Anti-Inflammatory Effects of Catalpalactone Isolated from Catalpa ovata in LPS-Induced RAW264.7 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Catalpalactone on Cell Cytotoxicity
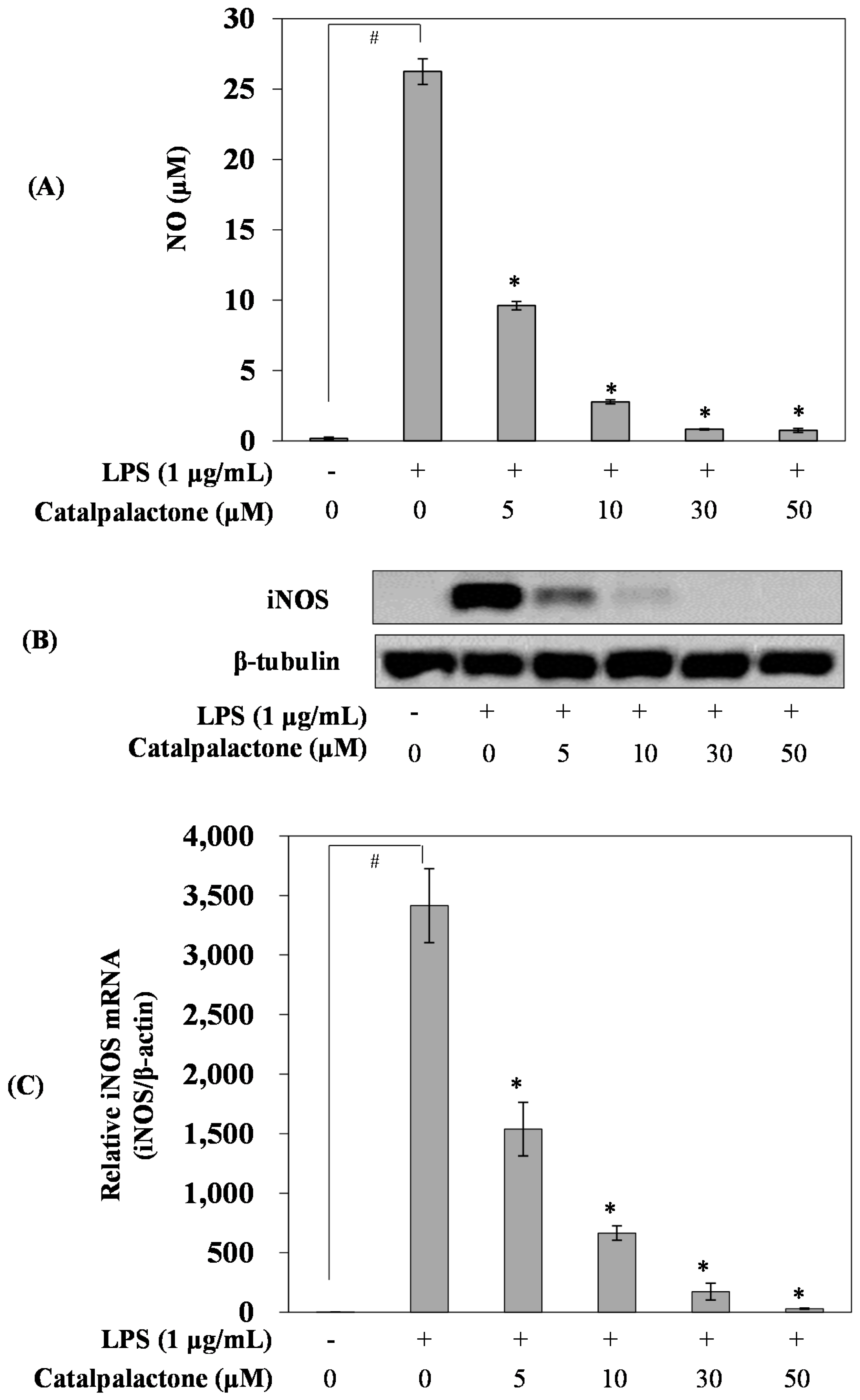
2.2. Effects of Catalpalactone on NO Production and iNOS Expression in LPS-Stimulated RAW264.7 Cells
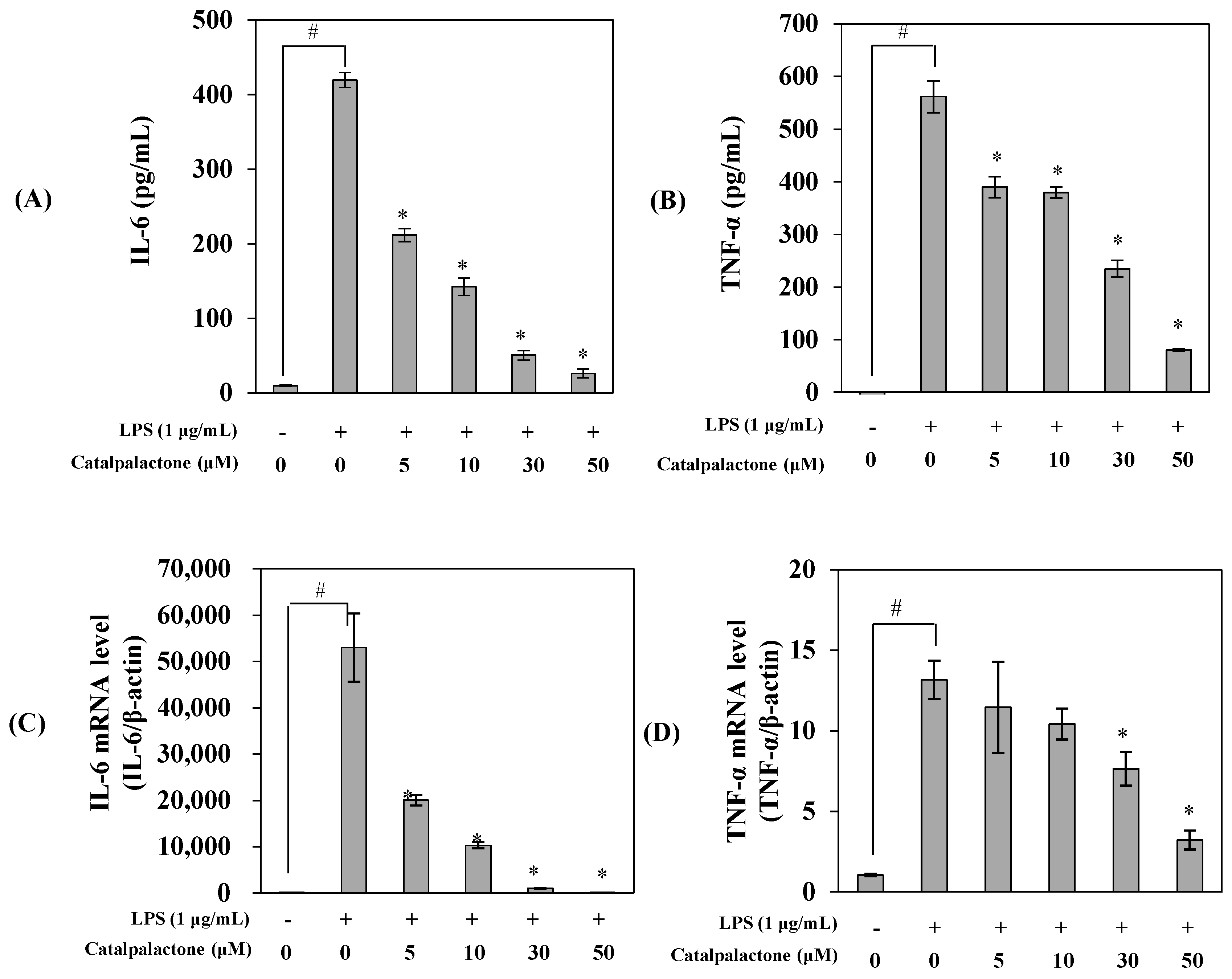
2.3. Effects of Catalpalactone on the Production and mRNA Expression of Interleukin-6 and TNF-α in LPS-Stimulated RAW264.7 Cells
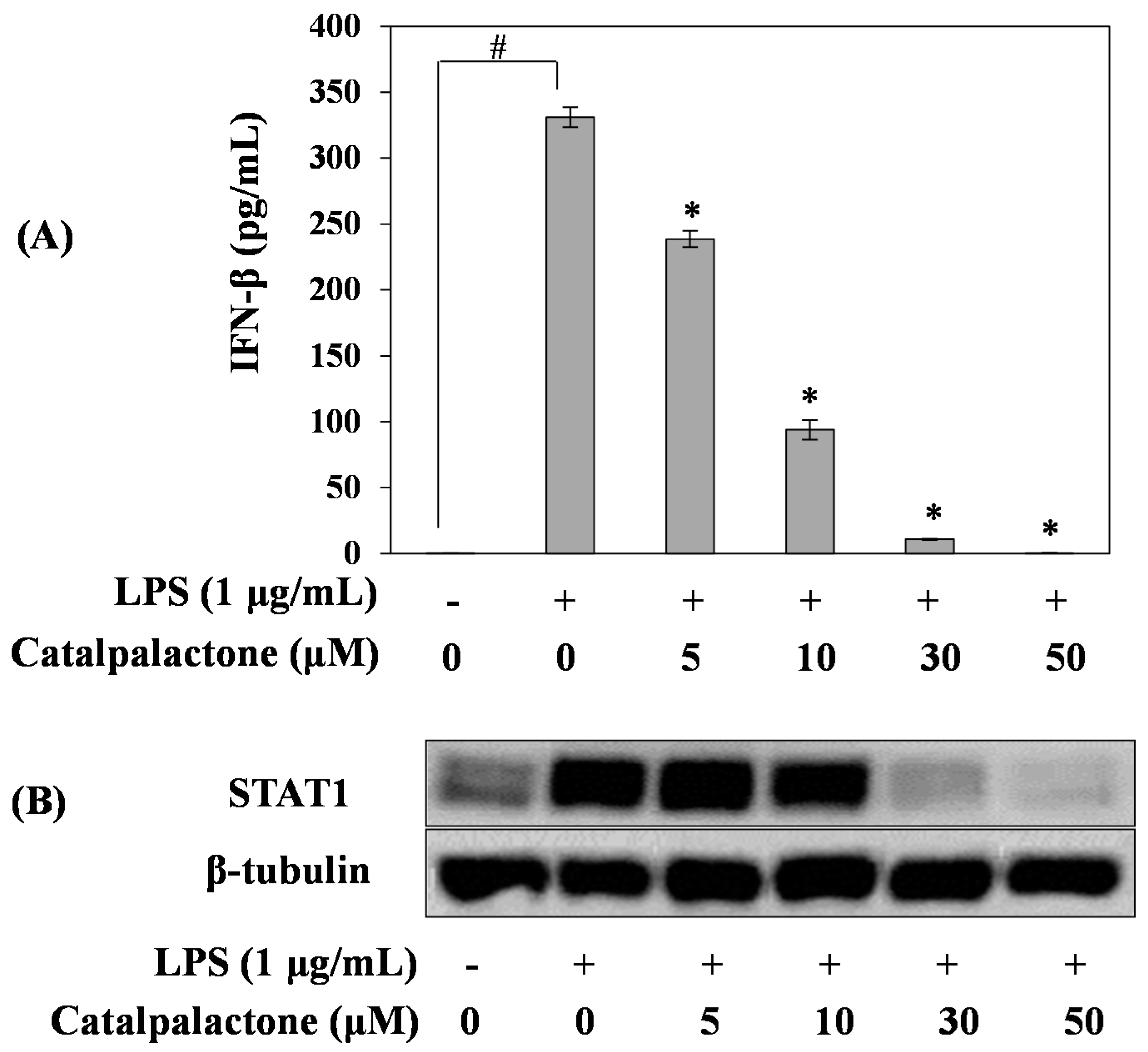
2.4. Effects of Catalpalactone on IFN-β Production and STAT1 Activation in LPS-Stimulated RAW264.7 Cells
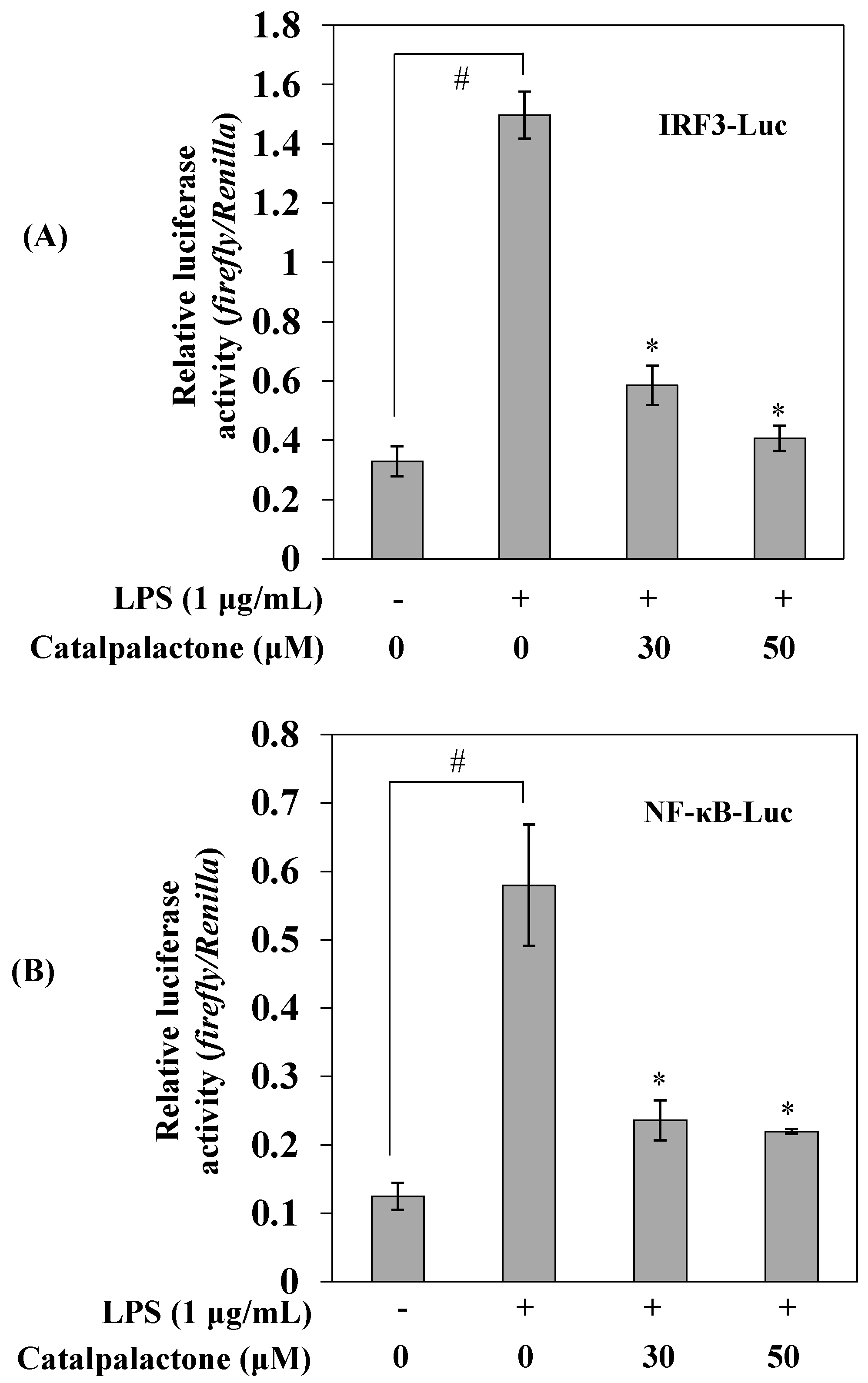
2.5. Effects of Catalpalactone on IRF3 and NF-κB Activation in LPS-Stimulated RAW264.7 Cells
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Cell Culture
3.5. Cytotoxicity Assay
3.6. Measurement of NO Production
3.7. Western Blotting
3.8. Measurements of IL-6, TNF-α, and IFN-β
3.9. Luciferase Assay
3.10. Quantitative Real-Time PCR Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar] [CrossRef]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Affan, A.; Oh, C.H.; Jung, W.K.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. IkappaB kinases: Key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sugiyama, M.; Yamamoto, M.; Watanabe, Y.; Kawai, T.; Takeda, K.; Akira, S. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 2003, 171, 4304–4310. [Google Scholar] [PubMed]
- Toshchakov, V.; Jones, B.W.; Perera, P.Y.; Thoms, K.; Cody, M.J.; Zhang, S.; Williams, B.R.; Major, J.; Hamilton, T.A.; Fenton, M.J.; et al. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat. Immunol. 2002, 3, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.T. Coloured Standard Illustration of Korean Plants; Academy Publishing Company: Seoul, Korea, 1996; p. 326. [Google Scholar]
- Inouye, H.; Hayashi, T.; Shingu, T. Quinones and related compounds in higher plants. III. Absolute structure of catalponol, a naphthoquinone congener of Catalpa ovata. Chem. Pharm. Bull. 1975, 23, 392–399. [Google Scholar] [CrossRef]
- Fujiwara, A.; Mori, T.; Iida, A.; Ueda, S.; Hano, Y.; Nomura, T.; Nishino, H. Antitumor-promoting naphthoquinones from Catalpa ovata. J. Nat. Prod. 1998, 61, 629–632. [Google Scholar] [CrossRef]
- Young, H.S.; Kim, M.S.; Park, H.J.; Chung, H.Y.; Choi, J.S. Phytochemical study on Catalpa ovata. Arch. Pharm. Res. 1992, 15, 322–327. [Google Scholar] [CrossRef]
- Machida, K.; Hishinuma, E.; Kikuchi, M. Studies on the constituents of Catalpa species. IX. Iridoids from the fallen leves of Catalpa ovata G. Don. Chem. Pharm. Bull. 2004, 52, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Machida, K.; Ando, M.; Yaoita, Y.; Kakuda, R.; Kikuchi, M. Phenolic compounds of the leaves of Catalpa ovata G. Don. Nat. Med. 2001, 55, 64–67. [Google Scholar]
- Pae, H.O.; Oh, G.S.; Choi, B.M.; Shin, S.; Chai, K.Y.; Oh, H.; Kim, J.M.; Kim, H.J.; Jang, S.I.; Chung, H.T. Inhibitory effects of the stem bark of Catalpa ovata G. Don. (Bignoniaceae) on the productions of tumor necrosis factor-α and nitric oxide by the lipopolysaccharide-stimulated RAW264.7 macrophages. J. Ethnopharmacol. 2003, 88, 287–291. [Google Scholar] [CrossRef]
- An, S.J.; Pae, H.O.; Oh, G.S.; Choi, B.M.; Jeong, S.; Jang, S.I.; Oh, H.; Kwon, T.O.; Song, C.E.; Chung, H.T. Inhibition of TNF-α, IL-1β, and IL-6 productions and NF-κB activation in lipopolysaccharide-activated RAW264.7 macrophages by catalposide, an iridoid glycoside isolated from Catalpa ovata G. Don (Bignoniaceae). Int. Immunopharmacol. 2002, 2, 1173–1181. [Google Scholar] [CrossRef]
- Park, B.M.; Hong, S.S.; Lee, C.; Lee, M.S.; Kang, S.J.; Shin, Y.S.; Hwang, B.Y. Naphthoquinones from Catalpa ovata and their inhibitory effects on the production of nitric oxide. Arch. Pharm. Res. 2010, 33, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Kil, Y.S.; So, Y.K.; Choi, M.J.; Han, A.R.; Jin, C.H.; Seo, E.K. Cytoprotective dihydronaphthalenones from the wood of Catalpa ovata. Phytochemistry 2018, 147, 14–20. [Google Scholar] [CrossRef]
- Wolf, G. Nitric oxide and nitric oxide synthase: Biology, pathology, localization. Histol. Histopathol. 1997, 12, 251–261. [Google Scholar]
- KrÖche, K.D.; Fensel, K.; Kolb-bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147–156. [Google Scholar]
- Tunctan, B.; Uludag, O.; Altug, S.; Abacioglu, N. Effects of nitric oxide synthase inhibition in lipopolysaccharide-induced sepsis in mice. Pharmacol. Res. 1998, 38, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Han, A.R.; Min, H.Y.; Nam, J.W.; Lee, N.Y.; Wiryawan, A.; Suprapto, W.; Lee, S.K.; Lee, K.R.; Seo, E.K. Identification of a new naphthalene and its derivatives from the bulb of Eleutherine americana with inhibitory activity on lipopolysaccharide-induced nitric oxide production. Chem. Pharm. Bull. 2008, 56, 1314–1316. [Google Scholar] [CrossRef]
- Huang, H.S.; Han, X.H.; Hwang, B.Y.; Park, J.I.; Yoo, S.K.; Lee, H.J.; Lim, S.C.; Lee, M.K. Effects of catalpalactone on dopamine biosynthesis and L-DOPA-induced cytotoxicity in PC12 cells. Environ. Toxicol. Pharmacol. 2008, 26, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Ryu, H.W.; So, Y.K.; Cho, J.K.; Woo, H.S.; Jin, C.H.; Seo, K.I.; Park, J.C.; Jeong, I.Y. Anti-inflammatory effect of austroinulin and 6-O-acetyl-austroinulin from Stevia rebaudiana in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 62, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.X.; Gu, Y.F.; Zhao, Y.F.; Song, Y.L.; Li, J.; Tu, P.F. GYF-17, a chloride substituted 2-(2-phenethyl)-chromone, suppresses LPS-induced inflammatory mediator production in RAW264.7 cells by inhibiting STAT1/3 and ERK1/2 signaling pathways. Int. Immunopharmacol. 2016, 35, 185–192. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, S.Y.; Kim, Y.S.; Lee, W.H.; Hwang, D.H.; Lee, J.Y. Suppression of the TRIF-dependent signaling pathway of Toll-like receptors by luteolin. Biochem. Pharmacol. 2009, 77, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Inouye, H.; Okuda, T.; Hirata, Y.; Nagakura, N.; Yoshizaki, M. The structure of catalpalactone. Tetrahedron Lett. 1965, 18, 1261–1264. [Google Scholar] [CrossRef]
- Canesi, L.; Borghi, C.; Ciacci, C.; Fabbri, R.; Vergani, L.; Gallo, G. Bisphenol-A alters gene expression and functional parameters in molluscan hepatopancreas. Mol. Cell. Endocrinol. 2007, 276, 36–44. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Gene | Sequence (5′ to 3′) |
|---|---|
| iNOS | Forward: TCCTACACACCAAACTGTGTGC Reverse: CTCCAATCTCTGCCTATCCGTCTC |
| IL-6 | Forward: GTTCTCTGGGAAATCGTGGAA Reverse: GCAAGTCCATCATCGTTGTTC |
| TNF-α | Forward: GCCACCACGCTCTTCTGTCTAC Reverse: GGGCTACAGGCTTGTCACTCG |
| β-actin | Forward: TGAGAGGGAAATCGTGCGTGAC Reverse: GCTCGTTGCCAATAGTGATGACC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-Y.; Han, A.-R.; Kil, Y.-S.; Seo, E.K.; Jin, C.H. Anti-Inflammatory Effects of Catalpalactone Isolated from Catalpa ovata in LPS-Induced RAW264.7 Cells. Molecules 2019, 24, 1236. https://doi.org/10.3390/molecules24071236
Kim H-Y, Han A-R, Kil Y-S, Seo EK, Jin CH. Anti-Inflammatory Effects of Catalpalactone Isolated from Catalpa ovata in LPS-Induced RAW264.7 Cells. Molecules. 2019; 24(7):1236. https://doi.org/10.3390/molecules24071236
Chicago/Turabian StyleKim, Hyo-Young, Ah-Reum Han, Yun-Seo Kil, Eun Kyoung Seo, and Chang Hyun Jin. 2019. "Anti-Inflammatory Effects of Catalpalactone Isolated from Catalpa ovata in LPS-Induced RAW264.7 Cells" Molecules 24, no. 7: 1236. https://doi.org/10.3390/molecules24071236
APA StyleKim, H.-Y., Han, A.-R., Kil, Y.-S., Seo, E. K., & Jin, C. H. (2019). Anti-Inflammatory Effects of Catalpalactone Isolated from Catalpa ovata in LPS-Induced RAW264.7 Cells. Molecules, 24(7), 1236. https://doi.org/10.3390/molecules24071236







