Phytoestrogenic Effects of Blackcurrant Anthocyanins Increased Endothelial Nitric Oxide Synthase (eNOS) Expression in Human Endothelial Cells and Ovariectomized Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microarray Gene Expression Profiling of HUVECs Exposed to BCE
2.2. eNOS mRNA Expression in Human Endothelial Cells Determined by Reverse Transcription (RT)-qPCR Analysis
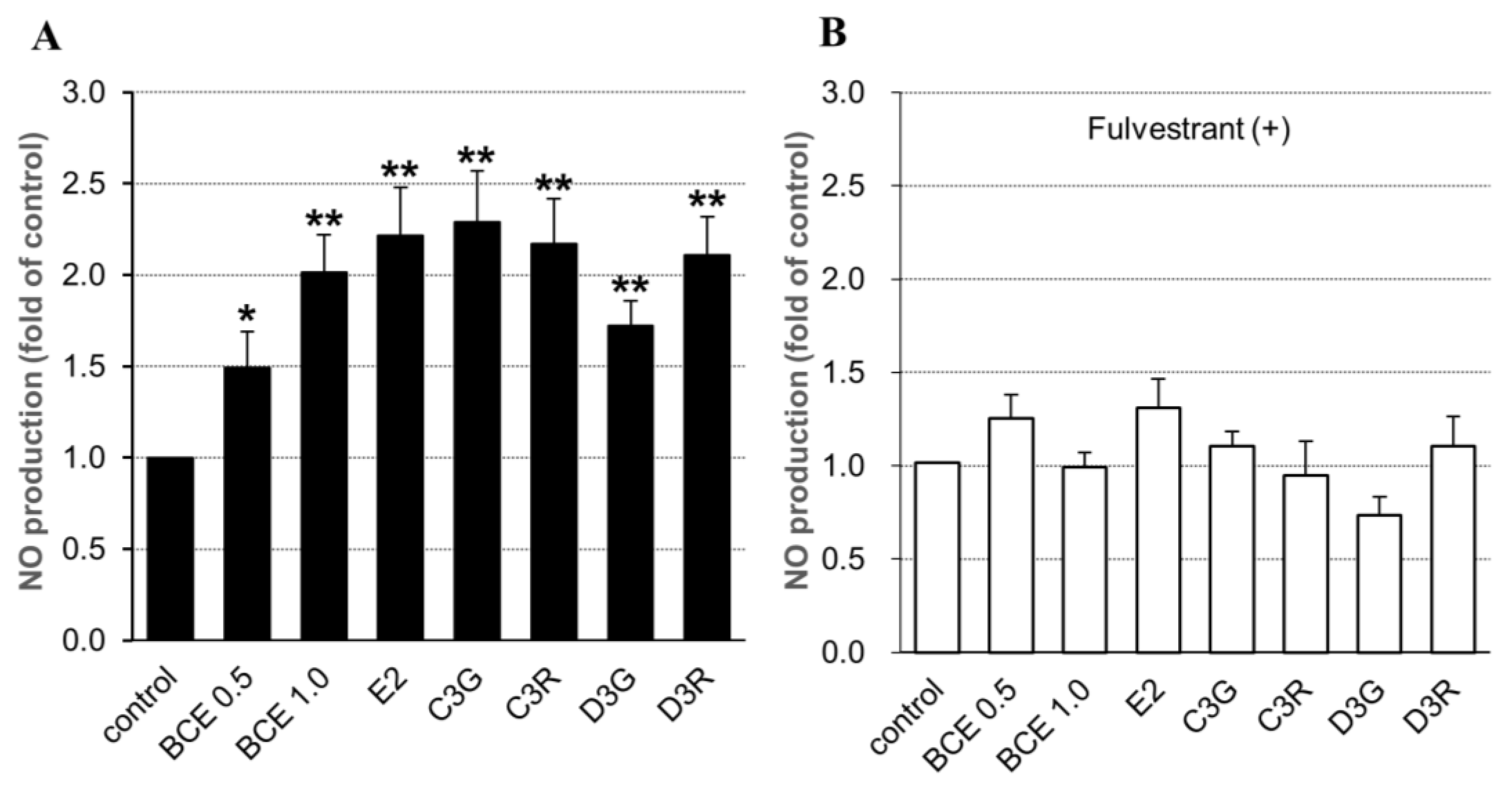
2.3. NO Synthesis in Human Endothelial Cells
2.4. NO Synthesis-Related Gene Expression in HUVECs Exposed to BCE
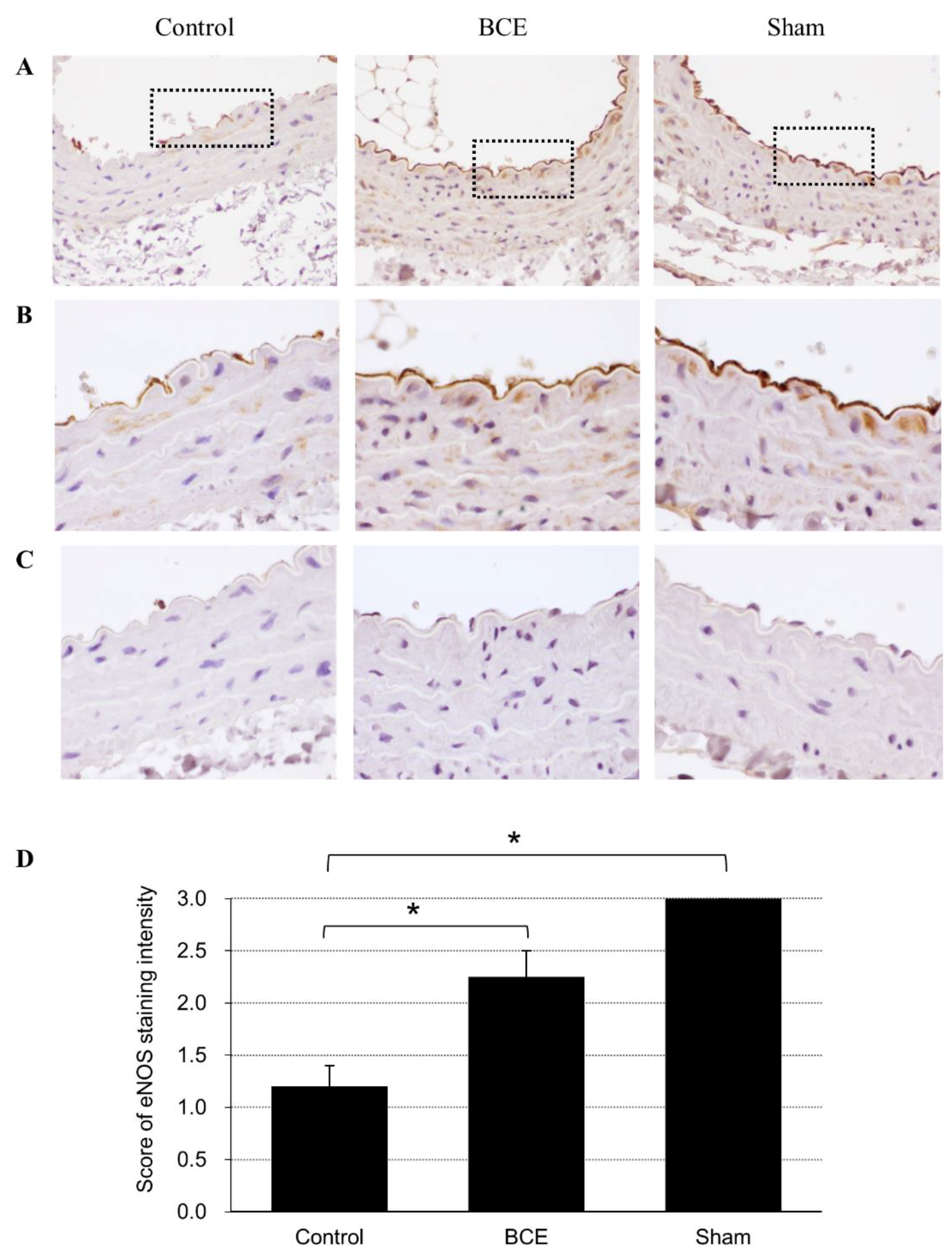
2.5. eNOS Protein Expression in BCE-Treated OVX Rats Determined by Immunohistochemical Staining
3. Materials and Methods
3.1. Materials and Cell Culture
3.2. Microarray Gene Expression Profiling
3.3. IPA
3.4. RT-qPCR
3.5. NO Measurement
3.6. Animals and Treatments
3.7. Immunohistochemical Staining of eNOS Protein
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muka, T.; Oliver-Williams, C.; Kunutsor, S.; Laven, J.S.; Fauser, B.C.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016, 1, 767–776. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Spiegelhalter, D.J.; Georgakopoulos, D.; Robinson, J.; Deanfield, J.E. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J. Am. Coll. Cardiol. 1994, 24, 471–476. [Google Scholar]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Mattei, P.; Sudano, I.; Bernini, G.; Pinto, S.; Salvetti, A. Menopause is associated with endothelial dysfunction in women. Hypertension 1996, 28, 576–582. [Google Scholar] [CrossRef]
- Jousilahti, P.; Vartiainen, E.; Tuomilehto, J.; Puska, P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation 1999, 99, 1165–1172. [Google Scholar] [CrossRef]
- Rice, S.; Whitehead, S.A. Phytoestrogens and breast cancer--promoters or protectors? Endocr. Relat. Cancer 2006, 13, 995–1015. [Google Scholar] [CrossRef]
- Hickey, M.; Saunders, C.M.; Stuckey, B.G. Management of menopausal symptoms in patients with breast cancer: an evidence-based approach. Lancet Oncol. 2005, 6, 687–695. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [PubMed]
- Schmitt, E.; Stopper, H. Estrogenic activity of naturally occurring anthocyanidins. Nutr. Cancer 2001, 41, 145–149. [Google Scholar]
- Nanashima, N.; Horie, K.; Tomisawa, T.; Chiba, M.; Nakano, M.; Fujita, T.; Maeda, H.; Kitajima, M.; Takamagi, S.; Uchiyama, D.; Watanabe, J.; Nakamura, T.; Kato, Y. Phytoestrogenic activity of blackcurrant (Ribes nigrum) anthocyanins is mediated through estrogen receptor alpha. Mol. Nutr. Food Res. 2015, 59, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Shaw, O.M.; Nyanhanda, T.; McGhie, T.K.; Harper, J.L.; Hurst, R.D. Blackcurrant anthocyanins modulate CCL11 secretion and suppress allergic airway inflammation. Mol. Nutr. Food Res. 2017, 61, 1600868. [Google Scholar] [CrossRef]
- Nanashima, N.; Horie, K.; Chiba, M.; Nakano, M.; Maeda, H.; Nakamura, T. Anthocyanin-rich blackcurrant extract inhibits proliferation of the MCF10A healthy human breast epithelial cell line through induction of G0/G1 arrest and apoptosis. Mol. Med. Rep. 2017, 16, 6134–6141. [Google Scholar] [CrossRef]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef]
- Iizuka, Y.; Ozeki, A.; Tani, T.; Tsuda, T. Blackcurrant extract ameliorates hyperglycemia in type 2 diabetic mice in association with increased basal secretion of glucagon-like peptide-1 and activation of AMP-activated protein kinase. J. Nutr. Sci. Vitaminol. 2018, 64, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Nishikawa, S.; Kato, M.; Tsuda, T. Delphinidin 3-rutinoside-rich blackcurrant extract ameliorates glucose tolerance by increasing the release of glucagon-like peptide-1 secretion. Food Sci. Nutr. 2017, 5, 929–933. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; McCarthy, D.; Burton-Freeman, B.M. Effect of black currant anthocyanins on the activation of endothelial nitric oxide synthase (eNOS) in vitro in human endothelial cells. J. Agric. Food Chem. 2011, 59, 8616–8624. [Google Scholar] [CrossRef]
- Willems, M.E.; Myers, S.D.; Gault, M.L.; Cook, M.D. Beneficial physiological effects with blackcurrant intake in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.E.T.; Parktin, N.; Widjaja, W.; Ajjimaporn, A. Effect of New Zealand blackcurrant extract on physiological responses at rest and during brisk walking in Southeast Asian men: a randomized, double-blind, placebo-controlled, crossover study. Nutrients 2018, 10, 1732. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K.; Maeda, H. Phytoestrogenic activity of blackcurrant anthocyanins is partially mediated through estrogen receptor beta. Molecules 2017, 23, 74. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K.; Maeda, H.; Tomisawa, T.; Kitajima, M.; Nakamura, T. Blackcurrant anthocyanins increase the levels of collagen, elastin, and hyaluronic acid in human skin fibroblasts and ovariectomized rats. Nutrients 2018, 10, 495. [Google Scholar] [CrossRef]
- Simoncini, T.; Varone, G.; Fornari, L.; Mannella, P.; Luisi, M.; Labrie, F.; Genazzani, A.R. Genomic and nongenomic mechanisms of nitric oxide synthesis induction in human endothelial cells by a fourth-generation selective estrogen receptor modulator. Endocrinology 2002, 143, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Usselman, C.W.; Stachenfeld, N.S.; Bender, J.R. The molecular actions of oestrogen in the regulation of vascular health. Exp. Physiol. 2016, 101, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Forstermann, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000, 190, 244–254. [Google Scholar] [CrossRef]
- Nevzati, E.; Shafighi, M.; Bakhtian, K.D.; Treiber, H.; Fandino, J.; Fathi, A.R. Estrogen induces nitric oxide production via nitric oxide synthase activation in endothelial cells. Acta Neurochir. Suppl. 2015, 120, 141–145. [Google Scholar] [PubMed]
- Kypreos, K.E.; Zafirovic, S.; Petropoulou, P.I.; Bjelogrlic, P.; Resanovic, I.; Traish, A.; Isenovic, E.R. Regulation of endothelial nitric oxide synthase and high-density lipoprotein quality by estradiol in cardiovascular pathology. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Gavin, K.M.; Seals, D.R.; Silver, A.E.; Moreau, K.L. Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J. Clin. Endocrinol. Metab. 2009, 94, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Schnitzler, G.R.; Ueda, K.; Iyer, L.K.; Diomede, O.I.; Andrade, T.; Karas, R.H. ER alpha rapid signaling is required for estrogen induced proliferation and migration of vascular endothelial cells. PLoS ONE 2016, 11, e0152807. [Google Scholar] [CrossRef]
- Simoncini, T.; Genazzani, A.R. Raloxifene acutely stimulates nitric oxide release from human endothelial cells via an activation of endothelial nitric oxide synthase. J. Clin. Endocrinol. Metab. 2000, 85, 2966–2969. [Google Scholar] [CrossRef]
- Hisamoto, K.; Ohmichi, M.; Kanda, Y.; Adachi, K.; Nishio, Y.; Hayakawa, J.; Mabuchi, S.; Takahashi, K.; Tasaka, K.; Miyamoto, Y.; Taniguchi, N.; Murata, Y. Induction of endothelial nitric-oxide synthase phosphorylation by the raloxifene analog LY117018 is differentially mediated by Akt and extracellular signal-regulated protein kinase in vascular endothelial cells. J. Biol. Chem. 2001, 276, 47642–47649. [Google Scholar] [CrossRef]
- Simoncini, T.; Genazzani, A.R.; Liao, J.K. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation 2002, 105, 1368–1373. [Google Scholar] [CrossRef]
- Leikert, J.F.; Rathel, T.R.; Wohlfart, P.; Cheynier, V.; Vollmar, A.M.; Dirsch, V.M. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation 2002, 106, 1614–1617. [Google Scholar] [CrossRef]
- Klinge, C.M.; Wickramasinghe, N.S.; Ivanova, M.M.; Dougherty, S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor α-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008, 22, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Liu, D. Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J. Nutr. 2008, 138, 297–304. [Google Scholar] [CrossRef]
- Xia, N.; Pautz, A.; Wollscheid, U.; Reifenberg, G.; Forstermann, U.; Li, H. Artichoke, cynarin and cyanidin downregulate the expression of inducible nitric oxide synthase in human coronary smooth muscle cells. Molecules 2014, 19, 3654–3668. [Google Scholar] [CrossRef]
- Luzak, B.; Boncler, M.; Rywaniak, J.; Dudzinska, D.; Rozalski, M.; Krajewska, U.; Balcerczak, E.; Podsedek, A.; Redzynia, M.; Watala, C. Extract from Ribes nigrum leaves in vitro activates nitric oxide synthase (eNOS) and increases CD39 expression in human endothelial cells. J. Physiol. Biochem. 2014, 70, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, A.; Garcia-Cardena, G.; Madri, J.A.; Sessa, W.C. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J. Clin. Invest. 1997, 100, 3131–3139. [Google Scholar] [CrossRef]
- Hood, J.D.; Meininger, C.J.; Ziche, M.; Granger, H.J. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am. J. Physiol. 1998, 274, H1054–H1058. [Google Scholar] [CrossRef]
- Kroll, J.; Waltenberger, J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR). Biochem. Biophys. Res. Commun. 1998, 252, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, D.S.; Bernatchez, P.N.; Rollin, S.; Bazan, N.G.; Sirois, M.G. Immediate and delayed VEGF-mediated NO synthesis in endothelial cells: role of PI3K, PKC and PLC pathways. Br. J. Pharmacol. 2002, 137, 1021–1030. [Google Scholar] [CrossRef]
- Garcia-Cardena, G.; Fan, R.; Shah, V.; Sorrentino, R.; Cirino, G.; Papapetropoulos, A.; Sessa, W.C. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 1998, 392, 821–824. [Google Scholar] [CrossRef]
- Brouet, A.; Sonveaux, P.; Dessy, C.; Balligand, J.L.; Feron, O. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J. Biol. Chem. 2001, 276, 32663–32669. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, J.; Han, B.; Liu, J.; Xiang, X.; Zhang, M.; Xia, S.; Zhang, W.; Zhang, X. Novel zinc finger transcription factor ZFP580 facilitates all-trans retinoic acid -induced vascular smooth muscle cells differentiation by raralpha-mediated PI3K/Akt and ERK signaling. Cell. Physiol. Biochem. 2018, 50, 2390–2405. [Google Scholar] [CrossRef]
- Shi, H.; Yuan, L.; Yang, H.; Zang, A. The mechanism of all-trans retinoic acid in the regulation of apelin expression in vascular endothelial cells. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Lai, L.; Bohnsack, B.L.; Niederreither, K.; Hirschi, K.K. Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development 2003, 130, 6465–6474. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (BCE and anthocyanins) are available from the authors. |

| Predicted Upstream Regulator | z-Score |
|---|---|
| ESR1 (ERα) | 2.7 |
| Raloxifene | 2.3 |
| Tretinoin | 2.2 |
| Nitric oxide (NO) | 2.1 |
| Gene Symbol | Gene Name | * Fold Change | Accession No. |
|---|---|---|---|
| VEGFA | Vascular endothelial growth factor A | 1.7 | NM_001025366 |
| HSP90AB1 | Heat shock protein 90kDa alpha (cytosolic), class B member 1 | 1.4 | NM_007355 |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha | 1.4 | NM_006218 |
| AKT1 | v-Akt murine thymoma viral oncogene homolog 1 | 1.1 | NM_005163 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horie, K.; Nanashima, N.; Maeda, H. Phytoestrogenic Effects of Blackcurrant Anthocyanins Increased Endothelial Nitric Oxide Synthase (eNOS) Expression in Human Endothelial Cells and Ovariectomized Rats. Molecules 2019, 24, 1259. https://doi.org/10.3390/molecules24071259
Horie K, Nanashima N, Maeda H. Phytoestrogenic Effects of Blackcurrant Anthocyanins Increased Endothelial Nitric Oxide Synthase (eNOS) Expression in Human Endothelial Cells and Ovariectomized Rats. Molecules. 2019; 24(7):1259. https://doi.org/10.3390/molecules24071259
Chicago/Turabian StyleHorie, Kayo, Naoki Nanashima, and Hayato Maeda. 2019. "Phytoestrogenic Effects of Blackcurrant Anthocyanins Increased Endothelial Nitric Oxide Synthase (eNOS) Expression in Human Endothelial Cells and Ovariectomized Rats" Molecules 24, no. 7: 1259. https://doi.org/10.3390/molecules24071259
APA StyleHorie, K., Nanashima, N., & Maeda, H. (2019). Phytoestrogenic Effects of Blackcurrant Anthocyanins Increased Endothelial Nitric Oxide Synthase (eNOS) Expression in Human Endothelial Cells and Ovariectomized Rats. Molecules, 24(7), 1259. https://doi.org/10.3390/molecules24071259







