Preparation of Salvianolic Acid B Disodium Salt Considering the Water Extract Quality Standard
Abstract
:1. Introduction
2. Results and Discussion
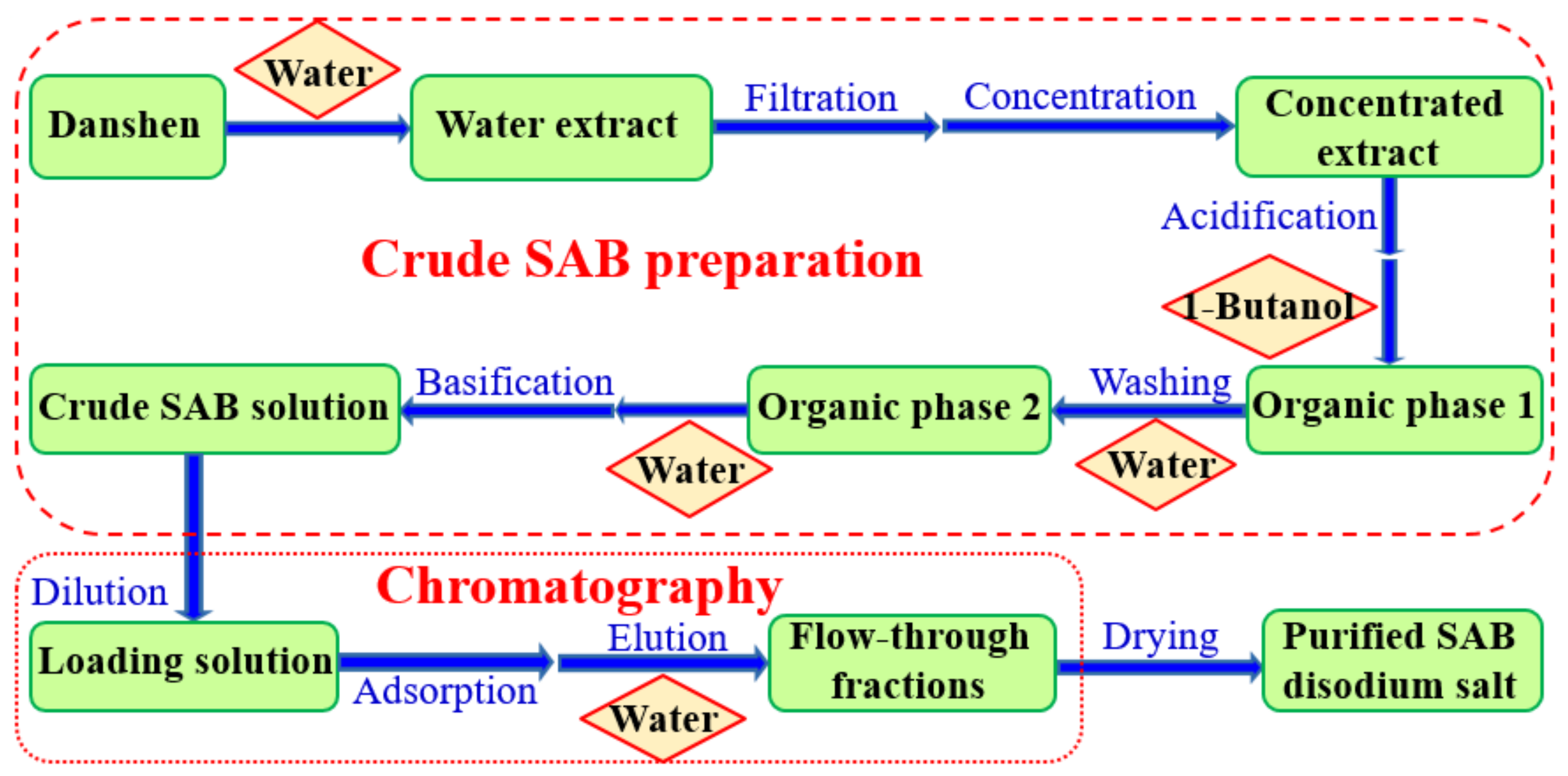
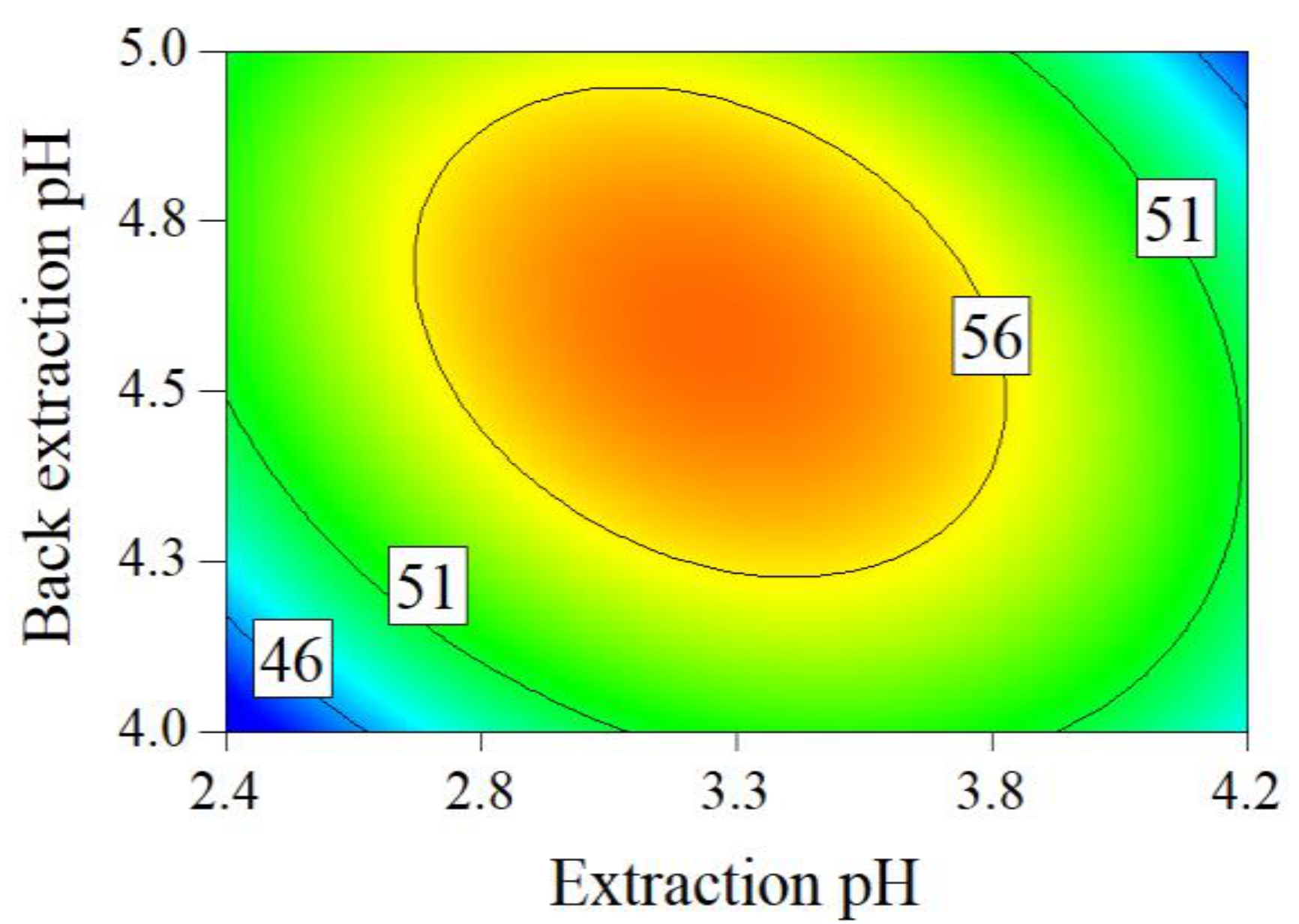
2.1. Crude SAB Preparation
2.2. SAB Purification with Chromatography Process
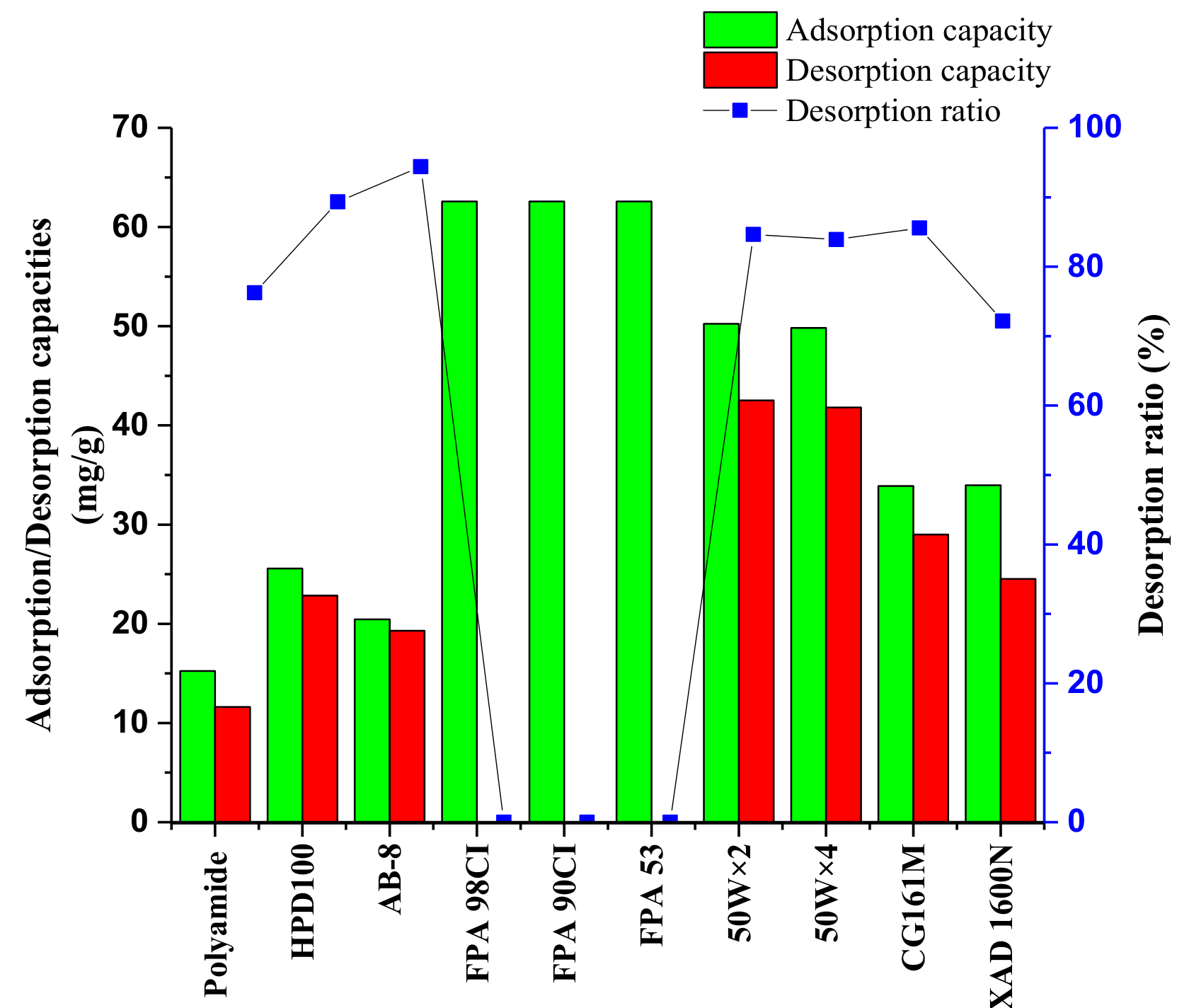
2.2.1. Resin Screening by Adsorption Capacity, Desorption Capacity, and Desorption Ratio
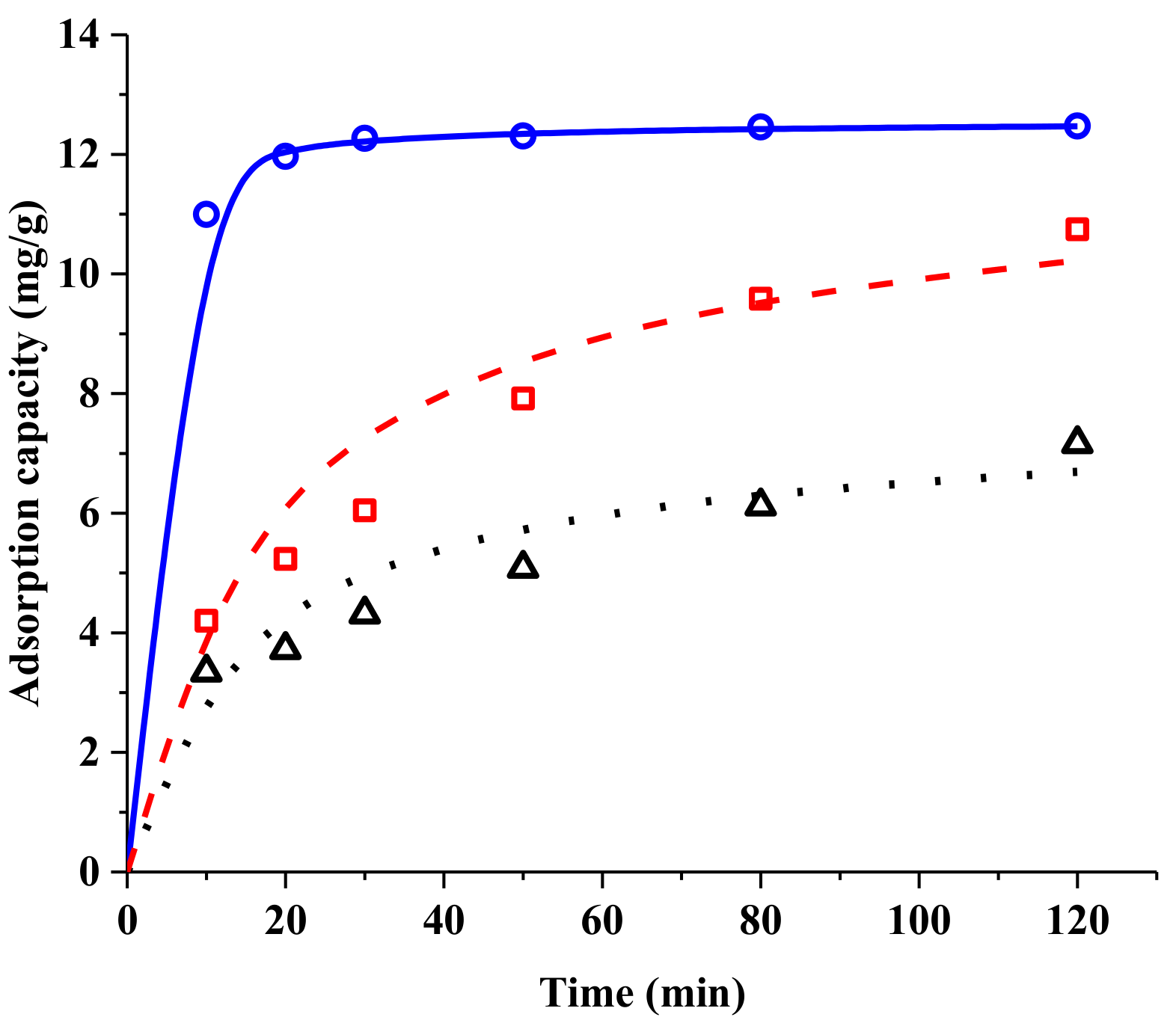
2.2.2. Resin Screening by Adsorption Kinetics
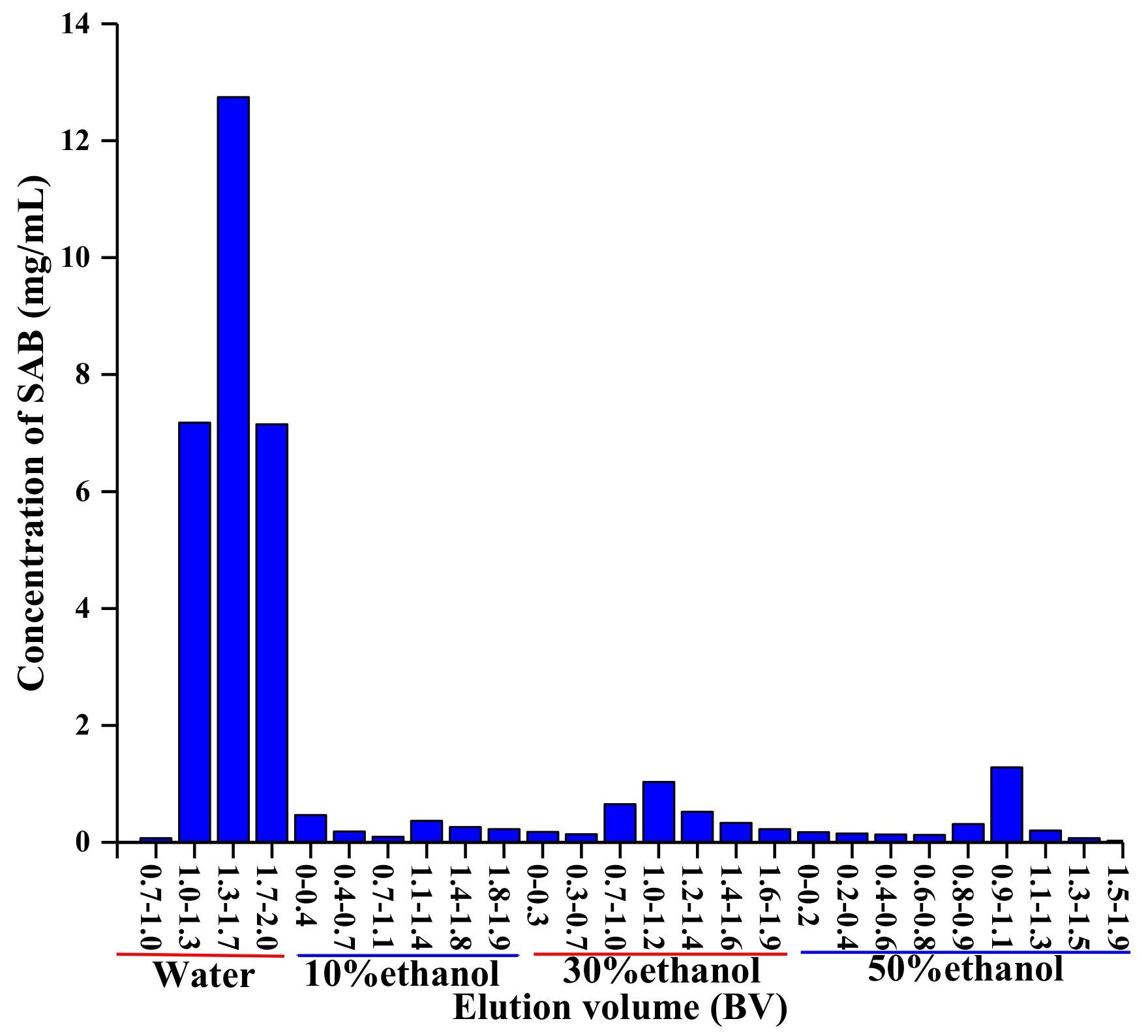
2.2.3. Chromatographic Process
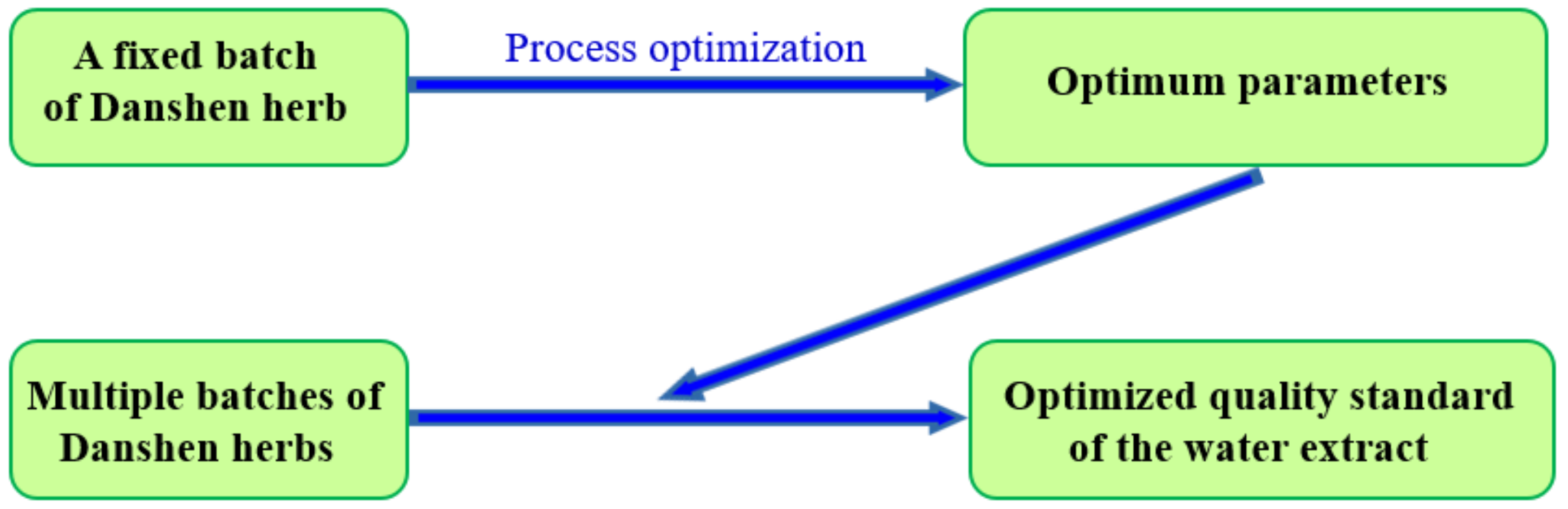
2.3. Water Extract Standard
2.4. Verification
2.5. Identification of SAB Disodium Salt
3. Materials and Methods
3.1. Materials and Reagents
3.2. Optimization of Crude SAB Preparation
3.2.1. Procedures
3.2.2. Experimental Design
3.3. Optimization of Chromatography
3.3.1. Selection of Resins
Adsorbents
Static Adsorption and Desorption Tests
3.3.2. Adsorption Kinetics Tests
3.3.3. Optimization of the Chromatographic Process
3.4. Standard Establishment of the Water Extract
3.5. Analytical Methods
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, W.Y.; Chen, W.J.; Wu, L.F.; Li, S.; Qi, Q.; Cui, Y.P.; Liang, L.J.; Ye, T.; Zhang, L.Z. Quality evaluation and chemical markers screening of salvia miltiorrhiza Bge. (Danshen) based on HPLC fingerprints and HPLC-MSn coupled with chemometrics. Molecules 2017, 22, 478. [Google Scholar] [CrossRef]
- Liu, A.H.; Li, L.; Xu, M.; Lin, Y.H.; Guo, H.Z.; Guo, D.A. Simultaneous quantification of six major phenolic acids in the roots of Salvia miltiorrhiza and four related traditional Chinese medicinal preparations by HPLC-DAD method. J. Pharm. Biomed. Anal. 2006, 41, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.T.; Su, S.L.; Xiang, X.; Sha, X.X.; Zhu, Z.H.; Wang, Y.Y.; Guo, S.; Yan, H.; Qian, D.W.; Duan, J.N. Comparative Analysis of the Major Chemical Constituents in Salvia miltiorrhiza Roots, Stems, Leaves and Flowers during Different Growth Periods by UPLC-TQ-MS/MS and HPLC-ELSD Methods. Molecules 2017, 22, 771. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wei, Y.J.; Qi, L.W.; Li, P.; Qian, Z.M.; Luo, H.W.; Chen, J.; Zhao, J. Determination of fifteen bioactive components in Radix et Rhizoma Salviae Miltiorrhizae by high-performance liquid chromatography with ultraviolet and mass spectrometric detection. Biomed. Chromatogr. 2008, 22, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sun, W.Y.; Sun, G.X.; Zhang, S.J. Total monitoring of the constituents of Danshen tablet using micellar electrokinetic chromatography fingerprinting for antioxidant activity profilin. J. Sep. Sci. 2016, 39, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Wang, Y.P.; Wang, W.; Sun, W.K.; Xu, Y.M. Free radical scavenging and inhibition of lipid peroxidation by magnesium lithospermate B. Acta. Pharmacol. Sin. 2000, 21, 855–858. [Google Scholar]
- Tang, M.K.; Ren, D.C.; Zhang, J.T.; Du, G.H. Effect of salvianolic acids from Radix Salviae miltiorrhizae on regional cerebral blood flow and platelet aggregation in rats. Phytomedicine 2002, 9, 405–409. [Google Scholar] [CrossRef]
- Yue, J.M.; Li, B.; Jing, Q.P.; Guan, Q.B. Salvianolic acid B accelerated ABCA1-dependent cholesterol efflux by targeting PPAR-γ and LXRα. Biochem. Bioph. Res. Co. 2015, 462, 233–238. [Google Scholar] [CrossRef]
- Gong, X.C.; Wang, S.S.; Qu, H.B. Comparison of two separation technologies applied in the manufacture of botanical injections: Second ethanol precipitation and solvent extraction. Ind. Eng. Chem. Res. 2011, 50, 7542–7548. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhu, H.F.; Wang, J.H.; Liu, Z.B.; Bi, J.J. Isolation and purification of salvianolic acid A and salvianolic acid B fromSalvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. J. Chromatogr. B 2009, 877, 733–737. [Google Scholar] [CrossRef]
- Ming, G.; Wang, X.L.; Su, Z.G.; Fan, O.Y. One-step separation and purification of 3,4-dihydroxyphenyllactic acid, salvianolic acid B and protocatechualdehyde from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography. J. Chromatogr. A 2007, 1140, 107–111. [Google Scholar]
- Chen, J.H.; Wang, F.M.; Lee, F.S.C.; Wang, X.R.; Xie, M.Y. Separation and identification of water-soluble salvianolic acids from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography and ESI-MS analysis. Talanta 2006, 69, 172–179. [Google Scholar] [CrossRef] [PubMed]
- LI, H.B.; Lai, J.P.; Jiang, Y.; Chen, F. Preparative isolation and purification of salvianolic acid B from the Chinese medicinal plant Salvia miltiorrhiza by high-speed counter-current chromatography. J. Chromatogr. A 2002, 943, 235–239. [Google Scholar] [CrossRef]
- Kan, S.D.; Li, J.A.; Huang, W.Y.; Shao, L.; Chen, D.J. Microsphere resin chromatography combined with microbial biotransformation for the separation and purification of salvianolic acid B in aqueous extract of roots of Salvia multiorrihza Bunge. J. Chromatogr. A 2009, 1216, 3881–3886. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Zhou, L.L.; Li, T.; Wang, L.H. Preparative separation of lithospermic acid B from Salvia miltiorrhiza by polyamide resin and preparative high-performance liquid chromatography. J. Chromatogr. A 2011, 1218, 4606–4611. [Google Scholar] [CrossRef]
- Xu, J.; Tan, T.W.; Janson, J.C. One-step simultaneous purification of three water-soluble constituents in crude extracts from Danshen by adsorption chromatography on oligo-b-cyclodextrin substituted agarose gel media. Process Biochem. 2007, 42, 480–485. [Google Scholar] [CrossRef]
- Nie, L.R.; Lu, J.; Zhang, W.; He, A.; Yao, S. Ionic liquid-modified silica gel as adsorbents for adsorption and separation of water-soluble phenolic acids from Salvia militiorrhiza Bunge. SEP. Purif. Technol. 2015, 155, 2–12. [Google Scholar] [CrossRef]
- Guo, Y.X.; Shi, C.Z.; Zhang, L.; Lv, L.; Zhang, Y.Y. Extraction and isolation of lithospermic acid B fromSalvia miltiorrhiza Bunge using aqueous two-phase extraction followed by high-performance liquid chromatography. J. Sep. Sci. 2016, 39, 3624–3630. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, N.; Zhang, M.; Ito, Y.; Zhang, H.Y.; Wang, Y.R.; Guo, X.; HU, P. Development of a method to extract and purify target compounds from medicinal plants in a single step: Online hyphenation of expanded bed adsorption chromatography and countercurrent chromatography. Anal. Chem. 2014, 86, 3373–3379. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Chen, S.; Zhang, J.; Wang, Y.S.; LIu, A. Which one is more important, raw materials or productive technology?—A case study for quality consistency control of Gegen Qinlian decoction. China J. Chin. Mater. Med. 2016, 41, 1027–1032. [Google Scholar]
- Chen, T.; Gong, X.C.; Chen, H.L.; Zhang, Y.; Qu, H.B. Chromatographic elution process design space development for the purification of saponins in Panax notoginseng extract using a probability-based approach. J. Sep. Sci. 2016, 39, 306–315. [Google Scholar] [CrossRef]
- Ni, L.J.; Gu, H.X.; Gao, X.J.; Zhang, L.G. A new technology of extracting salvianolic acid B from Salviamiltiorrhiza Bunge. J. East China Univ. Sci. Techno.:Nat. Sci. Ed. 2006, 32, 964–966. [Google Scholar]
- Darabi, S.F.; Bahramifar, N.; Khalilzadeh, M.A. Equilibrium, thermodynamic and kinetics studies on adsorption of eosin Y and red X-GRL from aqueous solution by treated rice husk. J. App. Res. Wat. Wast. 2018, 9, 392–398. [Google Scholar]
- Gao, C.; Zhao, S.M.; Yagiz, Y.; Gu, L.W. Static, kinetic, and isotherm adsorption performances of macroporous adsorbent resins for recovery and enrichment of bioactive procyanidins from cranberry pomace. J. Food Sci. 2018, 83, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, C.; Cortina, J.L.; Farran, A.; Gamisans, X.; Lao, C. Kinetics of sorption of polyaromatic hydrocarbons onto granular activated carbon and Macronet hyper-cross-linked polymers (MN200). J. Colloid Interf. Sci. 2007, 310, 35–46. [Google Scholar] [CrossRef]
- Committee, N.P. Pharmacopoeia of the People’s Republic of China, 2015 ed.; China medical science and technology press: Beijing, China, 2015; p. 202. [Google Scholar]
- Liu, S.Y.; Shen, J.J.; Li, W.L.; Qu, H.B.; Gong, X.C. Comparison on three methods for identification of critical process parameters. Chin. Tradit. Herbal Drugs 2016, 47, 3193–3198. [Google Scholar]
- Yan, B.J.; Guo, Z.T.; Qu, H.B.; Zhao, B.C.; Zhao, T. An approach to determine critical process parameters for ethanol precipitation process of danhong injection. China J. Chin. Mater. Med. 2013, 38, 1672–1675. [Google Scholar]
- Shao, J.Y.; Cao, W.; Qu, H.B.; Pan, J.Y.; Gong, X.C. A novel quality by design approach for developing an HPLC method to analyze herbal extracts: A case study of sugar content analysis. PLoS ONE 2018. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Run | Process Parameters | Process Indices | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 (°C) | X2 | X3 | X4 (h) | X5 (mL/g) | X6 (mL) | X7 (mL) | Y1 (mg/g Medicinal Slices) | Y2 (%) | |
| 1 | 50 | 4.2 | 5.0 | 2.0 | 8.0 | 60 | 100 | 0.516 | 37.7 |
| 2 | 50 | 2.4 | 4.0 | 2.0 | 10.0 | 80 | 100 | 2.72 | 53.9 |
| 3 | 100 | 2.4 | 5.0 | 4.0 | 10.0 | 60 | 50 | 9.23 | 38.7 |
| 4 | 75 | 3.3 | 4.5 | 3.0 | 9.0 | 70 | 75 | 5.17 | 65.2 |
| 5 | 50 | 2.4 | 4.0 | 2.0 | 8.0 | 60 | 50 | 2.69 | 57.2 |
| 6 | 50 | 4.2 | 5.0 | 4.0 | 8.0 | 80 | 50 | 0.970 | 58.1 |
| 7 | 100 | 2.4 | 5.0 | 2.0 | 8.0 | 80 | 50 | 10.2 | 49.0 |
| 8 | 100 | 2.4 | 4.0 | 4.0 | 8.0 | 60 | 100 | 5.09 | 45.9 |
| 9 | 75 | 3.3 | 4.5 | 3.0 | 9.0 | 70 | 75 | 5.33 | 63.8 |
| 10 | 100 | 4.2 | 5.0 | 2.0 | 10.0 | 60 | 100 | 0.881 | 33.1 |
| 11 | 50 | 2.4 | 5.0 | 4.0 | 10.0 | 80 | 100 | 10.8 | 64.2 |
| 12 | 75 | 3.3 | 4.5 | 3.0 | 9.0 | 70 | 75 | 5.61 | 62.9 |
| 13 | 100 | 4.2 | 4.0 | 2.0 | 10.0 | 80 | 50 | 0.300 | 38.8 |
| 14 | 100 | 4.2 | 4.0 | 4.0 | 8.0 | 80 | 100 | 0.238 | 28.5 |
| 15 | 50 | 4.2 | 4.0 | 4.0 | 10.0 | 60 | 50 | 0.205 | 39.5 |
| Run | Key Parameters of Crude SAB Preparation Process | Process Indices | |||
|---|---|---|---|---|---|
| X1 (°C) | X2 | X3 | Y1 (mg/g Medicinal Slices) | Y2 (%) | |
| S1 | 100 | 3.3 | 4.0 | 1.59 | 47.0 |
| S2 | 75 | 3.3 | 4.5 | 5.46 | 59.2 |
| S3 | 75 | 2.4 | 5.0 | 10.5 | 54.1 |
| S4 | 100 | 3.3 | 5.0 | 7.67 | 48.9 |
| S5 | 50 | 2.4 | 4.5 | 5.28 | 53.6 |
| S6 | 100 | 2.4 | 4.5 | 8.19 | 43.5 |
| S7 | 100 | 4.2 | 4.5 | 0.741 | 50.2 |
| S8 | 50 | 3.3 | 4.0 | 1.06 | 55.7 |
| S9 | 50 | 4.2 | 4.5 | 0.506 | 52.1 |
| S10 | 75 | 3.3 | 4.5 | 5.26 | 59.4 |
| S11 | 75 | 4.2 | 4.0 | 0.227 | 46.8 |
| S12 | 50 | 3.3 | 5.0 | 5.11 | 57.8 |
| S13 | 75 | 2.4 | 4.0 | 2.72 | 43.9 |
| S14 | 75 | 3.3 | 4.5 | 5.55 | 59.9 |
| S15 | 75 | 4.2 | 5.0 | 1.09 | 44.2 |
| S16 | 75 | 3.3 | 4.5 | 6.07 | 58.1 |
| Resin | Pseudo-Second-Order | ||
|---|---|---|---|
| Qe(cal) (mg/g Dry Resin) | k2 (min−1) | R2 | |
| CG161M | 12.55 | 0.103 | 0.99 |
| 50 W × 2 | 7.519 | 0.00900 | 0.96 |
| 50 W × 4 | 11.74 | 0.00479 | 0.95 |
| Run | Habitat | Phenolic Compounds Yield of Water Extract | Phenolic Compounds Purity of Water Extract | Final Product Indices | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ⅰ | Bozhou, Anhui Province | 0.295 | 1.08 | 5.54 | 1.15 | 0.685 | 22.6 | 0.301 | 9.81 | 0.358 | 1.84 | 0.381 | 0.228 | 7.53 | 0.100 | 95.9 | 1.42 |
| Ⅱ | Minxian, Gansu Province | 0.428 | 2.54 | 27.9 | 1.34 | 0.0746 | 0.809 | 0.0322 | 23.7 | 1.41 | 15.4 | 0.742 | 0.0413 | 0.447 | 0.0178 | 22.5 | 0.00483 |
| Ⅲ | Wanrong, Shanxi Province | 0.252 | 2.61 | 8.30 | 0.428 | 0.707 | 21.1 | 0.392 | 7.00 | 0.725 | 2.31 | 0.119 | 0.196 | 5.87 | 0.109 | 97.5 | 0.717 |
| Ⅳ | Dancheng, Henan Province | 0.377 | 1.92 | 6.40 | 0.709 | 0.976 | 20.1 | 0.372 | 12.1 | 0.617 | 2.05 | 0.228 | 0.313 | 6.45 | 0.119 | 88.3 | 0.784 |
| Ⅴ | Zhongjiang, Sichuan Province | 0.310 | 1.83 | 7.35 | 0.452 | 0.845 | 11.2 | 0.230 | 15.7 | 0.928 | 3.72 | 0.229 | 0.428 | 5.66 | 0.116 | 99.2 | 0.659 |
| Ⅵ | Wanrong, Shanxi Province (1) | 0.359 | 0.923 | 6.43 | 0.238 | 0.800 | 11.3 | 0.132 | 10.7 | 0.275 | 1.92 | 0.0710 | 0.238 | 3.38 | 0.0393 | 96.0 | 0.559 |
| Ⅶ | Wanrong, Shanxi Province (2) | 0.322 | 0.842 | 6.20 | 0.231 | 0.781 | 11.1 | 0.128 | 9.83 | 0.257 | 1.89 | 0.0704 | 0.238 | 3.38 | 0.0391 | 96.7 | 0.492 |
| Ⅷ | Wanrong, Shanxi Province (3) | 0.333 | 0.904 | 6.50 | 0.249 | 0.821 | 11.6 | 0.136 | 10.0 | 0.271 | 1.95 | 0.0746 | 0.246 | 3.50 | 0.0409 | 98.1 | 0.564 |
| Habitat | ||||
|---|---|---|---|---|
| Julu, Hebei Province | 10.30 | 2.31 | 99.3/95.3 | 0.732/0.487 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Qu, H.; Gong, X. Preparation of Salvianolic Acid B Disodium Salt Considering the Water Extract Quality Standard. Molecules 2019, 24, 1269. https://doi.org/10.3390/molecules24071269
Ye T, Qu H, Gong X. Preparation of Salvianolic Acid B Disodium Salt Considering the Water Extract Quality Standard. Molecules. 2019; 24(7):1269. https://doi.org/10.3390/molecules24071269
Chicago/Turabian StyleYe, Tiantian, Haibin Qu, and Xingchu Gong. 2019. "Preparation of Salvianolic Acid B Disodium Salt Considering the Water Extract Quality Standard" Molecules 24, no. 7: 1269. https://doi.org/10.3390/molecules24071269
APA StyleYe, T., Qu, H., & Gong, X. (2019). Preparation of Salvianolic Acid B Disodium Salt Considering the Water Extract Quality Standard. Molecules, 24(7), 1269. https://doi.org/10.3390/molecules24071269





