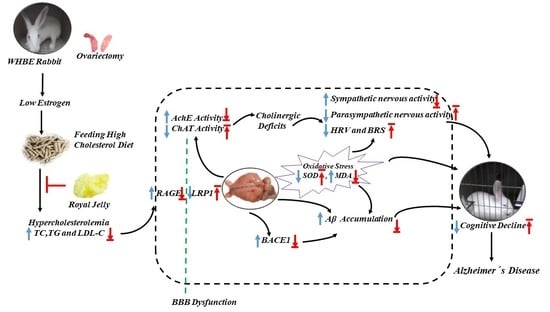
Royal Jelly Ameliorates Behavioral Deficits, Cholinergic System Deficiency, and Autonomic Nervous Dysfunction in Ovariectomized Cholesterol-Fed Rabbits
Abstract
:1. Introduction
2. Results
2.1. RJ Reduced Blood Lipid Levels in OVX Cholesterol-Fed Rabbit
2.2. RJ Improved Behavioral Deficits in OVX Cholesterol-Fed Rabbits
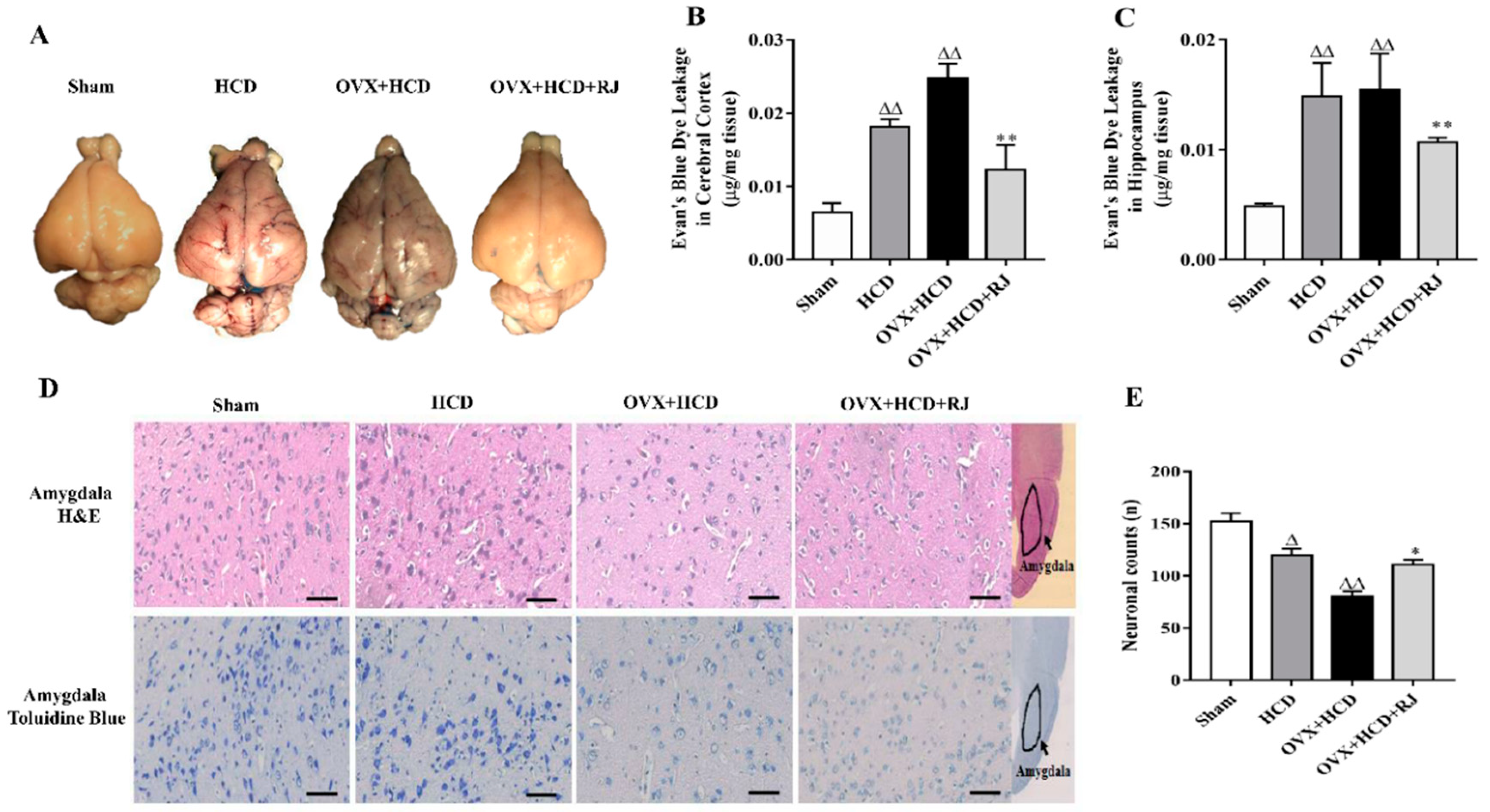
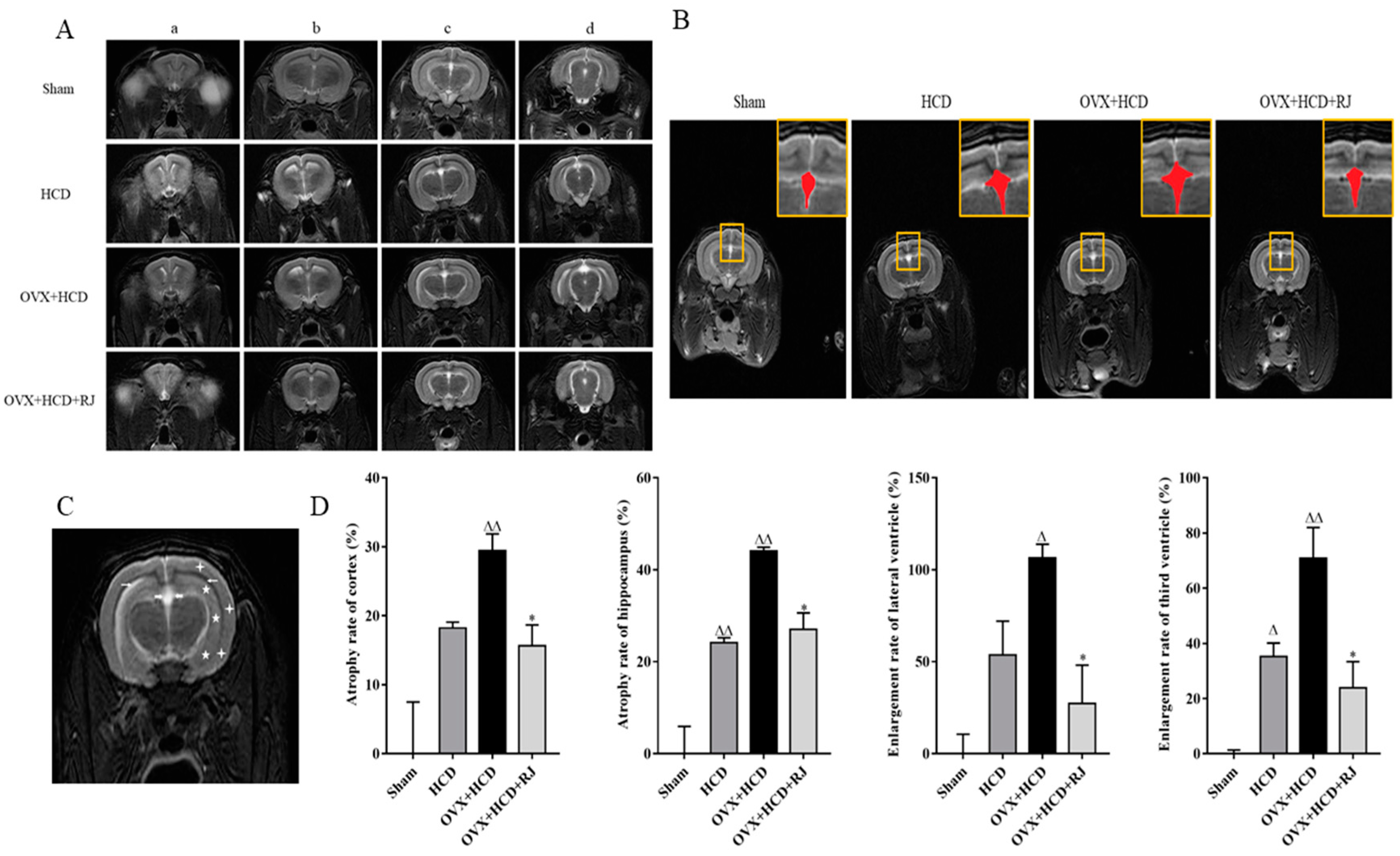
2.3. RJ Ameliorated BBB Permeability and Reduced Neuronal Loss in OVX Cholesterol-Fed Rabbits
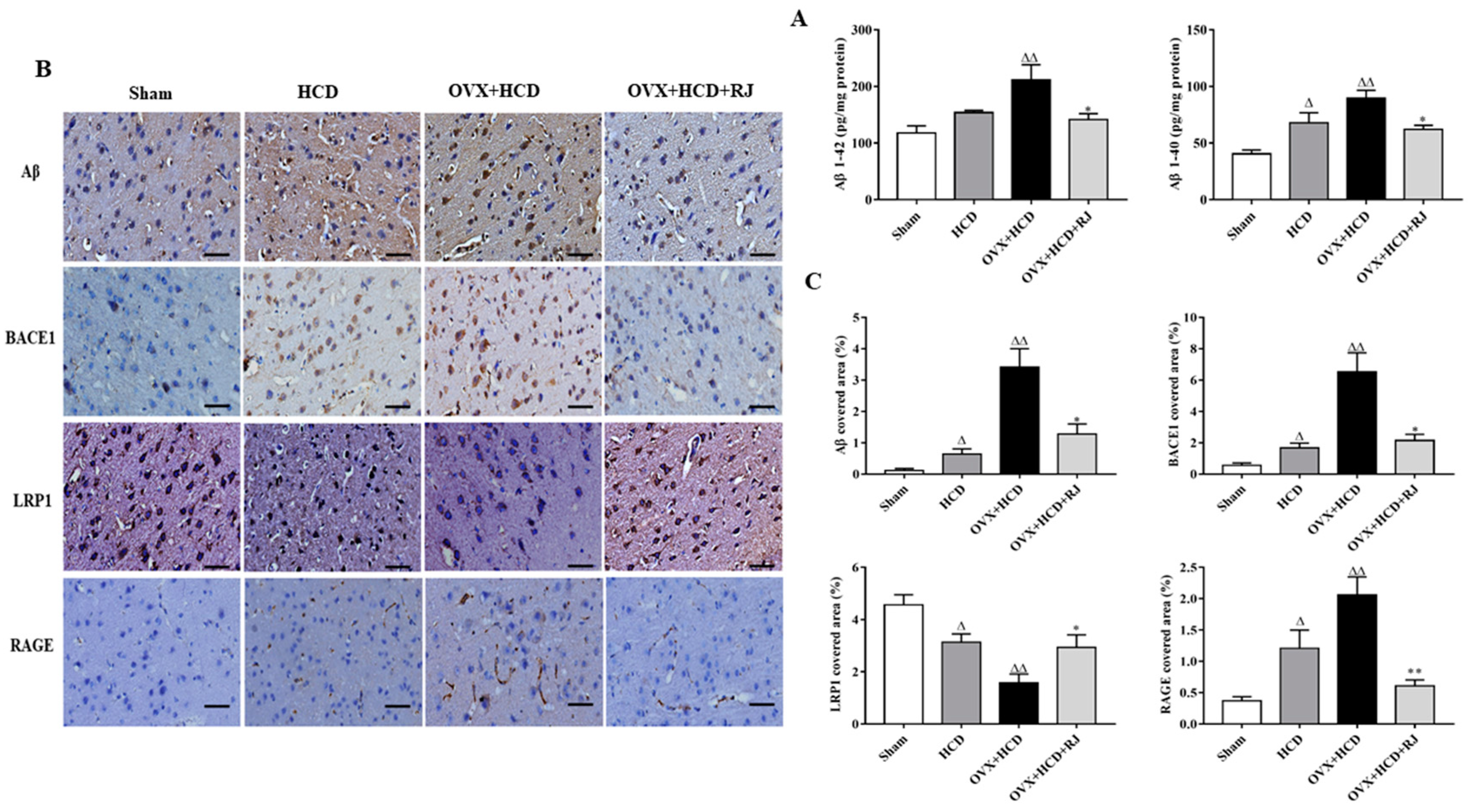
2.4. RJ Regulated the Expression of LRP1/RAGE and Reduced BACE1 Activity and Aβ Deposition in the Brains of OVX Cholesterol-Fed Rabbits
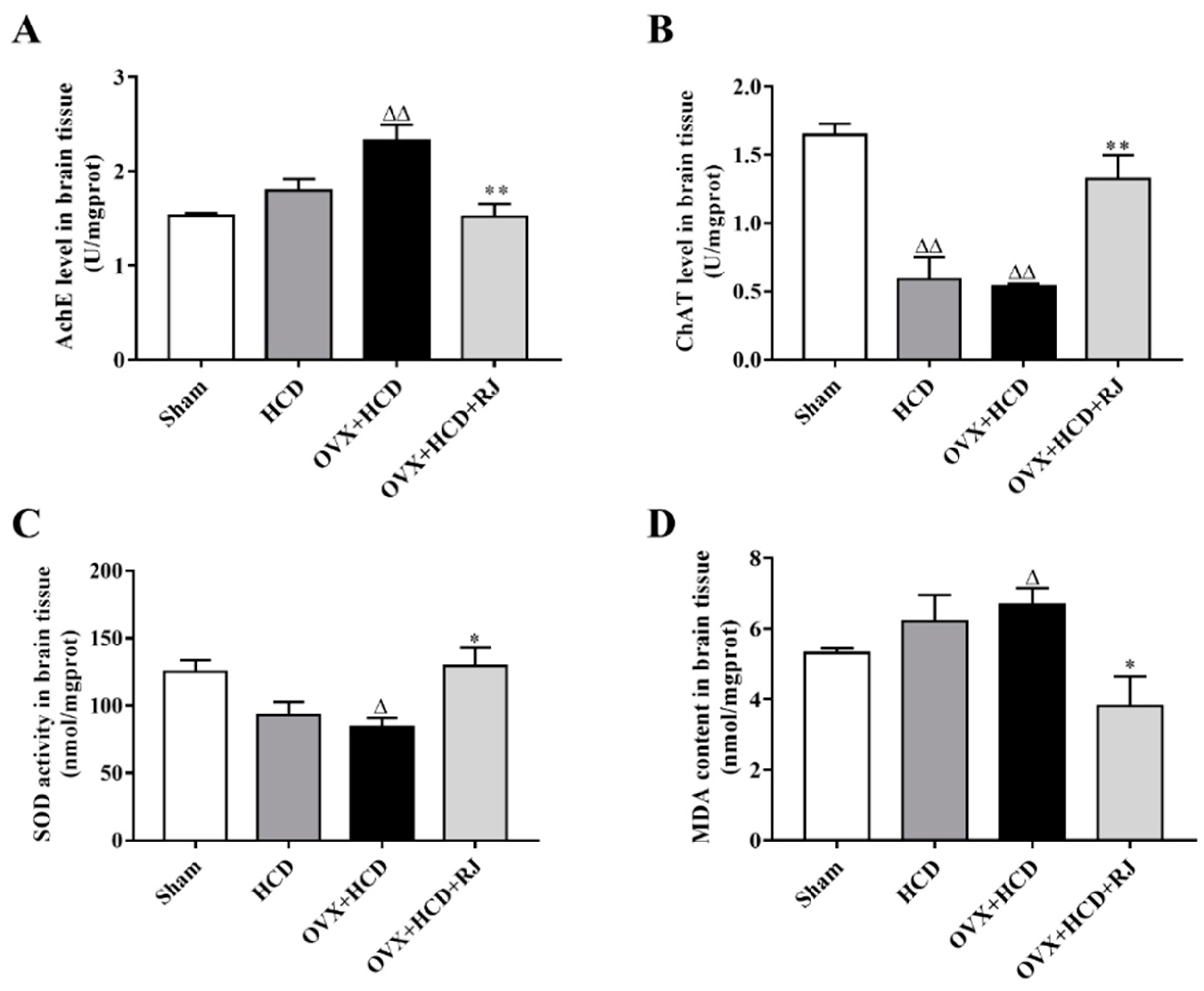
2.5. RJ Enhanced Cholinergic System Activities and Antioxidant Abilities in OVX Cholesterol-Fed Rabbit Brains
2.6. Royal Jelly Improved the Structure of OVX Cholesterol-Fed Rabbit Brains
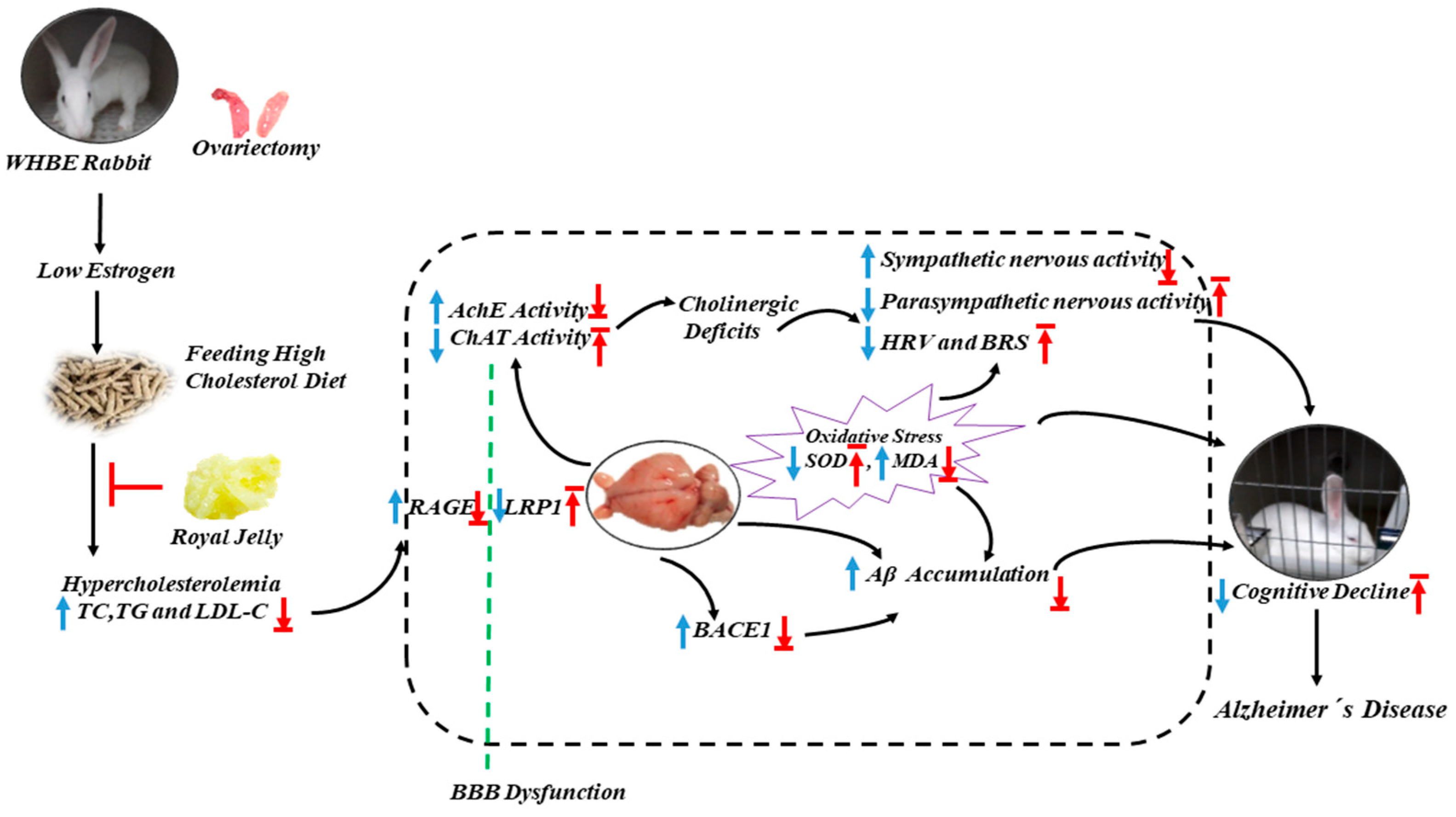
3. Discussion
4. Materials and Methods
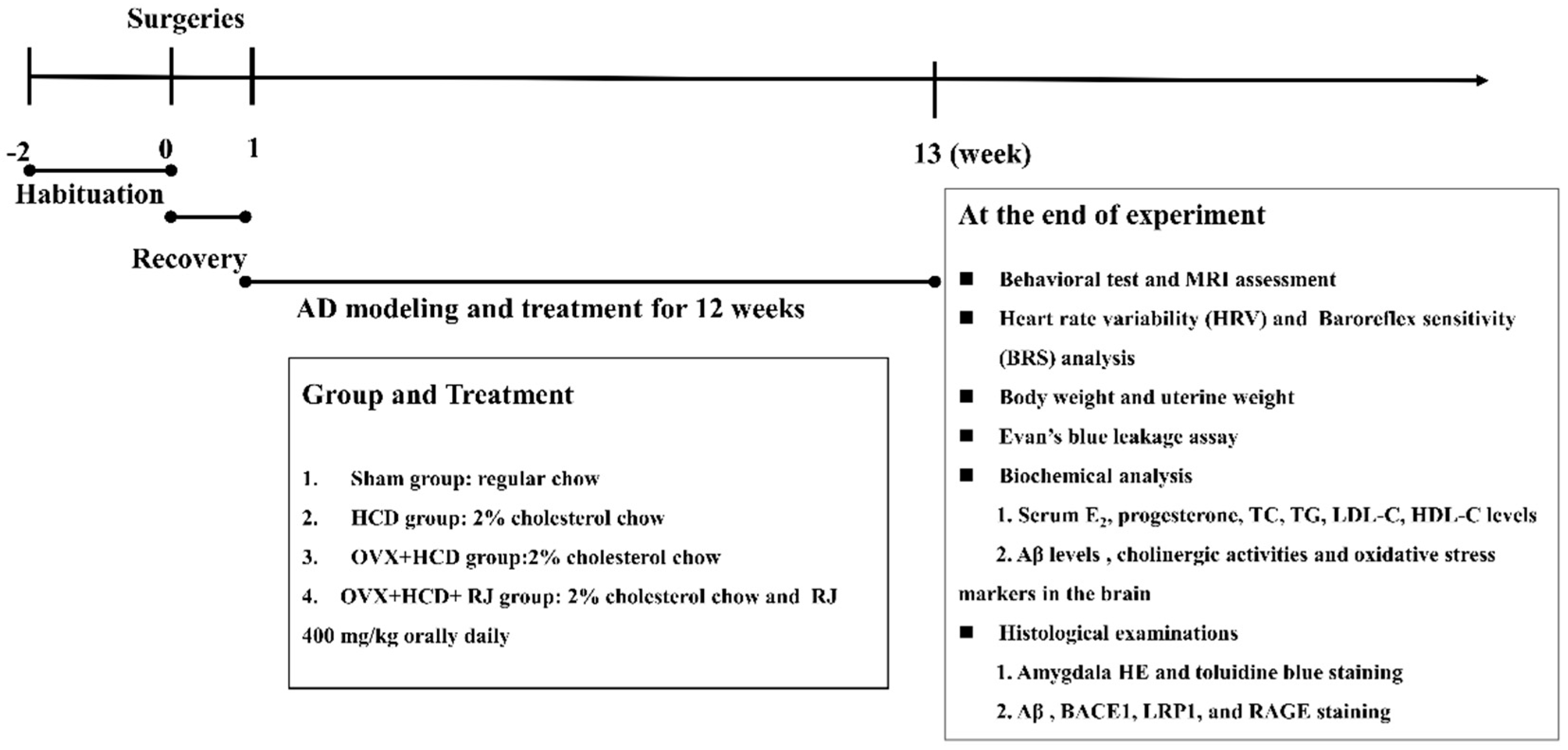
4.1. Laboratory Animals
4.2. Blood Lipid Levels Measurement
4.3. Behavioral Test
4.4. Evans Blue Permeability Determination
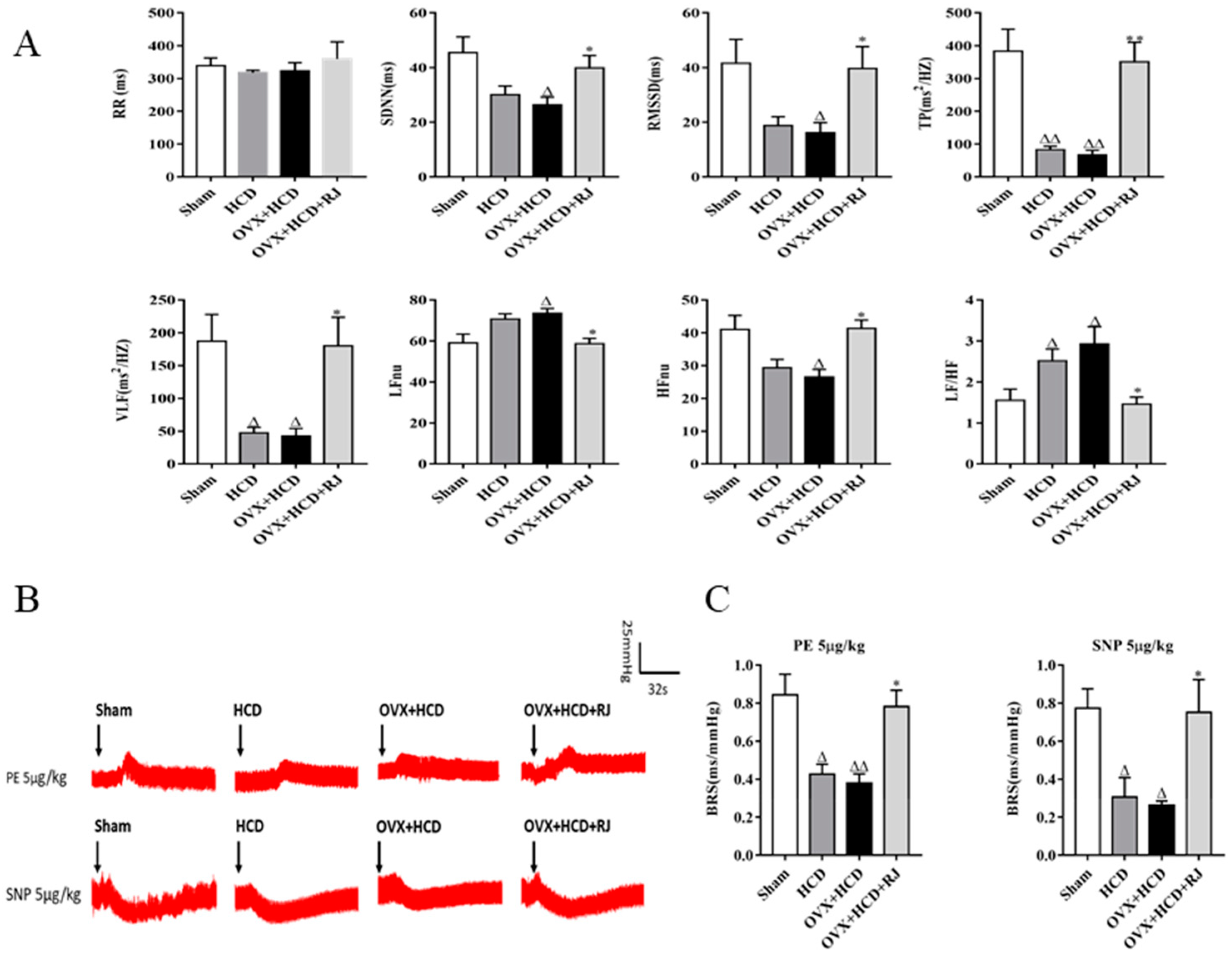
4.5. Electrocardiogram (ECG) Detection and Heart Rate Variability (HRV) Analysis
4.6. Baroreflex Sensitivity (BRS) Analysis
4.7. Determination of Aβ Level by ELISA
4.8. Determination of AchE, ChAT, SOD, and MDA Contents in Brains
4.9. Histopathological Examination
4.10. MRI Scanning
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid-beta |
| CNS | Central nervous system |
| HRT | Hormone replacement therapy |
| ER | Estrogen receptor |
| RJ | Royal jelly |
| APP | Amyloid precursor protein |
| MRI | Magnetic resonance imaging |
| HCD | High cholesterol diet |
| OVX | Ovariectomy |
| E2 | Estradiol |
| TC | Total cholesterol |
| TG | Triglycerides |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| BBB | Blood-brain barrier |
| ECG | Electrocardiogram |
| HRV | Heart rate variability |
| SDNN | Standard deviation of the RR interval |
| RMSSD | The mean square root of the adjacent RR interval |
| VLF | Very-low-frequency |
| LF | low-frequency |
| HF | high-frequency |
| BRS | Baroreflex sensitivity |
| PE | Phenylephrine |
| SNP | Sodium nitroprusside |
| SBP | Systolic blood pressure |
| AchE | Acetylcholinesterase |
| ChAT | Choline Acetyltransferase |
| SOD | Superoxide dismutase |
| MDA | Malonaldehyde |
| BACE1 | beta-site APP cleaving enzyme 1 |
| LRP1 | LDL receptor-related protein 1 |
| RAGE | Receptor for advanced glycation end products |
References
- Jia, J.; Wang, F.; Wei, C.; Zhou, A.; Jia, X.; Li, F.; Tang, M.; Chu, L.; Zhou, Y.; Zhou, C. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. J. Alzheimers Assoc. 2014, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Brinton, R.D. Estrogen regulation of mitochondrial bioenergetics: Implications for prevention of Alzheimer’s disease. Adv. Pharmacol. 2012, 64, 327–371. [Google Scholar]
- Congdon, E.E. Sex Differences in Autophagy Contribute to Female Vulnerability in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Idiaquez, J.; Roman, G.C. Autonomic dysfunction in neurodegenerative dementias. J. Neurol. Sci. 2011, 305, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Tranel, D.; Damasio, A.R.; Van Hoesen, G.W. The autonomic-related cortex: Pathology in Alzheimer’s disease. Cereb. Cortex 1997, 7, 86–95. [Google Scholar] [CrossRef]
- Royall, D.R. Insular Alzheimer disease pathology and the psychometric correlates of mortality. Clevel. Clin. J. Med. 2008, 75 (Suppl. 2), S97–S99. [Google Scholar] [CrossRef]
- Earnest, C.P.; Poirier, P.; Carnethon, M.R.; Blair, S.N.; Church, T.S. Autonomic function and change in insulin for exercising postmenopausal women. Maturitas 2010, 65, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Allan, L.M.; Ballard, C.G.; Allen, J.; Murray, A.; Davidson, A.W.; Mckeith, I.G.; Kenny, R.A. Autonomic dysfunction in dementia. J. Neurol. Neurosurg. Psychiatry 2007, 78, 671–677. [Google Scholar] [CrossRef]
- Richter, N.; Allendorf, I.; Onur, O.A.; Kracht, L.; Dietlein, M.; Tittgemeyer, M.; Neumaier, B.; Fink, G.R.; Kukolja, J. The integrity of the cholinergic system determines memory performance in healthy elderly. NeuroImage 2014, 100, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Newhouse, P.; Albert, K.; Astur, R.; Johnson, J.; Naylor, M.; Dumas, J. Tamoxifen improves cholinergically modulated cognitive performance in postmenopausal women. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013, 38, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Collins, O.; Dillon, S.; Finucane, C.; Lawlor, B.; Kenny, R.A. Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol. Aging 2012, 33, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Levin-Allerhand, J.; McEwen, B.S.; Lominska, C.E.; Lubahn, D.B.; Korach, K.S.; Smith, J.D. Brain region-specific up-regulation of mouse apolipoprotein E by pharmacological estrogen treatments. J. Neurochem. 2001, 79, 796–803. [Google Scholar] [CrossRef]
- Yun, J.; Yeo, I.J.; Hwang, C.J.; Choi, D.Y.; Im, H.S.; Kim, J.Y.; Choi, W.R.; Jung, M.H.; Han, S.B.; Hong, J.T. Estrogen deficiency exacerbates Abeta-induced memory impairment through enhancement of neuroinflammation, amyloidogenesis and NF-kB activation in ovariectomized mice. Brain Behav. Immun. 2018, 73, 282–293. [Google Scholar] [CrossRef]
- Anderson, G.L.; Chlebowski, R.T.; Rossouw, J.E.; Rodabough, R.J.; McTiernan, A.; Margolis, K.L.; Aggerwal, A.; David Curb, J.; Hendrix, S.L.; Allan Hubbell, F.; et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas 2006, 55, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Rapp, S.R.; Shumaker, S.A.; Brunner, R.; Manson, J.A.E.; Sherwin, B.B.; Hsia, J.; Margolis, K.L.; Hogan, P.E.; Wallace, R. Conjugated Equine Estrogens and Global Cognitive Function in Postmenopausal Women: Women’s Health Initiative Memory Study. JAMA 2004, 291, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, S.A.; Legault, C.; Rapp, S.R.; Thal, L.; Wallace, R.B.; Ockene, J.K.; Hendrix, S.L.; Jones, B.N., 3rd; Assaf, A.R.; Jackson, R.D.; et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA 2003, 289, 2651–2662. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Quesenberry, C.P.; Zhou, J.; Yaffe, K. Timing of hormone therapy and dementia: The critical window theory revisited. Ann. Neurol. 2011, 69, 163–169. [Google Scholar] [CrossRef]
- Maki, P.M. Critical window hypothesis of hormone therapy and cognition: A scientific update on clinical studies. Menopause 2013, 20, 695–709. [Google Scholar] [CrossRef]
- Moodithaya, S.; Avadhany, S. Comparison of cardiac autonomic activity between pre and post menopausal women using heart rate variability. Indian J. Physiol. Pharmacol. 2009, 53, 227–234. [Google Scholar]
- Harvey, P.; O’Donnell, E.; Picton, P.; Morris, B.; Notarius, C.; Floras, J. After-exercise heart rate variability is attenuated in postmenopausal women and unaffected by estrogen therapy. Menopause 2016, 23, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Hautamäki, H.; Mikkola, T.; Sovijärvi, A.; Piirilä, P.; Haapalahti, P. Menopausal hot flushes do not associate with changes in heart rate variability in controlled testing: A randomized trial on hormone therapy. Acta Obs. Gynecol. Scand. 2013, 92, 902–908. [Google Scholar] [CrossRef] [PubMed]
- De Rezende Barbosa, M.P.C.; Junior, J.N.; Cassemiro, B.M.; Bernardo, A.F.B.; Franca da Silva, A.K.; Vanderlei, F.M.; Pastre, C.M.; Vanderlei, L.C.M. Effects of functional training on geometric indices of heart rate variability. J Sport Health Sci 2016, 5, 183–189. [Google Scholar] [CrossRef]
- Jacome, L.F.; Gautreaux, C.; Inagaki, T.; Mohan, G.; Alves, S.; Lubbers, L.S.; Luine, V. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol. Learn. Mem. 2010, 94, 488–498. [Google Scholar] [CrossRef] [Green Version]
- Luine, V.N.; Frankfurt, M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front. Neuroendocrinol. 2012, 33, 388–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korach, K.S. Estrogen receptor knock-out mice: Molecular and endocrine phenotypes. J. Soc. Gynecol. Investig. 2000, 7 (Suppl. 1), S16–S17. [Google Scholar] [CrossRef]
- Andruska, N.; Zheng, X.; Yang, X.; Helferich, W.G.; Shapiro, D.J. Anticipatory Estrogen Activation of the Unfolded Protein Response is Linked to Cell Proliferation and Poor Survival in Estrogen Receptor α Positive Breast Cancer. Oncogene 2015, 34, 3760–3769. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.; Weiderpass, E.; Bergkvist, L. Risks of breast and endometrial cancer after estrogen and estrogen–progestin replacement. Cancer Causes Control 1999, 10, 253–260. [Google Scholar] [CrossRef]
- Beral, V.; Gaitskell, K.; Hermon, C.; Moser, K.; Reeves, G.; Peto, R. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 2015, 385, 1835–1842. [Google Scholar]
- You, M.; Chen, Y.; Pan, Y.; Liu, Y.; Tu, J.; Wang, K.; Hu, F. Royal Jelly Attenuates LPS-Induced Inflammation in BV-2 Microglial Cells through Modulating NF-B and p38/JNK Signaling Pathways. Mediat. Inflamm. 2018, 2018, 7834381. [Google Scholar] [CrossRef]
- Bincoletto, C.; Eberlin, S.; Figueiredo, C.A.; Luengo, M.B.; Queiroz, M.L. Effects produced by Royal Jelly on haematopoiesis: Relation with host resistance against Ehrlich ascites tumour challenge. Int. Immunopharmacol. 2005, 5, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Suzuki, K.M.; Isohama, Y.; Maruyama, H.; Yamada, Y.; Narita, Y.; Ohta, S.; Araki, Y.; Miyata, T.; Mishima, S. Estrogenic Activities of Fatty Acids and a Sterol Isolated from Royal Jelly. Evid. Based Complementary Altern. Med. eCAM 2008, 5, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.; Tsiapara, A.; Melliou, E.; Chrousos, G.P.; Chinou, I.; Karshikoff, A. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef]
- Notkola, I.L.; Sulkava, R.; Pekkanen, J.; Erkinjuntti, T.; Ehnholm, C.; Kivinen, P.; Tuomilehto, J.; Nissinen, A. Serum Total Cholesterol, Apolipoprotein E e4 Allele, and Alzheimer’s Disease. Neuroepidemiology 1998, 17, 14–20. [Google Scholar] [CrossRef]
- Kölsch, H.; Lütjohann, D.; Tulke, A.; Björkhem, I.; Rao, M.L. The neurotoxic effect of 24-hydroxycholesterol on SH-SY5Y human neuroblastoma cells. Brain Res. 1999, 818, 171–175. [Google Scholar] [CrossRef]
- Sparks, D.L.; Scheff, S.W.; Liu, H.; Landers, T.; Gross, D.R. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp. Neurol. 1994, 126, 88–94. [Google Scholar] [CrossRef]
- Jaya Prasanthi, R.P.; Schommer, E.; Thomasson, S.; Thompson, A.; Feist, G.; Ghribi, O. Regulation of beta-amyloid levels in the brain of cholesterol-fed rabbit, a model system for sporadic Alzheimer’s disease. Mech. Ageing Dev. 2008, 129, 649–655. [Google Scholar] [CrossRef]
- Caufriez, A.; Leproult, R.; Copinschi, G. Circadian profiles of progesterone, gonadotropins, cortisol and corticotropin in cycling and postmenopausal women. Chronobiol. Int. 2018, 35, 72–79. [Google Scholar] [CrossRef]
- Frizzell, C.; Ndossi, D.; Kalayou, S.; Eriksen, G.S.; Verhaegen, S.; Sorlie, M.; Elliott, C.T.; Ropstad, E.; Connolly, L. An in vitro investigation of endocrine disrupting effects of the mycotoxin alternariol. Toxicol. Appl. Pharmacol. 2013, 271, 64–71. [Google Scholar] [CrossRef]
- Mishima, S.; Suzuki, K.M.; Isohama, Y.; Kuratsu, N.; Araki, Y.; Inoue, M.; Miyata, T. Royal jelly has estrogenic effects in vitro and in vivo. J. Ethnopharmacol. 2005, 101, 215–220. [Google Scholar] [CrossRef]
- Jing, S.; Shusen, L.; Weilin, S. The Effects of 10-HDA on Immune Funciton of Mice. Chin. Pharmacol. Bull. 1990, 6, 175–177. [Google Scholar]
- Ghanbari, E.; Khazaei, M.R.; Khazaei, M.; Nejati, V. Royal Jelly Promotes Ovarian Follicles Growth and Increases Steroid Hormones in Immature Rats. Int. J. Fertil. Steril. 2018, 11, 263–269. [Google Scholar]
- Husein, M.Q.; Kridli, R.T. Reproductive responses following royal jelly treatment administered orally or intramuscularly into progesterone-treated Awassi ewes. Anim. Reprod. Sci. 2002, 74, 45–53. [Google Scholar] [CrossRef]
- Yoon, B.K.; Chin, J.; Kim, J.W.; Shin, M.H.; Ahn, S.; Lee, D.Y.; Seo, S.W.; Na, D.L. Menopausal hormone therapy and mild cognitive impairment: A randomized, placebo-controlled trial. Menopause 2018, 25, 870–876. [Google Scholar] [CrossRef]
- Kurita, T.; Lee, K.; Saunders, P.T.; Cooke, P.S.; Taylor, J.A.; Lubahn, D.B.; Zhao, C.; Mäkelä, S.; Gustafsson, J.A.; Dahiya, R. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-alpha knockout mouse. Biol. Reprod. 2001, 64, 272–283. [Google Scholar] [CrossRef]
- Guo, H.; Saiga, A.; Sato, M.; Miyazawa, I.; Shibata, M.; Takahata, Y.; Morimatsu, F. Royal jelly supplementation improves lipoprotein metabolism in humans. J. Nutr. Sci. Vitaminol. 2007, 53, 345–348. [Google Scholar] [CrossRef]
- Munstedt, K.; Henschel, M.; Hauenschild, A.; von Georgi, R. Royal jelly increases high density lipoprotein levels but in older patients only. J. Altern. Complementary Med. 2009, 15, 329–330. [Google Scholar]
- Shen, X.; Lu, R.; He, G. Effects of lyophilized royal jelly on experimental hyperlipidemia and thrombosis. Chin. J. Prev. Med. 1995, 29, 27–29. [Google Scholar]
- Chiu, H.F.; Chen, B.K.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharm. Biol. 2017, 55, 497–502. [Google Scholar] [CrossRef]
- Panza, F.; Capurso, C.; D’Introno, A.; Colacicco, A.M.; Vasquez, F.; Pistoia, G.; Capurso, A.; Solfrizzi, V. Serum total cholesterol as a biomarker for Alzheimer’s disease: Mid-life or late-life determinations? Exp. Gerontol. 2006, 41, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Larry, S.D. Cholesterol, copper, and accumulation of thioflavine S-reactive Alzheimer’s-like amyloid beta in rabbit brain. J. Mol. Neurosci. 2004, 24, 97–104. [Google Scholar] [CrossRef]
- Mosconi, L.; Rahman, A. Increased Alzheimer’s risk during the menopause transition: A 3-year longitudinal brain imaging study. PLoS ONE 2018, 13, e0207885. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Setalo, G., Jr.; Guan, X.; Warren, M.; Toran-Allerand, C.D. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: Convergence of estrogen and neurotrophin signaling pathways. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 1179–1188. [Google Scholar] [CrossRef]
- Jeon, K.; Lee, S.; Hwang, M. Effect of combined circuit exercise on arterial stiffness in hypertensive postmenopausal women: A local public health center-based pilot study. Menopause 2018, 25, 1442–1447. [Google Scholar] [CrossRef]
- Kawasaki, K.; Annicchiarico, I.; Glueck, A.C.; Moron, I.; Papini, M.R. Reward loss and the basolateral amygdala: A function in reward comparisons. Behav. Brain Res. 2017, 331, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Capoccia, E.; Iuvone, T.; Cuomo, R.; Sarnelli, G.; Steardo, L.; Esposito, G. S100B Inhibitor Pentamidine Attenuates Reactive Gliosis and Reduces Neuronal Loss in a Mouse Model of Alzheimer’s Disease. Biomed. Res. Int. 2015, 2015, 508342. [Google Scholar] [CrossRef]
- Sparks, D.L.; Kuo, Y.M.; Roher, A.; Martin, T.; Lukas, R.J. Alterations of Alzheimer’s disease in the cholesterol-fed rabbit, including vascular inflammation. Preliminary observations. Ann. N. Y. Acad. Sci. 2000, 903, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, Z.; Umegaki, H.; Makino, T.; Suzuki, Y.; Kuzuya, M. Relationship between cardiac autonomic function and cognitive function in Alzheimer’s disease. Geriatr. Gerontol. Int. 2015, 17, 92–98. [Google Scholar] [CrossRef]
- Van Ravenswaaij-Arts, C.M.; Kollée, L.A.; Hopman, J.C.; Stoelinga, G.B.; van Geijn, H.P. Heart rate variability. Clin. Cardiol. 1993, 13, 570–576. [Google Scholar] [CrossRef]
- Mpdc, D.R.B.; Lcm, V.; Neves, L.M.; Takahashi, C.; Prds, T.; Acs, F.; Freitas Júnior, I.F.; Ice, S.; Abreu, L.C.; Pérez Riera, A.R. Impact of functional training on geometric indices and fractal correlation property of heart rate variability in postmenopausal women. Ann. Noninvasive Electrocardiol. 2018, 23, e12469. [Google Scholar]
- Saint Martin, M.; Sforza, E.; Thomas-Anterion, C.; Barthélémy, J.C.; Roche, F.; PROOF Study Group. Baroreflex Sensitivity, Vascular Risk Factors, and Cognitive Function in a Healthy Elderly Population: The PROOF Cohort. J. Am. Geriatr. Soc. 2013, 61, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Bellelli, G.; Pezzini, A.; Bianchetti, A.; Trabucchi, M. Increased blood pressure variability may be associated with cognitive decline in hypertensive elderly subjects with no dementia. Arch. Intern. Med. 2002, 162, 483. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Hansen, A.L.; Sausrose, E.; Johnsen, B.H. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Matzek, L.; Charkoudian, N.; Joyner, M.; Curry, T.; Hart, E. Association of cardiac baroreflex sensitivity with blood pressure transients: Influence of sex and menopausal status. Front. Physiol. 2012, 3, 187. [Google Scholar] [CrossRef] [PubMed]
- Giubilei, F.; Strano, S.; Imbimbo, B.P.; Tisei, P.; Calcagnini, G.; Lino, S.; Frontoni, M.; Santini, M.; Fieschi, C. Cardiac autonomic dysfunction in patients with Alzheimer disease: Possible pathogenetic mechanisms. Alzheimer Dis. Assoc. Disord. 1998, 12, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Mufson, E.J.; Wuu, J.; Bennett, D.A.; Dekosky, S.T. Reduction of choline acetyltransferase activity in primary visual cortex in mild to moderate Alzheimer’s disease. Arch. Neurol. 2005, 62, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Yan-Hong, H.; Qing-Hong, Z. Genistein reduced the neural apoptosis in the brain of ovariectomised rats by modulating mitochondrial oxidative stress. Br. J. Nutr. 2010, 104, 1297–1303. [Google Scholar] [CrossRef] [Green Version]
- Clausen, A.; Doctrow, S.; Baudry, M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol. Aging 2010, 31, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.S.; Chiu, Y.J.; Wu, L.Y.; Lu, T.C.; Huang, T.H.; Hsieh, M.T.; Lu, C.Y.; Peng, W.H. Diosgenin ameliorates cognition deficit and attenuates oxidative damage in senescent mice induced by D-galactose. Am. J. Chin. Med. 2011, 39, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Zhao, S.J.; Wang, Y.S.; Yu, T.; Luo, M. Effects of estrogen intervention on the biomechanical characteristics of serum SOD, MDA, and middle cerebral artery in aged female rats. Clin. Exp. Obstet. Gynecol. 2015, 42, 295–299. [Google Scholar] [PubMed]
- Xu, Q.; Tian, M.; Chen, S.; Jia, C.; Wang, M. Effect of electric acucpunture on behaviour and GAA content of rabbits with aluminium induced dementia. J. Shaanxi Coll. Tradit. Chin. Med. 2011, 34, 68–69. [Google Scholar]
- Zagzag, D.; Brem, S.; Robert, F. Neovascularization and tumor growth in the rabbit brain. A model for experimental studies of angiogenesis and the blood-brain barrier. Am. J. Pathol. 1988, 131, 361–372. [Google Scholar]
- Pellegrino, P.R.; Schiller, A.M.; Zucker, I.H. Validation of pulse rate variability as a surrogate for heart rate variability in chronically instrumented rabbits. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H97. [Google Scholar] [CrossRef] [PubMed]
- Baptiste, D.C.; Hartwick, A.T.; Jollimore, C.A.; Baldridge, W.H.; Seigel, G.M.; Kelly, M.E. An investigation of the neuroprotective effects of tetracycline derivatives in experimental models of retinal cell death. Mol. Pharmacol. 2004, 66, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Wang, Y.Z.; Zhang, K.Y.; Shen, J.H.; Zhou, H.Q.; Qiu, X.Y. Effect of superoxide dismutase and malondialdehyde metabolic changes on carcinogenesis of gastric carcinoma. World J. Gastroenterol. 2005, 11, 4305–4310. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Sham | HCD | OVX + HCD | OVX + HCD + RJ |
|---|---|---|---|---|
| Body Weight (kg) | 2.70 ± 0.06 | 3.03 ± 0.07ΔΔ | 3.05 ± 0.04ΔΔ | 2.84 ± 0.03* |
| Uterine Weight (g) | 11.63 ± 0.31 | 11.75 ± 0.36 | 7.46 ± 0.62ΔΔ | 7.27 ± 0.43 |
| E2 (ng/L) | 65.04 ± 4.91 | 63.86 ± 12.61 | 25.39 ± 8.71ΔΔ | 50.79 ± 6.36* |
| Progesterone (ng/mL) | 2.90 ± 0.47 | 2.50 ± 0.29 | 1.06 ± 0.41Δ | 2.15 ± 0.19* |
| TC (mmol/L) | 1.73 ± 0.22 | 48.57 ± 4.61ΔΔ | 51.55 ± 11.83ΔΔ | 30.00 ± 2.94* |
| HDL-C (mmol/L) | 0.49 ± 0.04 | 2.98 ± 0.12ΔΔ | 2.86 ± 0.09ΔΔ | 2.47 ± 0.10 |
| LDL-C (mmol/L) | 1.02 ± 0.19 | 35.23 ± 2.94ΔΔ | 35.29 ± 4.24ΔΔ | 22.76 ± 2.59* |
| TG (mmol/L) | 0.61 ± 0.07 | 1.24 ± 0.18Δ | 2.07 ± 0.77ΔΔ | 1.12 ± 0.16* |
| Group | N | Spontaneously Searching Food–Water Behavior Within 5 Minutes | Sudden Sound Stimulus Response | ||||
|---|---|---|---|---|---|---|---|
| Success | Failure | Successful Rate (%) | Yes | No | Response Rate (%) | ||
| Sham | 6 | 6 | 0 | 100 | 6 | 0 | 100 |
| HCD | 6 | 3 | 3 | 50% | 3 | 3 | 50% |
| OVX + HCD | 6 | 0 | 6 | 0%ΔΔ | 0 | 6 | 0%ΔΔ |
| OVX + HCD + RJ | 6 | 5 | 1 | 83.3%* | 5 | 1 | 83.3%* |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Xu, J.; Jin, P.; Yang, Q.; Zhu, K.; You, M.; Hu, F.; Chen, M. Royal Jelly Ameliorates Behavioral Deficits, Cholinergic System Deficiency, and Autonomic Nervous Dysfunction in Ovariectomized Cholesterol-Fed Rabbits. Molecules 2019, 24, 1149. https://doi.org/10.3390/molecules24061149
Pan Y, Xu J, Jin P, Yang Q, Zhu K, You M, Hu F, Chen M. Royal Jelly Ameliorates Behavioral Deficits, Cholinergic System Deficiency, and Autonomic Nervous Dysfunction in Ovariectomized Cholesterol-Fed Rabbits. Molecules. 2019; 24(6):1149. https://doi.org/10.3390/molecules24061149
Chicago/Turabian StylePan, Yongming, Jianqin Xu, Ping Jin, Qinqin Yang, Keyan Zhu, Mengmeng You, Fuliang Hu, and Minli Chen. 2019. "Royal Jelly Ameliorates Behavioral Deficits, Cholinergic System Deficiency, and Autonomic Nervous Dysfunction in Ovariectomized Cholesterol-Fed Rabbits" Molecules 24, no. 6: 1149. https://doi.org/10.3390/molecules24061149
APA StylePan, Y., Xu, J., Jin, P., Yang, Q., Zhu, K., You, M., Hu, F., & Chen, M. (2019). Royal Jelly Ameliorates Behavioral Deficits, Cholinergic System Deficiency, and Autonomic Nervous Dysfunction in Ovariectomized Cholesterol-Fed Rabbits. Molecules, 24(6), 1149. https://doi.org/10.3390/molecules24061149