The Effects of Ergosta-7,9(11),22-trien-3β-ol from Antrodia camphorata on the Biochemical Profile and Exercise Performance of Mice
Abstract
:1. Introduction
2. Results
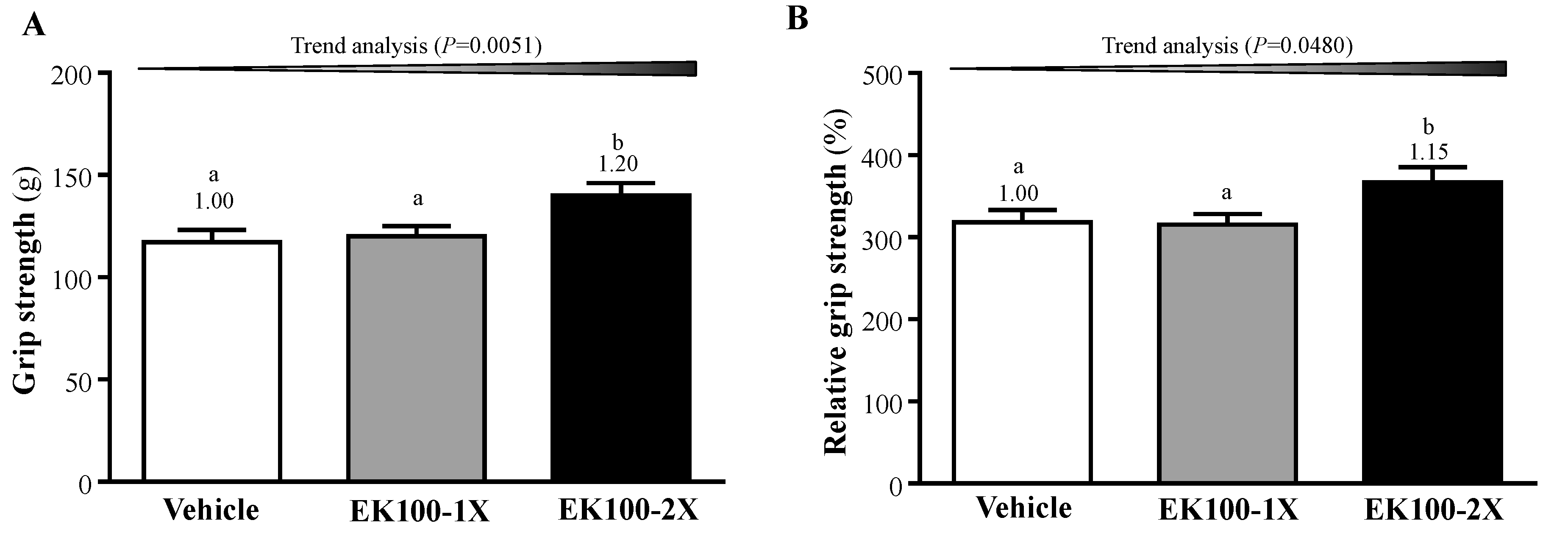
2.1. Effect of EK100 Supplementation on Forelimb Grip Strength
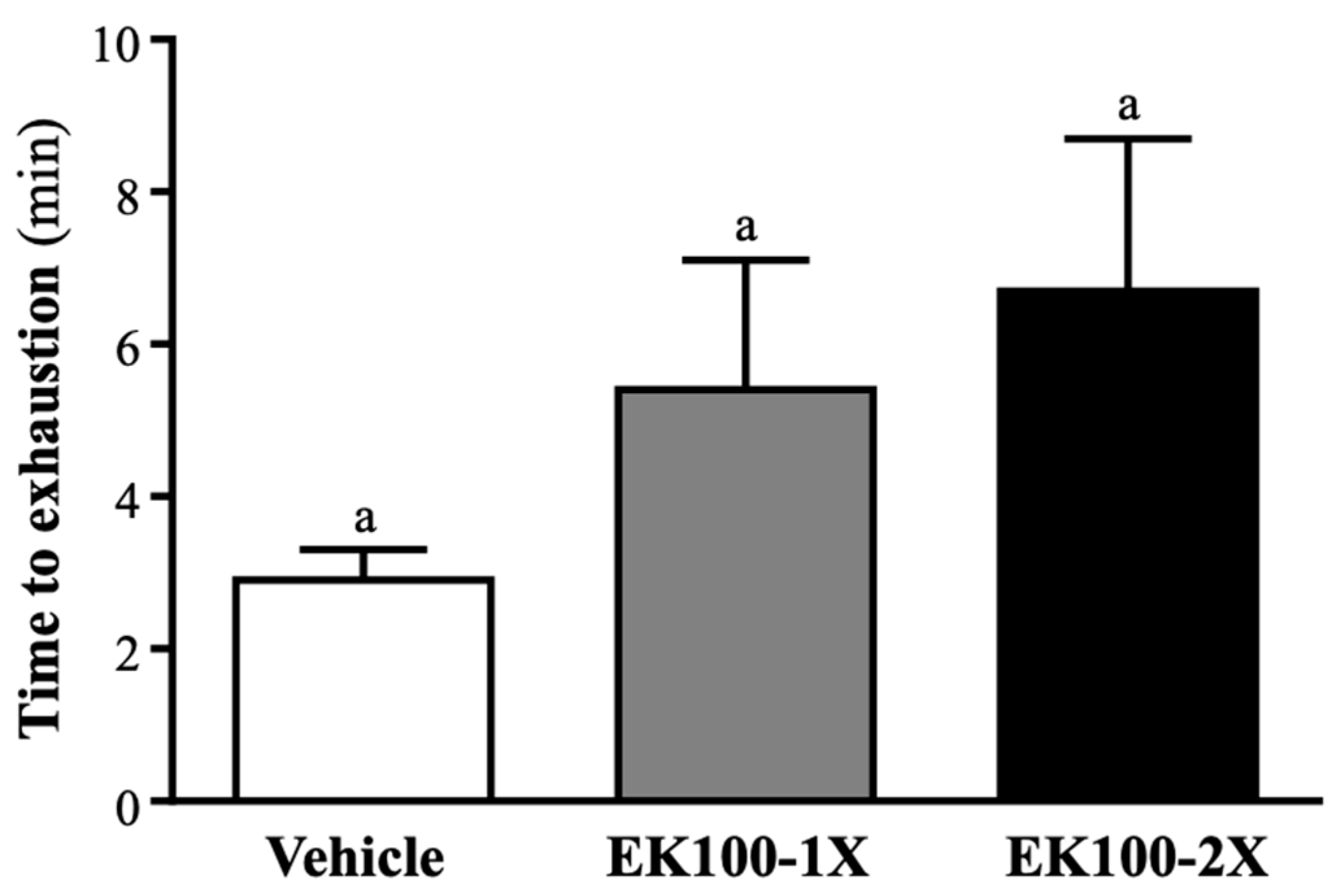
2.2. Effect of EK100 Supplementation on Exercise Performance in a Weight-loaded Swimming Test
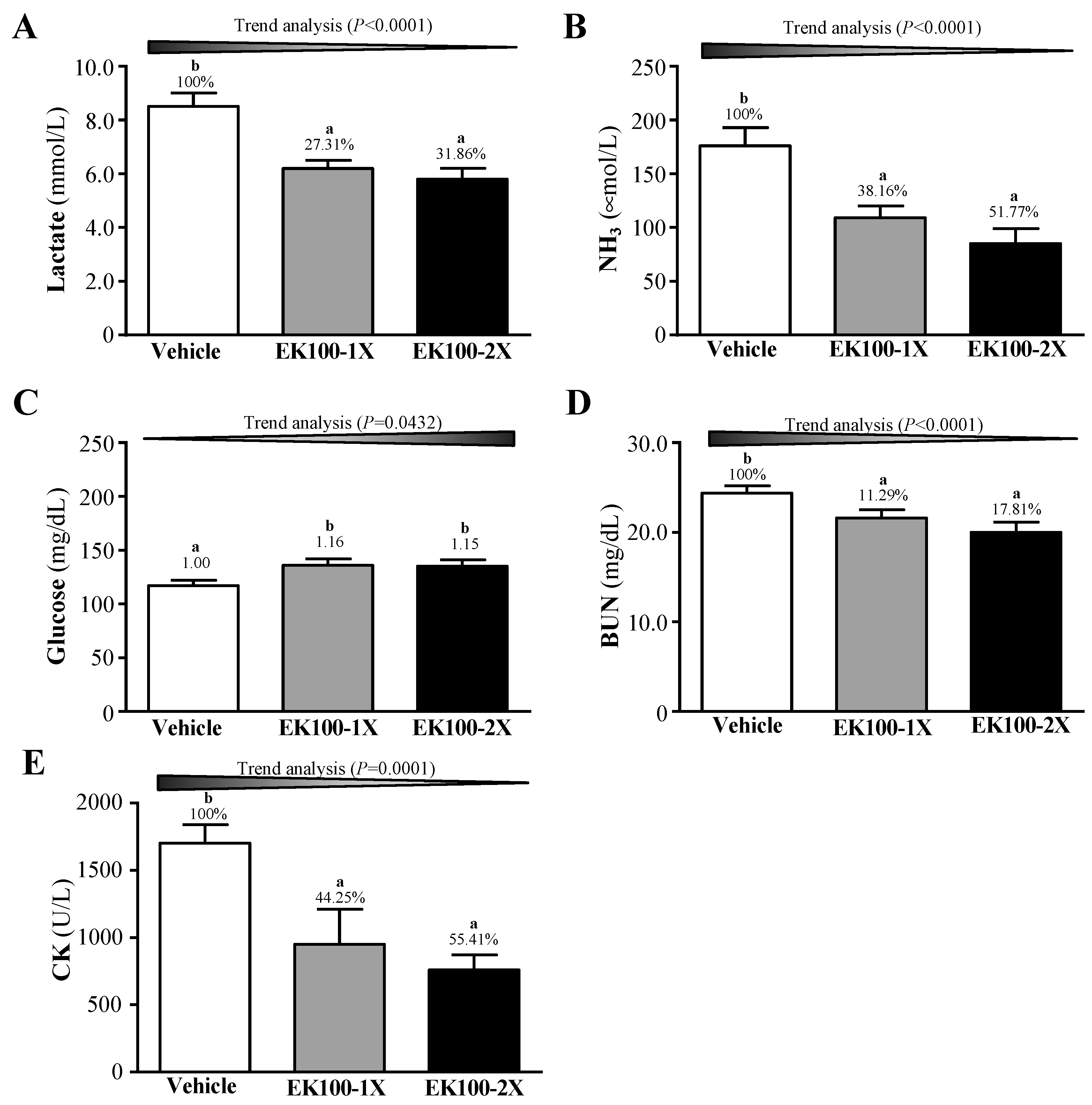
2.3. Effect of EK100 Supplementation on Serum Lactate, Ammonia, Glucose, Blood Urea Nitrogen (BUN), and Creatine Kinase (CK) Levels after Acute Exercise Challenge
2.4. Effect of EK100 Supplementation on Liver and Muscle Glycogen Levels
2.5. Subacute Oral Toxicity Evaluation of EK100 Supplementation
2.6. Effect of EK100 Supplementation on Biochemical Markers
3. Discussion
4. Materials and Methods
4.1. Preparation of Ergosta-7,9(11),22-trien-3β-ol (EK100) from Antrodia camphorata
4.2. Animals and Experimental Design
4.3. Forelimb Grip Strength
4.4. Exhaustive Swimming Test
4.5. Fatigue-Associated Biochemical Indices
4.6. Tissue Glycogen Determination and Visceral Organ Weight
4.7. Histological Staining of Tissues
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 364741. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Ergostatrien-3beta-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and amp-activated protein kinase phosphorylation. J. Agric. Food Chem. 2015, 63, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y.M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid. Based Complement. Alternat. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef]
- Mille-Lindblom, C.; von Wachenfeldt, E.; Tranvik, L.J. Ergosterol as a measure of living fungal biomass: Persistence in environmental samples after fungal death. J. Microbiol. Methods 2004, 59, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Takaku, T.; Kimura, Y.; Okuda, H. Isolation of an antitumor compound from Agaricus blazei murill and its mechanism of action. J. Nutr. 2001, 131, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Huang, S.S.; Shao, Y.Y.; Chen, C.C.; Kuo, Y.H. Protective effect of antrosterol from Antrodia camphorata submerged whole broth against carbon tetrachloride-induced acute liver injury in mice. Food Chem. 2012, 132, 709–716. [Google Scholar] [CrossRef]
- Huang, G.J.; Huang, S.S.; Lin, S.S.; Shao, Y.Y.; Chen, C.C.; Hou, W.C.; Kuo, Y.H. Analgesic effects and the mechanisms of anti-inflammation of ergostatrien-3beta-ol from Antrodia camphorata submerged whole broth in mice. J. Agric. Food Chem. 2010, 58, 7445–7452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Firempong, C.K.; Wang, Y.W.; Xu, W.Q.; Wang, M.M.; Cao, X.; Zhu, Y.; Tong, S.S.; Yu, J.N.; Xu, X.M. Ergosterol-loaded poly (lactide-co-glycolide) nanoparticles with enhanced in vitro antitumor activity and oral bioavailability. Acta. Pharmacol. Sin. 2016, 37, 834–844. [Google Scholar] [CrossRef]
- Hakkinen, K. Neuromuscular fatigue in males and females during strenuous heavy resistance loading. Electromyogr. Clin. Neurophysiol. 1994, 34, 205–214. [Google Scholar] [PubMed]
- MacDougall, J.D.; Ray, S.; Sale, D.G.; McCartney, N.; Lee, P.; Garner, S. Muscle substrate utilization and lactate production. Can. J. Appl. Physiol. 1999, 24, 209–215. [Google Scholar] [CrossRef]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Time to move beyond a brainless exercise physiology: The evidence for complex regulation of human exercise performance. Appl. Physiol. Nutr. Metab. 2011, 36, 23–35. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Liu, Y.C.; Kuo, Y.H.; Lin, Y.L.; Wu, Y.S.; Chen, J.W.; Chen, Y.C. Effects of antrosterol from Antrodia camphorata submerged whole broth on lipid homeostasis, antioxidation, alcohol clearance, and anti-inflammation in livers of chronic-alcohol fed mice. J. Ethnopharmacol. 2017, 202, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Huang, H.Y.; Hsu, Y.J.; Su, W.H.; Shen, S.Y.; Lee, M.C.; Lin, C.L.; Huang, C.C. The effects of thiamine tetrahydrofurfuryl disulfide on physiological adaption and exercise performance improvement. Nutrients 2018, 10, 851. [Google Scholar] [CrossRef]
- Ma, G.D.; Chiu, C.H.; Hsu, Y.J.; Hou, C.W.; Chen, Y.M.; Huang, C.C. Changbai mountain ginseng (Panax ginseng C.A. Mey) extract supplementation improves exercise performance and energy utilization and decreases fatigue-associated parameters in mice. Molecules 2017, 22, 237. [Google Scholar] [CrossRef]
- Cai, R.L.; Yang, M.H.; Shi, Y.; Chen, J.; Li, Y.C.; Qi, Y. Antifatigue activity of phenylethanoid-rich extract from Cistanche deserticola. Phytother. Res. 2010, 24, 313–315. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chen, S.C.; Yech, Y.J.; Wang, L.; Yang, H.L. Antioxidant activity of Antrodia camphorata on free radical-induced endothelial cell damage. J. Ethnopharmacol. 2008, 118, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Ken, C.F.; Wen, L.; Lin, C.T. An enzyme possessing both glutathione-dependent formaldehyde dehydrogenase and s-nitrosoglutathione reductase from Antrodia camphorata. Food Chem. 2009, 112, 795–802. [Google Scholar] [CrossRef]
- Mendes-Ribeiro, A.C.; Mann, G.E.; de Meirelles, L.R.; Moss, M.B.; Matsuura, C.; Brunini, T.M.C. The role of exercise on l-arginine nitric oxide pathway in chronic heart failure. Open Clin. Biochem. J. 2009, 3, 55–65. [Google Scholar] [CrossRef]
- Fitts, R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994, 74, 49–94. [Google Scholar] [CrossRef]
- Brooks, G.A. Amino acid and protein metabolism during exercise and recovery. Med. Sci. Sports Exerc. 1987, 19, S150–S156. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.; Wong, S.H.; Chen, Y. Glycaemic index, glycaemic load and exercise performance. Sports Med. 2010, 40, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Kanzaki, M. Heterotypic endosomal fusion as an initial trigger for insulin-induced glucose transporter 4 (glut4) translocation in skeletal muscle. J. Physiol. 2017, 595, 5603–5621. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef]
- Allen, D.; Lannergren, J.; Westerblad, H. Muscle cell function during prolonged activity: Cellular mechanisms of fatigue. Exp. Physiol. 1995, 80, 497–527. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.-T.; Huang, C.C.; Tung, Y.T. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.G.; Pizza, F.X.; Boone, J.B., Jr.; Andres, F.F.; Michaud, T.A.; Rodriguez-Zayas, J.R. Indices of training stress during competitive running and swimming seasons. Int. J. Sports Med. 1994, 15, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Balsom, P.D.; Gaitanos, G.C.; Soderlund, K.; Ekblom, B. High-intensity exercise and muscle glycogen availability in humans. Acta. Physiol. Scand. 1999, 165, 337–345. [Google Scholar] [CrossRef]
- Areta, J.L.; Hopkins, W.G. Skeletal muscle glycogen content at rest and during endurance exercise in humans: A meta-analysis. Sports Med. 2018, 48, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Du, Y.C.; Chuu, J.J.; Hwang, S.L.; Hsieh, P.C.; Hung, C.S.; Chang, F.R.; Wu, Y.C. Active extracts of wild fruiting bodies of Antrodia camphorata (eeac) induce leukemia HL 60 cells apoptosis partially through histone hypoacetylation and synergistically promote anticancer effect of trichostatin a. Arch. Toxicol. 2009, 83, 121–129. [Google Scholar] [CrossRef]
- Chen, C.C.; Li, I.C.; Lin, T.W.; Chang, H.L.; Lin, W.H.; Shen, Y.C. Efficacy and safety of oral Antrodia cinnamomea mycelium in mildly hypertensive adults: A randomized controlled pilot clinical study. Eur. J. Integr. Med. 2016, 8, 654–660. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Chen, C.C.; Wang, H.Y.; Chiu, H.L.; Hseu, T.H.; Kuo, Y.H. Chemical constituents of Antrodia camphorata submerged whole broth. Nat. Prod. Res. 2008, 22, 1151–1157. [Google Scholar] [CrossRef]
- Chen, Y.M.; Tsai, Y.H.; Tsai, T.Y.; Chiu, Y.S.; Wei, L.; Chen, W.C.; Huang, C.C. Fucoidan supplementation improves exercise performance and exhibits anti-fatigue action in mice. Nutrients 2014, 7, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Lee, H.C.; Chen, M.T.; Huang, C.C.; Chen, W.C. Dehydroepiandrosterone supplementation combined with weight-loading whole-body vibration training (wwbv) affects exercise performance and muscle glycogen storage in middle-aged c57bl/6 mice. Int. J. Med. Sci. 2018, 15, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Chen, Y.M.; Huang, C.C.; Hsiao, C.Y.; Hu, S.; Wang, I.L.; Sung, H.C. Ludwigia octovalvis (jacq.) raven extract supplementation enhances muscle glycogen content and endurance exercise performance in mice. J. Vet. Med. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Characteristic | Vehicle | EK100-1X | EK100-2X | Trend Analysis |
|---|---|---|---|---|
| Initial BW (g) | 29.6 ± 0.4 | 29.6 ± 0.3 | 29.6 ± 0.3 | 0.7824 |
| Final BW (g) | 36.9 ± 0.5 | 37.9 ± 0.3 | 38.3 ± 0.6 | 0.0382 |
| Food intake (g/day) | 6.47 ± 0.15 | 6.38 ± 0.12 | 6.37 ± 0.17 | 0.2899 |
| Water intake (g//day) | 7.76 ± 0.23 | 7.71 ± 0.17 | 7.69 ± 0.12 | 0.1086 |
| Liver (g) | 2.06 ± 0.05 | 2.07 ± 0.03 | 2.09 ± 0.06 | 0.8734 |
| Kidney (g) | 0.58 ± 0.03 | 0.58 ± 0.02 | 0.60 ± 0.02 | 0.6216 |
| EFP(g) | 0.491 ± 0.03 | 0.525 ± 0.03 | 0.520 ± 0.02 | 0.5455 |
| Heart (g) | 0.25 ± 0.01 | 0.23 ± 0.01 | 0.25 ± 0.02 | 0.7737 |
| Lung (g) | 0.26 ± 0.01 | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.3851 |
| Muscle (g) | 0.41 ± 0.01 | 0.41 ± 0.01 | 0.40 ± 0.01 | 0.5377 |
| BAT (g) | 0.109 ± 0.004 | 0.275 ± 0.102 | 0.211 ± 0.081 | 0.0586 |
| Relative Liver weight (%) | 5.58 ± 0.13 | 5.45 ± 0.07 | 5.44 ± 0.10 | 0.3738 |
| Relative Kidney weight (%) | 1.56 ± 0.09 | 1.52 ± 0.05 | 1.57 ± 0.05 | 0.7721 |
| Relative EFP weight (%) | 1.33 ± 0.08 | 1.39 ± 0.09 | 1.36 ± 0.06 | 0.8391 |
| Relative Heart weight (%) | 0.68 ± 0.04 | 0.61 ± 0.03 | 0.65 ± 0.04 | 0.4660 |
| Relative Lung weight (%) | 0.71 ± 0.04 | 0.65 ± 0.04 | 0.63 ± 0.03 | 0.1422 |
| Relative Muscle weight (%) | 1.12 ± 0.03 | 1.09 ± 0.02 | 1.04 ± 0.03 | 0.1285 |
| Relative BAT weight | 0.30 ± 0.01 | 0.72 ± 0.27 | 0.54 ± 0.20 | 0.0941 |
| Parameter | Vehicle | EK100-1X | EK100-2X | Trend Analysis |
|---|---|---|---|---|
| AST (U/L) | 185 ± 13b | 134 ± 12a | 136 ± 14a | 0.0053 |
| ALT (U/L) | 148 ± 24b | 81 ± 13a | 74 ± 7a | 0.0004 |
| ALP (U/L) | 78 ±5b | 66 ±3a | 61±4a | 0.0011 |
| LDH (U/L) | 983 ±57b | 735± 64a | 672 ± 43a | <0.0001 |
| CK (U/L) | 1744 ± 279 | 1385 ± 196 | 1360 ± 269 | 0.2599 |
| BUN (mg/dL) | 29.4 ± 1.7c | 24.8 ± 0.7b | 20.5 ± 0.7a | <0.0001 |
| Albumin (g/dL) | 3.5 ± 0.0 | 3.5 ± 0.1 | 3.5 ± 0.1 | 0.8996 |
| UA (mg/dL) | 1.43 ± 0.06 | 1.40 ± 0.10 | 1.34 ± 0.06 | 0.2954 |
| TC (mg/dL) | 157 ± 6 | 161 ± 5 | 148 ± 6 | 0.2894 |
| TG (mg/dL) | 180 ± 19 | 174 ± 10 | 146 ± 8 | 0.1637 |
| Creatinine (mg/dL) | 0.27 ± 0.01 | 0.25 ± 0.01 | 0.27 ± 0.02 | 0.9594 |
| TP (g/dL) | 4.66 ± 0.04 | 4.81 ± 0.03 | 4.84 ± 0.08 | 0.0061 |
| Glucose (mg/dL) | 152 ± 4 | 154 ± 4 | 167 ±7 | 0.0429 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-M.; Sung, H.-C.; Kuo, Y.-H.; Hsu, Y.-J.; Huang, C.-C.; Liang, H.-L. The Effects of Ergosta-7,9(11),22-trien-3β-ol from Antrodia camphorata on the Biochemical Profile and Exercise Performance of Mice. Molecules 2019, 24, 1225. https://doi.org/10.3390/molecules24071225
Chen Y-M, Sung H-C, Kuo Y-H, Hsu Y-J, Huang C-C, Liang H-L. The Effects of Ergosta-7,9(11),22-trien-3β-ol from Antrodia camphorata on the Biochemical Profile and Exercise Performance of Mice. Molecules. 2019; 24(7):1225. https://doi.org/10.3390/molecules24071225
Chicago/Turabian StyleChen, Yi-Ming, Hsin-Ching Sung, Yueh-Hsiung Kuo, Yi-Ju Hsu, Chi-Chang Huang, and Hsin-Li Liang. 2019. "The Effects of Ergosta-7,9(11),22-trien-3β-ol from Antrodia camphorata on the Biochemical Profile and Exercise Performance of Mice" Molecules 24, no. 7: 1225. https://doi.org/10.3390/molecules24071225
APA StyleChen, Y.-M., Sung, H.-C., Kuo, Y.-H., Hsu, Y.-J., Huang, C.-C., & Liang, H.-L. (2019). The Effects of Ergosta-7,9(11),22-trien-3β-ol from Antrodia camphorata on the Biochemical Profile and Exercise Performance of Mice. Molecules, 24(7), 1225. https://doi.org/10.3390/molecules24071225







