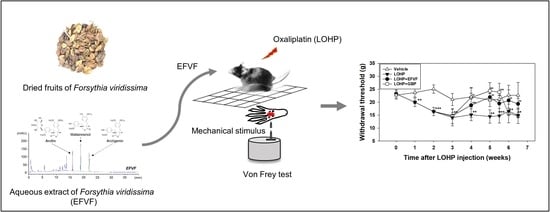
Neuroprotective Effects of an Aqueous Extract of Forsythia viridissima and Its Major Constituents on Oxaliplatin-Induced Peripheral Neuropathy
Abstract
:1. Introduction
2. Results
2.1. Ultra-High Performance Liquid Chromatography Analysis of EFVF
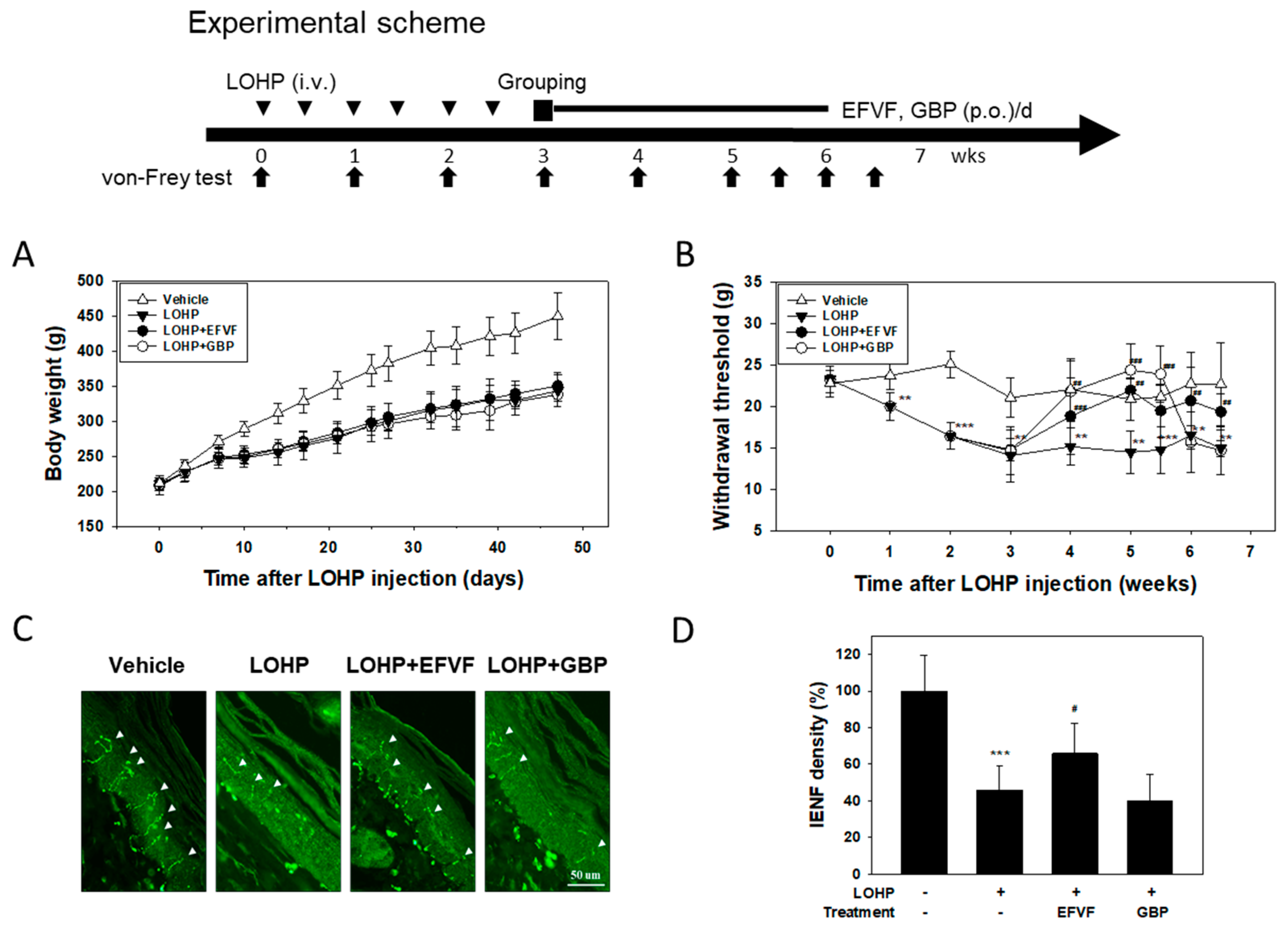
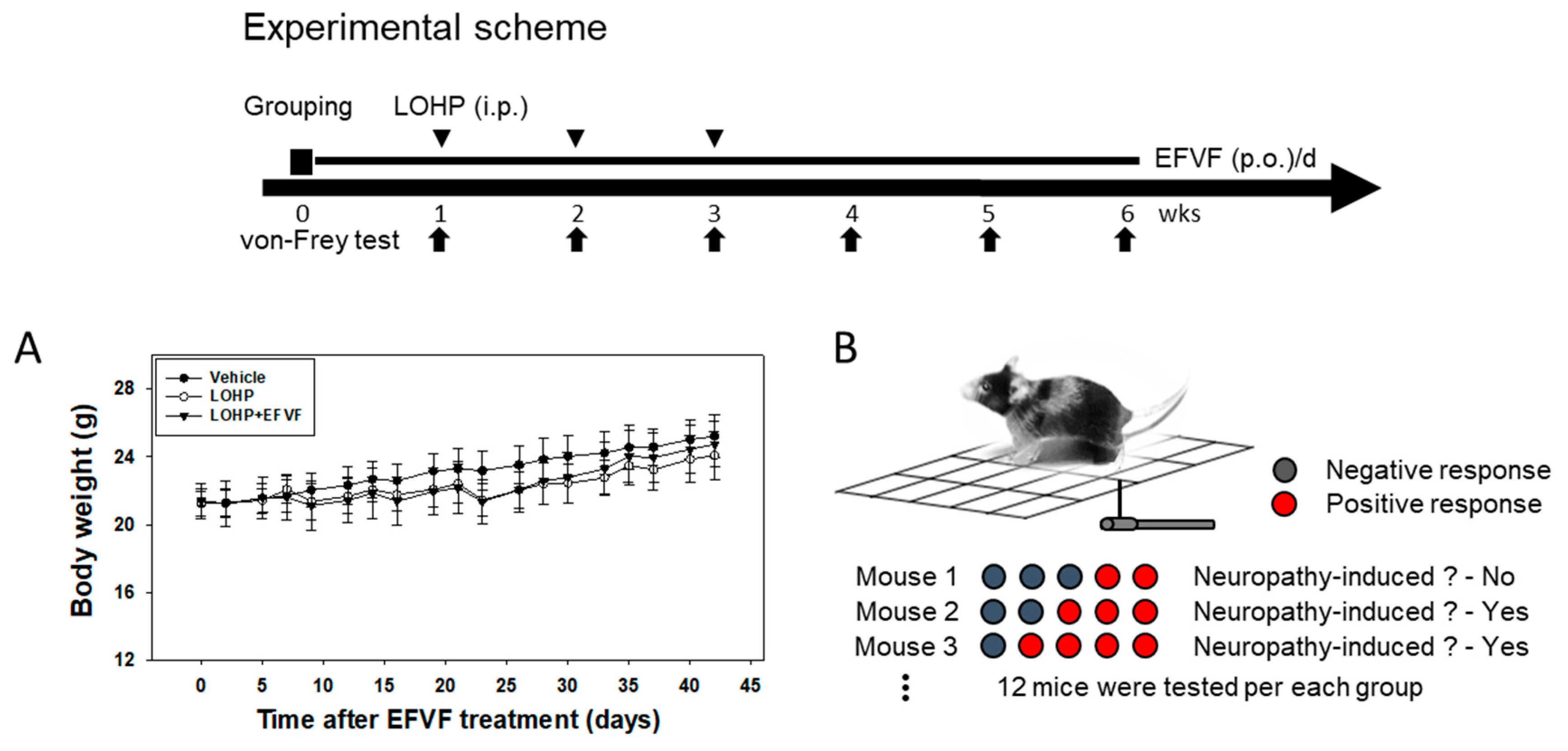
2.2. EFVF Attenuated LOHP-Induced Peripheral Neuropathy in Animal Models
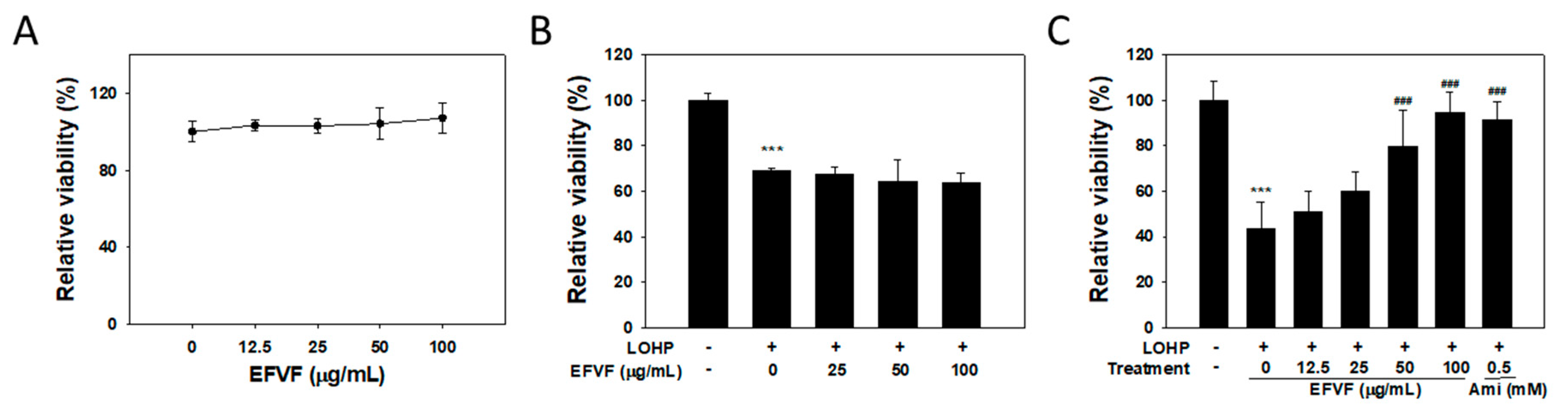
2.3. EFVF Relieved LOHP-Induced Cytotoxicity in Neuronal Differentiated PC12
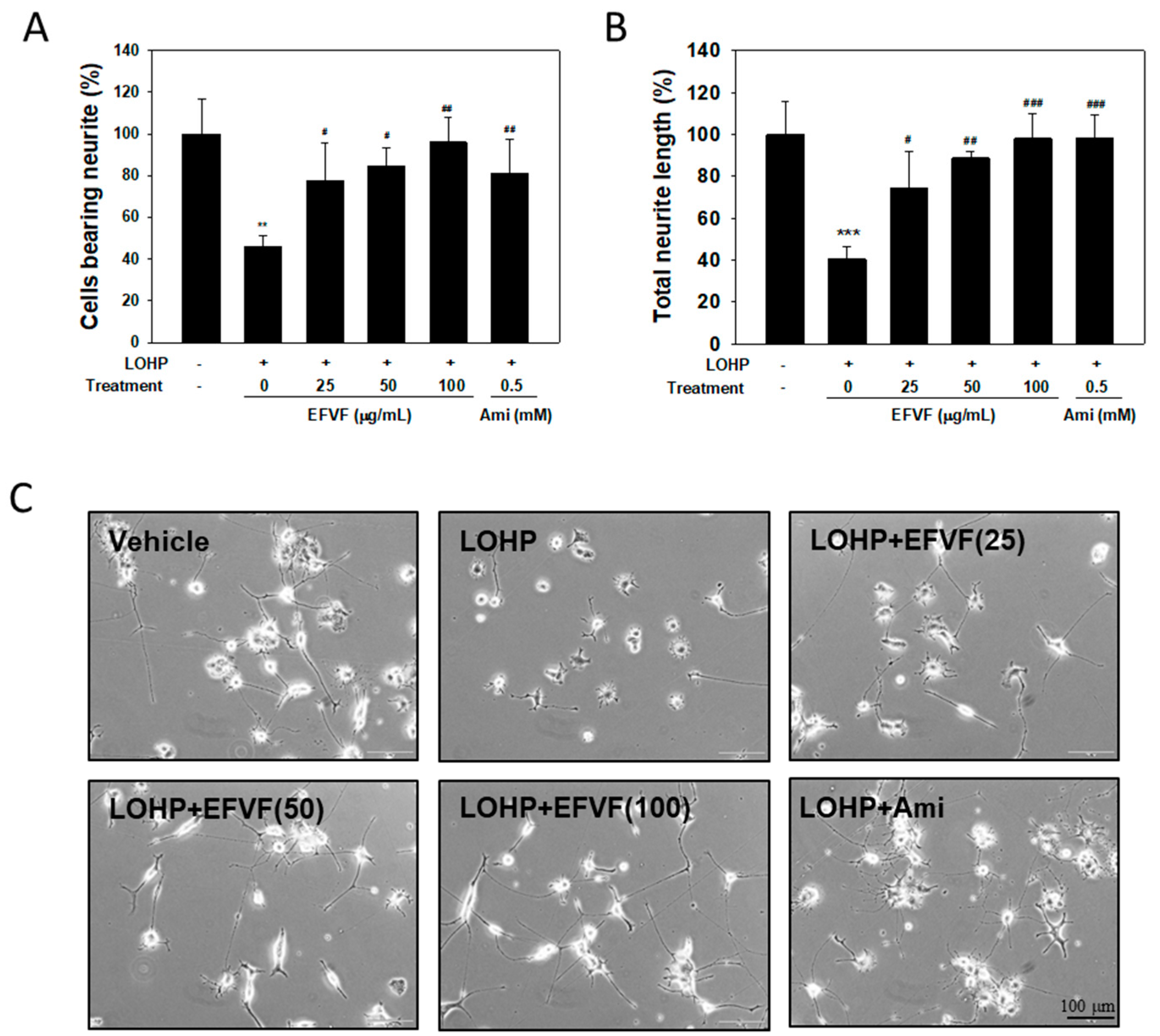
2.4. EFVF Attenuated LOHP-Induced Neurotoxicity in PC12 Cells.
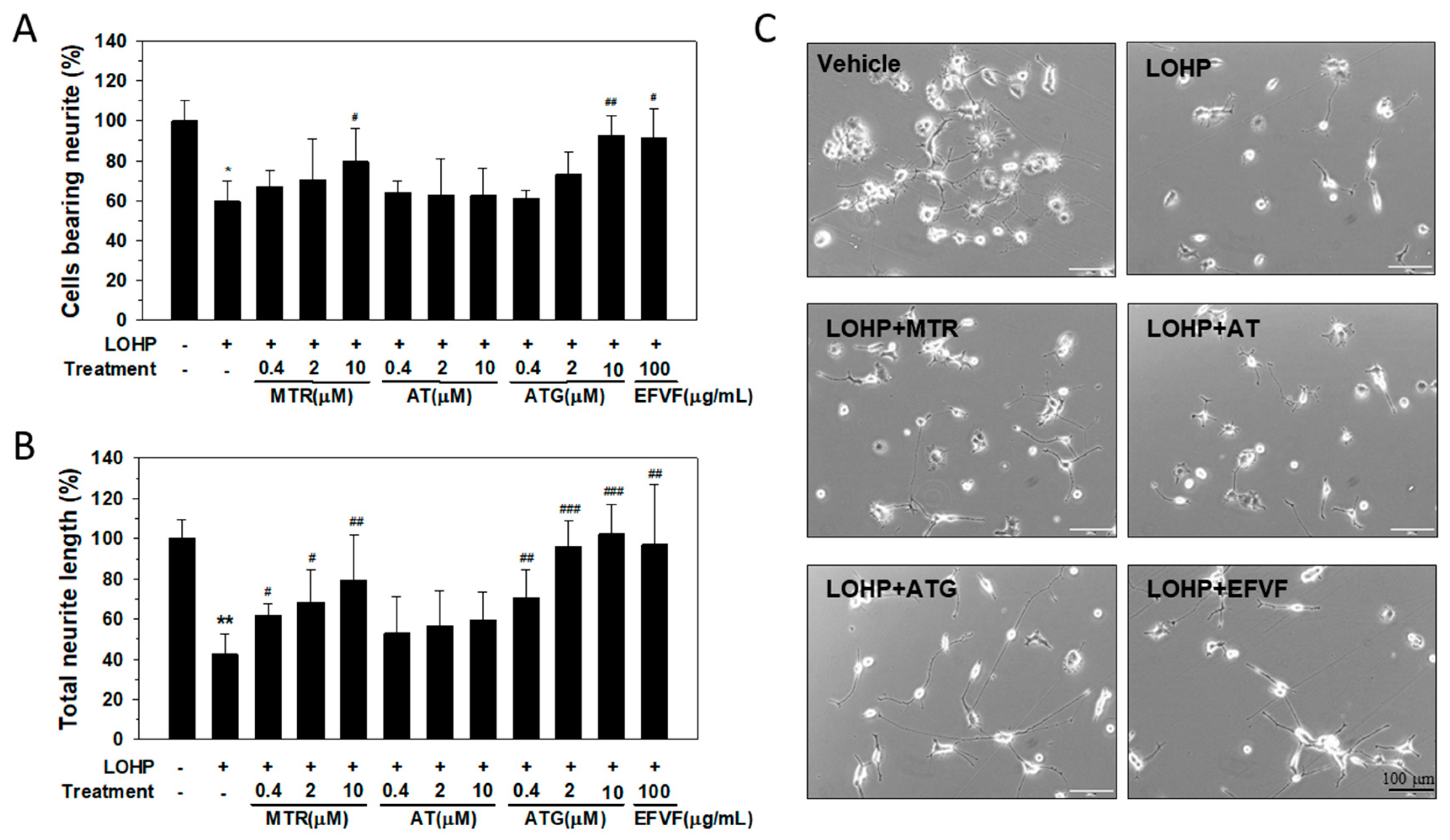
2.5. Effect of the Major Constituents of EFVF on LOHP-Induced Neurotoxicity
2.6. Protective Effect of EFVF on LOHP-Induced Neurotoxicity in DRG Cells
2.7. Protective Function of EFVF on LOHP-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials and Extract Preparations
4.3. Chromatographic Analysis
4.3.1. Chromatographic Condition
4.3.2. Quantitative analysis
4.4. Experimental Animals and Drug Administration
4.5. Assessment of Mechanical Allodynia
4.6. Cell Culture and Cell Viability Assay
4.7. Neurite Outgrowth Assay in PC12
4.8. Neurite Outgrowth Assay in DRGs
4.9. Apoptosis Assay
4.10. Mitochondrial Membrane Potential (MMP) Assay
4.11. Measurement of ROS Formation
4.12. Immunohistochemistry
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Curr. Opin. Neurol. 2015, 28, 500–507. [Google Scholar] [CrossRef]
- Flatters, S.J.L.; Dougherty, P.M.; Colvin, L.A. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Smith, E.M.L.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef]
- Von Hehn, C.A.; Baron, R.; Woolf, C.J. Deconstructing the Neuropathic Pain Phenotype to Reveal Neural Mechanisms. Neuron 2012, 73, 638–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, A.V.; Goldstein, D.; Friedlander, M.; Kiernan, M.C. Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle Nerve 2005, 32, 51–60. [Google Scholar] [CrossRef]
- Briani, C.; Argyriou, A.A.; Izquierdo, C.; Velasco, R.; Campagnolo, M.; Alberti, P.; Frigeni, B.; Cacciavillani, M.; Bergamo, F.; Cortinovis, D.; et al. Long-term course of oxaliplatin-induced polyneuropathy: A prospective 2-year follow-up study. J. Peripher. Nerv. Syst. 2014, 19, 299–306. [Google Scholar] [CrossRef]
- Park, S.B.; Goldstein, D.; Lin, C.S.Y.; Krishnan, A.V.; Friedlander, M.L.; Kiernan, M.C. Acute Abnormalities of Sensory Nerve Function Associated with Oxaliplatin-Induced Neurotoxicity. J. Clin. Oncol. 2009, 27, 1243–1249. [Google Scholar] [CrossRef]
- Tofthagen, C.; Donovan, K.A.; Morgan, M.A.; Shibata, D.; Yeh, Y.T. Oxaliplatin-induced peripheral neuropathy’s effects on health-related quality of life of colorectal cancer survivors. Support. Care Cancer 2013, 21, 3307–3313. [Google Scholar] [CrossRef]
- Chu, S.H.; Lee, Y.J.; Lee, E.S.; Geng, Y.M.; Wang, X.S.; Cleeland, C.S. Current use of drugs affecting the central nervous system for chemotherapy-induced peripheral neuropathy in cancer patients: A systematic review. Support. Care Cancer 2015, 23, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Olsen, Y. The CDC Guideline on Opioid Prescribing Rising to the Challenge. JAMA J. Am. Med. Assoc. 2016, 315, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Saiki, C.B. Cancer Pain Management. Mayo Clin. Proc. 2015, 90, 1428–1439. [Google Scholar] [CrossRef]
- Majithia, N.; Loprinzi, C.L.; Smith, T.J. New Practical Approaches to Chemotherapy-Induced Neuropathic Pain: Prevention, Assessment, and Treatment. Oncology 2016, 30, 1020–1029. [Google Scholar]
- Smith, E.M.L.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of Duloxetine on Pain, Function, and Quality of Life Among Patients with Chemotherapy-Induced Painful Peripheral Neuropathy A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2013, 309, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Korea Food and Drug Administration. South Korean Pharmacopoeia; Monographs Part II; Ministry of Health and Welfare: Se jong, Korea, 2017; pp. 1839–1840.
- Chen, H.Y.; Lin, Y.H.; Huang, J.W.; Chen, Y.C. Chinese herbal medicine network and core treatments for allergic skin diseases: Implications from a nationwide database. J. Ethnopharmacol. 2015, 168, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.L.; Lu, X.Y.; Tong, X.L.; Dong, Y.Q.; Tang, L.; Liu, M.H. Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics. Molecules 2017, 22, 1466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Xia, Q.; Liu, X.; Liu, W.X.; Huang, W.Z.; Mei, X.; Luo, J.; Shon, M.X.; Lin, R.C.; Zou, D.X.; et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. J. Ethnopharmacol. 2018, 210, 318–339. [Google Scholar] [CrossRef]
- Bao, J.L.; Ding, R.B.; Zou, L.D.; Zhang, C.; Wang, K.; Liu, F.; Li, P.; Chen, M.W.; Wan, J.B.; Su, H.X.; et al. Forsythiae Fructus Inhibits B16 Melanoma Growth Involving MAPKs/Nrf2/HO-1 Mediated Anti-Oxidation and Anti-Inflammation. Am. J. Chin. Med. 2016, 44, 1043–1061. [Google Scholar] [CrossRef]
- Kuo, P.C.; Chen, G.F.; Yang, M.L.; Lin, Y.H.; Peng, C.C. Chemical Constituents from the Fruits of Forsythia suspensa and Their Antimicrobial Activity. Biomed. Res. Int. 2014, 2014, 304830. [Google Scholar] [CrossRef]
- Kuo, P.C.; Hung, H.Y.; Nian, C.W.; Hwang, T.L.; Cheng, J.C.; Kuo, D.H.; Lee, E.J.; Tai, S.H.; Wu, T.S. Chemical Constituents and Anti-inflammatory Principles from the Fruits of Forsythia suspensa. J. Nat. Prod. 2017, 80, 1055–1064. [Google Scholar] [CrossRef]
- Kim, N.Y.; Kang, T.H.; Song, E.K.; Pae, H.O.; Chung, H.T.; Kim, Y.C. Inhibitory effects of butanol fraction of the aqueous extract of Forsythia koreana on the nitric oxide production by murine macrophage-like RAW 264.7 cells. J. Ethnopharmacol. 2000, 73, 323–327. [Google Scholar] [CrossRef]
- Lee, S.E.; Lim, C.; Ahn, S.C.; Cho, S. A Study of the Anti-Cancer Effects of the Hexane Fraction of the Methanol Extract of Forsythiae Fructus. Pharmacogn. Mag. 2017, 13, 719–724. [Google Scholar] [PubMed]
- Hao, Y.; Li, D.; Piao, X.; Piao, X. Forsythia suspensa extract alleviates hypersensitivity induced by soybean beta-conglycinin in weaned piglets. J. Ethnopharmacol. 2010, 128, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, A.H.; Li, L.; Guo, D.A. Simultaneous determination of 12 major constituents in Forsythia suspensa by high performance liquid chromatography—DAD method. J. Pharmaceut. Biomed. 2007, 43, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.-S.; Li, K.K.; Zheng, X.K. Studies on chemical constituents in Forsythia suspensa (Thunb.) vahl U. Chin. Pharmaceut. J. 2009, 44, 490–492. [Google Scholar]
- Piao, X.L.; Jang, M.H.; Cui, J.; Piao, X.S. Lignans from the fruits of Forsythia suspensa. Bioorg. Med. Chem. Lett. 2008, 18, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.; Song, J.H.; Kim, S.R.; Cho, H.M.; Ko, H.J.; Yang, H.; Sung, S.H. Lignan Dimers from Forsythia viridissima Roots and Their Antiviral Effects. J. Nat. Prod. 2019, 82, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Tokar, M.; Klimek, B. Isolation and identification of biologically active compounds from Forsythia viridissima flowers. Acta Pol. Pharmaceut. 2004, 61, 191–197. [Google Scholar]
- Ohsawa, M.; Otake, S.; Murakami, T.; Yamamoto, S.; Makino, T.; Ono, H. Gabapentin prevents oxaliplatin-induced mechanical hyperalgesia in mice. J. Pharmacol. Sci. 2014, 125, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, M.J.; Kautio, A.L.; Haanpaa, M.L.; Haapasalo, H.K.; Kellokumpu-Lehtinen, P.L.; Saarto, T.; Hietaharju, A.J. Intraepidermal Nerve Fibre Density in Cancer Patients Receiving Adjuvant Chemotherapy. Anticancer Res. 2011, 31, 4413–4416. [Google Scholar]
- Fujita, S.; Ushio, S.; Ozawa, N.; Masuguchi, K.; Kawashiri, T.; Oishi, R.; Egashira, N. Exenatide Facilitates Recovery from Oxaliplatin-Induced Peripheral Neuropathy in Rats. PLoS ONE 2015, 10, e0141921. [Google Scholar] [CrossRef]
- Kawashiri, T.; Miyagi, A.; Shimizu, S.; Shigematsu, N.; Kobayashi, D.; Shimazoe, T. Dimethyl fumarate ameliorates chemotherapy agent-induced neurotoxicity in vitro. J. Pharmacol. Sci. 2018, 137, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Dussmann, H.; Rehm, M.; Kogel, D.; Prehn, J.H.M. Outer mitochondrial membrane permeabilization during apoptosis triggers caspase-independent mitochondrial and caspase-dependent plasma membrane potential depolarization: A single-cell analysis. J. Cell Sci. 2003, 116, 525–536. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Qian, T.; He, L.H.; Kim, J.S.; Elmore, S.P.; Cascio, W.E.; Brenner, D.A. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid. Redox Signal. 2002, 4, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Beijers, A.J.M.; Mols, F.; Vreugdenhil, G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support. Care Cancer 2014, 22, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Mihara, Y.; Egashira, N.; Sada, H.; Kawashiri, T.; Ushio, S.; Yano, T.; Ikesue, H.; Oishi, R. Involvement of spinal NR2B-containing NMDA receptors in oxaliplatin-induced mechanical allodynia in rats. Mol. Pain 2011, 7, 8. [Google Scholar] [CrossRef]
- Cavaletti, G.; Tredici, G.; Petruccioli, M.G.; Donde, E.; Tredici, P.; Marmiroli, P.; Minoia, C.; Ronchi, A.; Bayssas, M.; Etienne, G.G. Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur. J. Cancer 2001, 37, 2457–2463. [Google Scholar] [CrossRef]
- Pasetto, L.M.; D’Andrea, M.R.; Rossi, E.; Monfardini, S. Oxaliplatin-related neurotoxicity: How and why? Crit. Rev. Oncol. Hematol. 2006, 59, 159–168. [Google Scholar] [CrossRef]
- Cho, E.S.; Yi, J.M.; Park, J.S.; Lee, Y.J.; Lim, C.J.; Bang, O.S.; Kim, N.S. Aqueous extract of Lithospermi radix attenuates oxaliplatin-induced neurotoxicity in both in vitro and in vivo models. BMC Complement. Altern. Med. 2016, 16, 419. [Google Scholar] [CrossRef]
- Hopkins, H.L.; Duggett, N.A.; Flatters, S.J.L. Chemotherapy-induced painful neuropathy: Pain-like behaviours in rodent models and their response to commonly used analgesics. Curr Opin. Support. Palliat. Care 2016, 10, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.; Patte-Mensah, C.; Taleb, O.; Mensah-Nyagan, A.G. Allopregnanolone prevents and suppresses oxaliplatin-evoked painful neuropathy: Multi-parametric assessment and direct evidence. Pain 2011, 152, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Renn, C.L.; Carozzi, V.A.; Rhee, P.; Gallop, D.; Dorsey, S.G.; Cavaletti, G. Multimodal assessment of painful peripheral neuropathy induced by chronic oxaliplatin-based chemotherapy in mice. Mol. Pain 2011, 7, 29. [Google Scholar] [CrossRef]
- Ceresa, C.; Avan, A.; Giovannetti, E.; Geldof, A.A.; Avan, A.; Cavaletti, G.; Peters, G.J. Characterization of and protection from neurotoxicity induced by oxaliplatin, bortezomib and epothilone-B. Anticancer Res. 2014, 34, 517–523. [Google Scholar]
- Gao, Q.; Yang, M.; Zuo, Z. Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L. Acta Pharmacol. Sin. 2018, 39, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, N.; Xia, Y.; Gao, Z.; Zou, S.F.; Kong, L.; Yao, Y.J.; Jiao, Y.N.; Yan, Y.H.; Li, S.H.; et al. Arctigenin Treatment Protects against Brain Damage through an Anti-Inflammatory and Anti-Apoptotic Mechanism after Needle Insertion. Front. Pharmacol. 2016, 7, 182. [Google Scholar] [CrossRef]
- Sun, G.D.; Hu, X.J.; Zhang, G.M.; Sun, C.H.; Zhang, R.W.; Tang, S.J.; Lin, Y.X.; Li, Z.Z. Arctigenin suppresses inflammation and plays a neuroprotective effect in mice with spinal cord injury. Int. J. Clin. Exp. Med. 2018, 11, 2100–2106. [Google Scholar]
- Zhang, N.; Wen, Q.P.; Ren, L.; Liang, W.B.; Xia, Y.; Zhang, X.D.; Zhao, D.; Sun, D.; Hu, Y.; Hao, H.G.; et al. Neuroprotective Effect of Arctigenin via Upregulation of P-CREB in Mouse Primary Neurons and Human SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2013, 14, 18657–18669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Duan, X.; Huang, F.; Cheng, X.; Zhang, L.; Liu, P.; Shulan, S.; Duan, J.A.; Dong, T.T.; Tsim, K.W. Kai-Xin-San, a traditional Chinese medicine formula, induces neuronal differentiation of cultured PC12 cells: Modulating neurotransmitter regulation enzymes and potentiating NGF inducing neurite outgrowth. J. Ethnopharmacol. 2016, 193, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Cheng, X.; Wink, M. Cytotoxicity of arctigenin and matairesinol against the T-cell lymphoma cell line CCRF-CEM. J. Pharm. Pharmacol. 2015, 67, 1316–1323. [Google Scholar] [CrossRef]
- Xu, P.; Huang, M.W.; Xiao, C.X.; Long, F.; Wang, Y.; Liu, S.Y.; Jia, W.W.; Wu, W.J.; Yang, D.; Hu, J.F.; et al. Matairesinol Suppresses Neuroinflammation and Migration Associated with Src and ERK1/2-NF-kappaB Pathway in Activating BV2 Microglia. Neurochem. Res. 2017, 42, 2850–2860. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.H.; Zheng, H.; Bennett, G.J. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 2012, 203, 194–206. [Google Scholar] [CrossRef] [PubMed]
- McCormick, B.; Lowes, D.A.; Colvin, L.; Torsney, C.; Galley, H.F. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. Br. J. Anaesth. 2016, 117, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K. Clinical Traditional Herbalogy, 5th ed.; YoungLim’s Publisher: Seoul, Korea, 1996; p. 322. [Google Scholar]
- US FDA Guidance for Industry. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Pharmacology and Toxicology. 2005. Available online: http://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf (accessed on 26 Feburuary 2019).
- Smiley, S.T.; Reers, M.; Mottola-Hartshorn, C.; Lin, M.; Chen, A.; Smith, T.W.; Steele, G.D., Jr.; Chen, L.B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3671–3675. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Constituents | Linear Range (μg/mL) | Regression Equation | r2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Arctiin | 5–200 | 0.9999 | 0.33 | 0.99 | |
| Matairesinol | 5–200 | 0.9999 | 0.12 | 0.37 | |
| Arctigenin | 5–200 | 0.9998 | 0.21 | 0.62 |
| Treatment | N | Hind Paw (N) | Time (Week) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 0.16 g Bending Force Filament | ||||||||
| Vehicle | 12 | Left | 2 | 3 | 2 | 2 | 2 | 2 |
| Right | 1 | 1 | 3 | 3 | 3 | 2 | ||
| Avg (%) 1 | 12.5 | 16.7 | 20.8 | 20.8 | 20.8 | 16.7 | ||
| LOHP | 12 | Left | 2 | 4 | 6 | 6 | 5 | 6 |
| Right | 1 | 2 | 6 | 6 | 5 | 4 | ||
| Avg (%) 1 | 12.5 | 25.0 | 50.0 | 50.0 | 41.7 | 41.7 | ||
| LOHP+EFVF | 12 | Left | 1 | 3 | 3 | 4 | 1 | 2 |
| Right | 2 | 3 | 3 | 3 | 4 | 4 | ||
| Avg (%) 1 | 12.5 | 25.0 | 25.0 | 29.2 | 20.8 | 25.0 | ||
| 0.4 g Bending Force Filament | ||||||||
| Vehicle | 12 | Left | 3 | 4 | 5 | 4 | 4 | 4 |
| Right | 4 | 5 | 3 | 4 | 5 | 4 | ||
| Avg (%) 1 | 29.2 | 37.5 | 33.3 | 33.3 | 37.5 | 33.3 | ||
| LOHP | 12 | Left | 4 | 8 | 9 | 9 | 9 | 7 |
| Right | 2 | 6 | 9 | 10 | 9 | 7 | ||
| Avg (%) 1 | 25.0 | 58.3 | 75.0 | 79.2 | 75.0 | 58.3 | ||
| LOHP+EFVF | 12 | Left | 3 | 4 | 3 | 4 | 7 | 4 |
| Right | 3 | 5 | 5 | 4 | 3 | 6 | ||
| Avg (%) 1 | 25.0 | 37.5 | 33.3 | 33.3 | 41.7 | 41.7 | ||
| Treatment | IC50 Value (μM) 1 | ||||
|---|---|---|---|---|---|
| HCT116 | COLO205 | KM12SM | MDA-MB-468 | MCF7 | |
| Vehicle | 1.6 ± 0.1 | 2.0 ± 1.4 | 4.9 ± 1.0 | 4.9 ± 0.2 | 7.2 ± 3.5 |
| EFVF 50 μg/mL | 2.1 ± 0.9 | 2.2 ± 1.8 | 5.9 ± 2.0 | 4.8 ± 0.4 | 6.5 ± 3.2 |
| EFVF 100 μg/mL | 2.8 ± 1.2 | 1.9 ± 1.5 | 4.4 ± 0.5 | 4.6 ± 0.1 | 6.8 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, J.-M.; Shin, S.; Kim, N.S.; Bang, O.-S. Neuroprotective Effects of an Aqueous Extract of Forsythia viridissima and Its Major Constituents on Oxaliplatin-Induced Peripheral Neuropathy. Molecules 2019, 24, 1177. https://doi.org/10.3390/molecules24061177
Yi J-M, Shin S, Kim NS, Bang O-S. Neuroprotective Effects of an Aqueous Extract of Forsythia viridissima and Its Major Constituents on Oxaliplatin-Induced Peripheral Neuropathy. Molecules. 2019; 24(6):1177. https://doi.org/10.3390/molecules24061177
Chicago/Turabian StyleYi, Jin-Mu, Sarah Shin, No Soo Kim, and Ok-Sun Bang. 2019. "Neuroprotective Effects of an Aqueous Extract of Forsythia viridissima and Its Major Constituents on Oxaliplatin-Induced Peripheral Neuropathy" Molecules 24, no. 6: 1177. https://doi.org/10.3390/molecules24061177
APA StyleYi, J.-M., Shin, S., Kim, N. S., & Bang, O.-S. (2019). Neuroprotective Effects of an Aqueous Extract of Forsythia viridissima and Its Major Constituents on Oxaliplatin-Induced Peripheral Neuropathy. Molecules, 24(6), 1177. https://doi.org/10.3390/molecules24061177