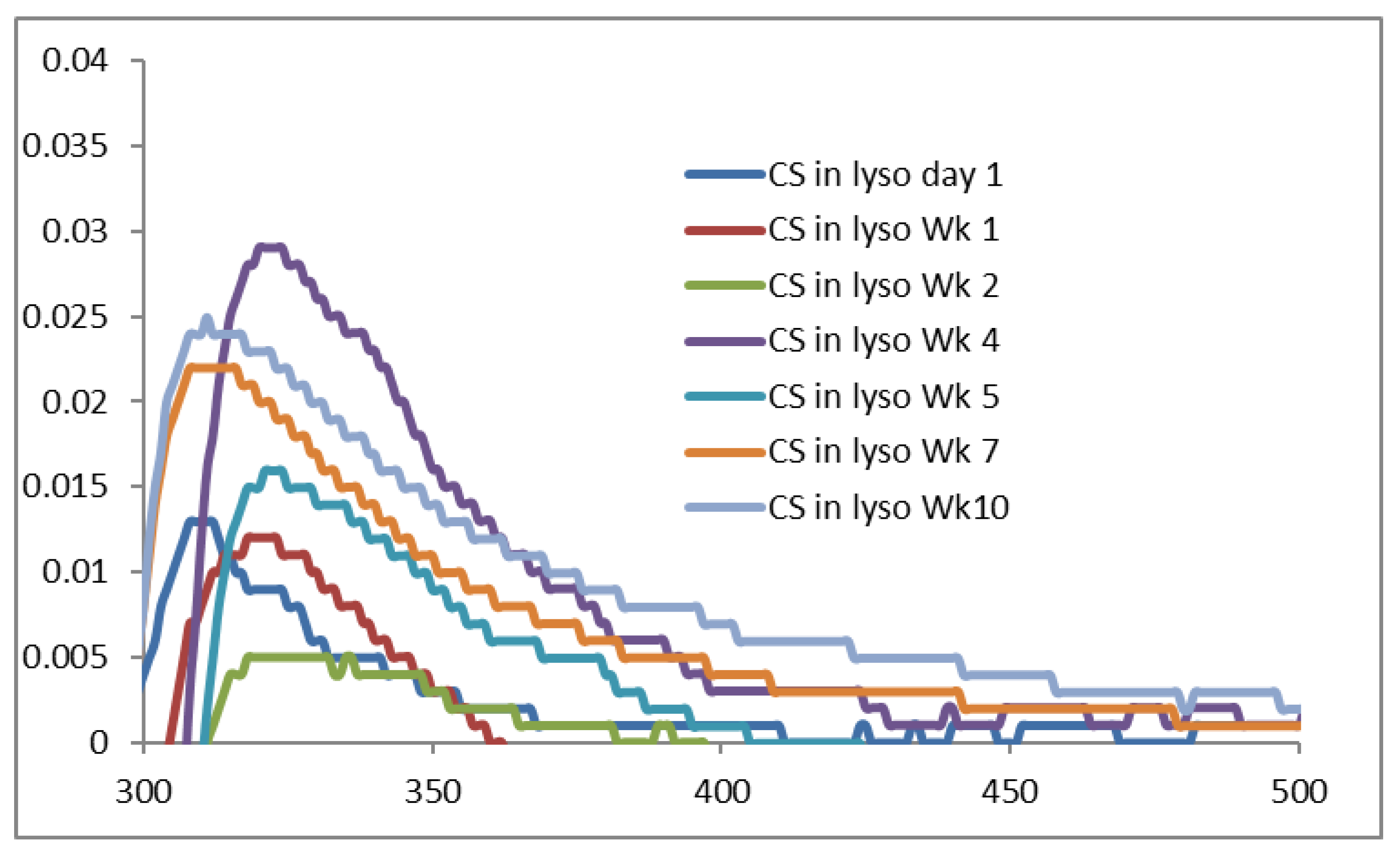
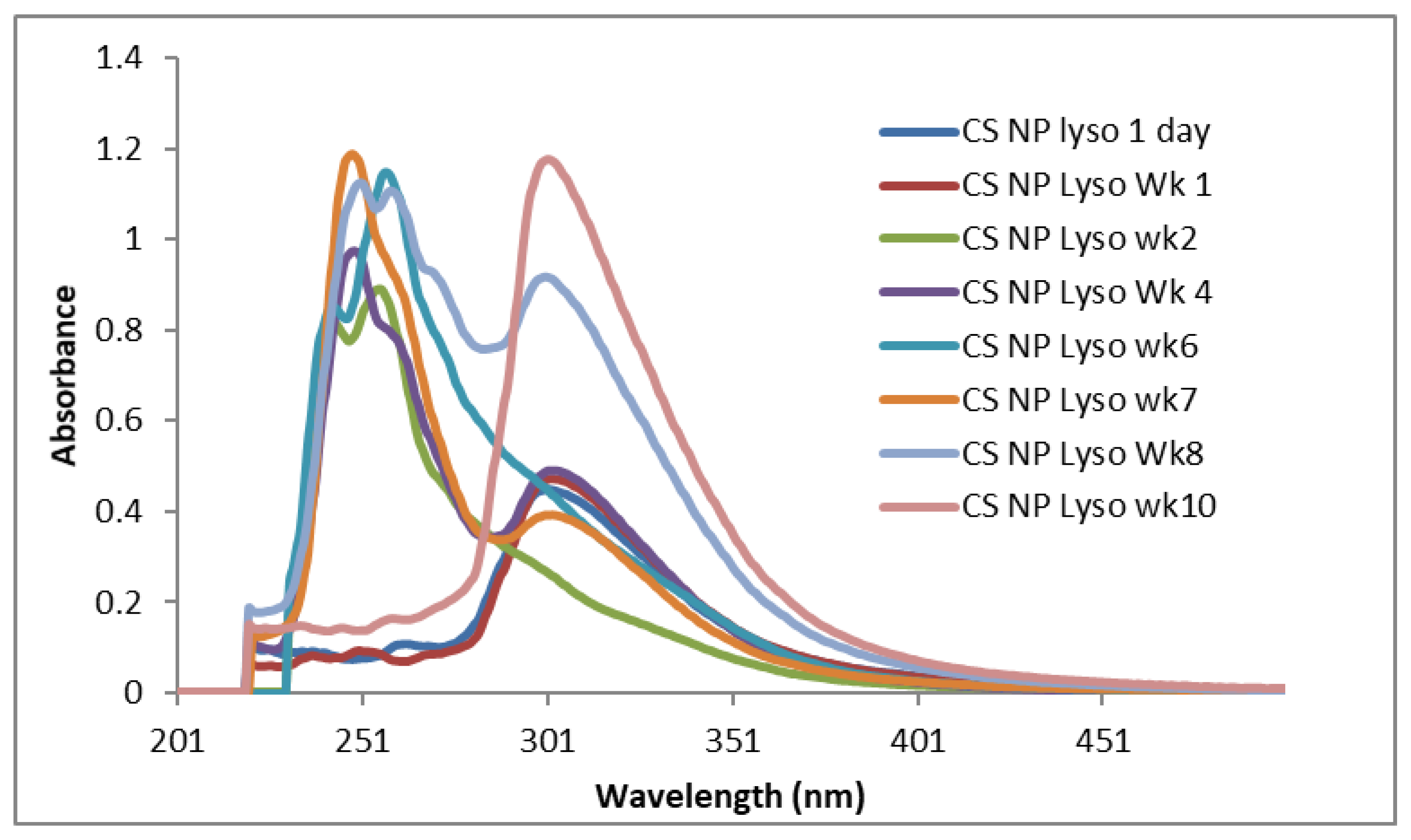
2.3. UV-VIS
2.4. Impact of pH
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Glutaraldehyde Crosslinked CS NPs
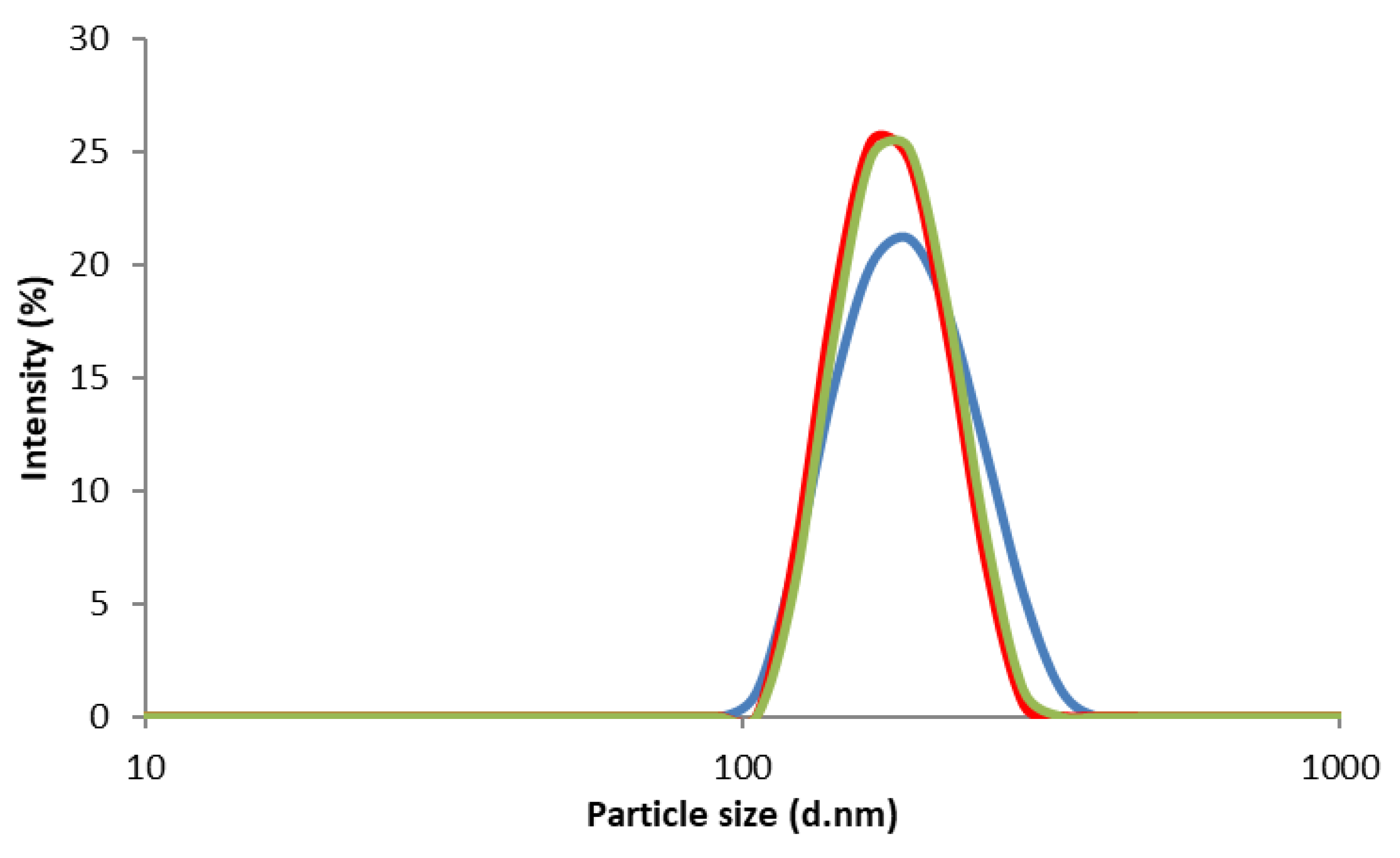
3.2.2. Particle Size and Size Distribution of CS NPs
3.2.3. Degradation Studies
3.2.4. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR)
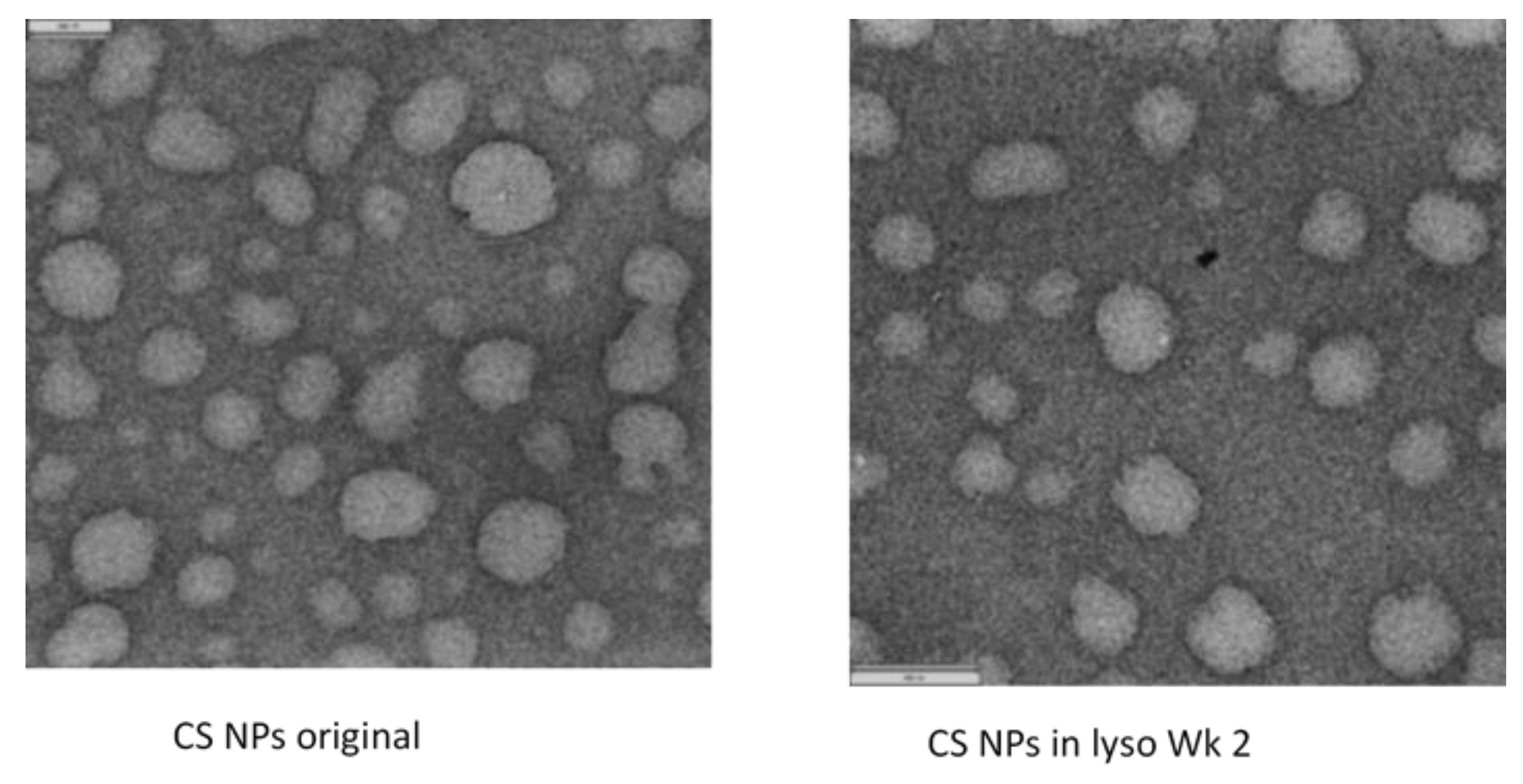
3.2.5. Transmission Electron Microscopy (TEM)
3.2.6. Scanning Electron Microscopy (SEM)
3.2.7. pH Analysis
3.2.8. UV-VIS Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carroll, E.C.; Jin, L.; Mori, A.; Munoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef]
- Islam, N.; Ferro, V. Recent Advances in Chitosan-Based Nanoparticulate Pulmonary Drug Delivery. Nanoscale 2016, 8, 14341–14358. [Google Scholar] [CrossRef]
- Islam, N.; Richard, D. Inhaled Micro/Nanoparticulate Anticancer Drug Formulations: An Emerging Targeted Drug Delivery Strategy for Lung Cancers. Curr. Cancer Drug Targets 2019, 19, 162–178. [Google Scholar] [CrossRef]
- Alpar, H.O.; Somavarapu, S.; Atuah, K.N.; Bramwell, V.W. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv. Drug Deliv. Rev. 2005, 57, 411–430. [Google Scholar] [CrossRef]
- He, P.; Davis, S.S.; Illum, L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Islam, M.A.; Park, T.-E.; Reesor, E.; Cherukula, K.; Hasan, A.; Firdous, J.; Singh, B.; Kang, S.-K.; Choi, Y.-J.; Park, I.-K.; et al. Mucoadhesive Chitosan Derivatives as Novel Drug Carriers. Curr. Pharm. Des. 2015, 21, 4285–4309. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kuno, Y.; Sugimoto, S.; Takeuchi, H.; Kawashima, Y. Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J. Controlled Release 2005, 102, 373–381. [Google Scholar] [CrossRef]
- Grenha, A.; Al-Qadi, S.; Seijo, B.; Remunan-Lopez, C. The potential of chitosan for pulmonary drug delivery. J. Drug Deliv. Sci. Technol. 2010, 20, 33–43. [Google Scholar] [CrossRef]
- Grenha, A.; Grainger, C.I.; Dailey, L.A.; Seijo, B.; Martin, G.P.; Remunan-Lopez, C.; Forbes, B. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur. J. Pharm. Sci. 2007, 31, 73–84. [Google Scholar] [CrossRef]
- Jabbal-Gill, I.; Watts, P.; Smith, A. Chitosan-based delivery systems for mucosal vaccines. Expert Opin Drug Deliv. 2012, 9, 1051–1067. [Google Scholar] [CrossRef]
- Muhsin, M.D.A.; George, G.; Beagley, K.; Ferro, V.; Wang, H.; Islam, N. Effects of Chemical Conjugation of L-Leucine to Chitosan on Dispersibility and Controlled Release of Drug from a Nanoparticulate Dry Powder Inhaler Formulation. Mol. Pharm. 2016, 13, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; George, G.; Bartlett, S.; Gao, C.; Islam, N. Nicotine hydrogen tartrate loaded chitosan nanoparticles: Formulation, characterization and in vitro delivery from dry powder inhaler formulation. Eur. J. Pharm. Biopharm. 2017, 113, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Soares, S.; Costa, A.; Andrade Jose, C.; Seabra, V.; Reis, S.; Sarmento, B. Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-drying. Biomatter 2012, 2, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Verheul, R.J.; Amidi, M.; van Steenbergen, M.J.; van Riet, E.; Jiskoot, W.; Hennink, W.E. Influence of the degree of acetylation on the enzymatic degradation and in vitro biological properties of trimethylated chitosans. Biomaterials 2009, 30, 3129–3135. [Google Scholar] [CrossRef]
- Jeong, E.J.; Choi, M.; Lee, J.; Rhim, T.; Lee, K.Y. The spacer arm length in cell-penetrating peptides influences chitosan/siRNA nanoparticle delivery for pulmonary inflammation treatment. Nanoscale 2015, 7, 20095–20104. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.D.; Frey, I.I.W.H.; Craft, S.; Danielyan, L.; Hallschmid, M.; Schioeth, H.B.; Benedict, C. Intranasal Treatment of Central Nervous System Dysfunction in Humans. Pharm. Res. 2013, 30, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, M.C.; Sousa, J.J.S.; Pais, A.A.C.C.; Cardoso, O.; Murtinho, D.; Serra, M.E.S.; Tewes, F.; Olivier, J.-C. Optimization of levofloxacin-loaded crosslinked chitosan microspheres for inhaled aerosol therapy. Eur. J. Pharm. Biopharm. 2015, 96, 65–75. [Google Scholar] [CrossRef]
- Wang, H.; George, G.; Islam, N. Nicotine-loaded chitosan nanoparticles for dry powder inhaler (DPI) formulations - Impact of nanoparticle surface charge on powder aerosolization. Adv. Powder Technol. 2018, 29, 3079–3086. [Google Scholar] [CrossRef]
- Muhsin, M.D.A.; George, G.A.; Beagley, K.; Ferro, V.; Armitage, C.; Islam, N. Synthesis and Toxicological Evaluation of a Chitosan-L-Leucine Conjugate for Pulmonary Drug Delivery Applications. Biomacromolecules 2014, 15, 3596–3607. [Google Scholar] [CrossRef]
- Monteiro, O.A.C., Jr.; Airoldi, C. Some studies of crosslinking chitosan-glutaraldehyde interaction in a homogeneous system. Int. J. Biol. Macromol. 1999, 26, 119–128. [Google Scholar] [CrossRef]
- US Department of Health and Human Services: Agency for Toxic Substances and Disease Registry. Toxicological Profile for Glutaraldehyde. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=1467&tid=284 (accessed on 28 March 2019).
- Zissu, D.; Bonnet, P.; Binet, S. Histopathological study in B6C3F1 mice chronically exposed by inhalation to glutaraldehyde. Toxicol. Lett. 1998, 95, 131–139. [Google Scholar] [CrossRef]
- Halatek, T.; Opalska, B.; Swiercz, R.; Palczynski, C.; Gorski, P.; Rydzynski, K.; Bernard, A. Glutaraldehyde Inhalation Exposure of Rats: Effects on Lung Morphology, Clara-Cell Protein, and Hyaluronic Acid Levels in BAL. Inhalation Toxicol. 2003, 15, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Jin, H.-E.; Kim, D.-D.; Chung, S.-J.; Shim, W.-S.; Shim, C.-K. Chitosan microspheres as an alveolar macrophage delivery system of ofloxacin via pulmonary inhalation. Int. J. Pharm. 2013, 441, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Y.; Zhou, X. Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: in vitro and in vivo evaluation. Int. J. Mol. Sci. 2014, 15, 3519–3532. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Smyth, H.D.C. Novel cryomilled physically cross-linked biodegradable hydrogel microparticles as carriers for inhalation therapy. J. Microencapsul. 2010, 27, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Poth, N.; Seiffart, V.; Gross, G.; Menze, H.; Dempwolf, W. Biodegradable chitosan nanoparticle coatings on titanium for the delivery of BMP-2. Biomolecules 2015, 5, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Seijo, B.; Remunan-Lopez, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Peniche, C.; Arguelles-Monal, W.; Davidenko, N.; Sastre, R.; Gallardo, A.; San Roman, J. Self-curing membranes of chitosan/PAA IPNs obtained by radical polymerization: preparation, characterization and interpolymer complexation. Biomaterials 1999, 20, 1869–1878. [Google Scholar] [CrossRef]
- Qasim, S.B.; Delaine-Smith, R.M.; Fey, T.; Rawlinson, A.; Rehman, I.U. Freeze gelated porous membranes for periodontal tissue regeneration. Acta Biomater. 2015, 23, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–796, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Thormann, E.; Claesson, P.M.; Tyrode, E. Surface Grafted Chitosan Gels. Part II. Gel Formation and Characterization. Langmuir 2014, 30, 8878–8888. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Jabrail, F.H. Glutaraldehyde cross-linked chitosan microspheres for controlled release of centchroman. Carbohydr. Res. 2007, 342, 2244–2252. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Baldassarre, V.; Conti, F.; Ferrara, P.; Biagini, G.; Gazzanelli, G.; Vasi, V. Biological activity of chitosan: ultrastructural study. Biomaterials 1988, 9, 247–252. [Google Scholar] [CrossRef]
- Nordtveit, R.J.; Vaarum, K.M.; Smidsroed, O. Degradation of partially N-acetylated chitosans with hen egg white and human lysozyme. Carbohydr. Polym. 1996, 29, 163–167. [Google Scholar] [CrossRef]
- Qasim, S.B.; Husain, S.; Huang, Y.; Pogorielov, M.; Deineka, V.; Lyndin, M.; Rawlinson, A.; Ur Rehman, I. In-vitro and in-vivo degradation studies of freeze gelated porous chitosan composite scaffolds for tissue engineering applications. Polym. Degrad. Stab. 2017, 136, 31–38. [Google Scholar] [CrossRef]
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr. Polym. 2017, 155, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Czechowska-Biskup, R.; Rokita, B.; Lotfy, S.; Ulanski, P.; Rosiak, J.M. Degradation of chitosan and starch by 360-kHz ultrasound. Carbohydr. Polym. 2005, 60, 175–184. [Google Scholar] [CrossRef]
- Cho, Y.-W.; Jang, J.; Park, C.R.; Ko, S.-W. Preparation and Solubility in Acid and Water of Partially Deacetylated Chitins. Biomacromolecules 2000, 1, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Skovstrup, S.; Hansen, S.G.; Skrydstrup, T.; Schiott, B. Conformational Flexibility of Chitosan: A Molecular Modeling Study. Biomacromolecules 2010, 11, 3196–3207. [Google Scholar] [CrossRef] [PubMed]
- Nordtveit, R.J.; Vaarum, K.M.; Smidsroed, O. Degradation of fully water-soluble, partially N-acetylated chitosans with lysozyme. Carbohydr. Polym. 1994, 23, 253–260. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Z.; Zhang, F.; Peng, X. In-Vitro Degradation and Cytotoxicity of Gelatin/Chitosan Microspheres for Drug Controlled Release. J. Macromol. Sci. Part A 2014, 51, 646–652. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, J.; Park, H.; Lee, M. Chitosan-based nanoparticles as a sustained protein release carrier for tissue engineering applications. J. Biomed. Mat. Res. Part A 2012, 100A, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, J.; Van Leeuwen-Herberts, T.; Otting-Van de Ruit, M. Determination of lysozyme in serum, urine, cerebrospinal fluid and feces by enzyme immunoassay. Clin. Chim. Acta 1984, 142, 21–30. [Google Scholar] [CrossRef]
- Mazancova, P.; Nemethova, V.; Trelova, D.; Klescikova, L.; Lacik, I.; Razga, F. Dissociation of chitosan/tripolyphosphate complexes into separate components upon pH elevation. Carbohydr. Polym. 2018, 192, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Tomihata, K.; Ikada, Y. In vitro and in vivo degradation of films in chitin and its deacetylated derivatives. Biomaterials 1997, 18, 567–575. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Biodegradable chitosan matrix for the controlled release of steroids. Biomater. Artif. Cells Immobilization Biotechnol. 1991, 19, 745–760. [Google Scholar] [CrossRef]
- Deng, Q.-Y.; Zhou, C.-R.; Luo, B.-H. Preparation and characterization of chitosan nanoparticles containing lysozyme. Pharm. Biol. 2006, 44, 336–342. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Samples | Wk1 | Wk2 | Wk3 | Wk4 | Wk5 | Wk6 | Wk7 | Wk8 | Wk9 | Wk10 |
|---|---|---|---|---|---|---|---|---|---|---|
| CS in Lyso | 7.45 | 7.24 | 7.25 | 7.25 | 7.08 | 6.66 | 6.60 | 6.93 | 7.04 | 7.18 |
| CS NP in Lyso | 7.23 | 7.24 | 7.27 | 7.02 | 6.99 | 6.70 | 6.69 | 6.88 | 6.66 | 6.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, N.; Wang, H.; Maqbool, F.; Ferro, V. In Vitro Enzymatic Digestibility of Glutaraldehyde-Crosslinked Chitosan Nanoparticles in Lysozyme Solution and Their Applicability in Pulmonary Drug Delivery. Molecules 2019, 24, 1271. https://doi.org/10.3390/molecules24071271
Islam N, Wang H, Maqbool F, Ferro V. In Vitro Enzymatic Digestibility of Glutaraldehyde-Crosslinked Chitosan Nanoparticles in Lysozyme Solution and Their Applicability in Pulmonary Drug Delivery. Molecules. 2019; 24(7):1271. https://doi.org/10.3390/molecules24071271
Chicago/Turabian StyleIslam, Nazrul, Hui Wang, Faheem Maqbool, and Vito Ferro. 2019. "In Vitro Enzymatic Digestibility of Glutaraldehyde-Crosslinked Chitosan Nanoparticles in Lysozyme Solution and Their Applicability in Pulmonary Drug Delivery" Molecules 24, no. 7: 1271. https://doi.org/10.3390/molecules24071271
APA StyleIslam, N., Wang, H., Maqbool, F., & Ferro, V. (2019). In Vitro Enzymatic Digestibility of Glutaraldehyde-Crosslinked Chitosan Nanoparticles in Lysozyme Solution and Their Applicability in Pulmonary Drug Delivery. Molecules, 24(7), 1271. https://doi.org/10.3390/molecules24071271






