Chiral Selectors in Capillary Electrophoresis: Trends during 2017–2018
Abstract
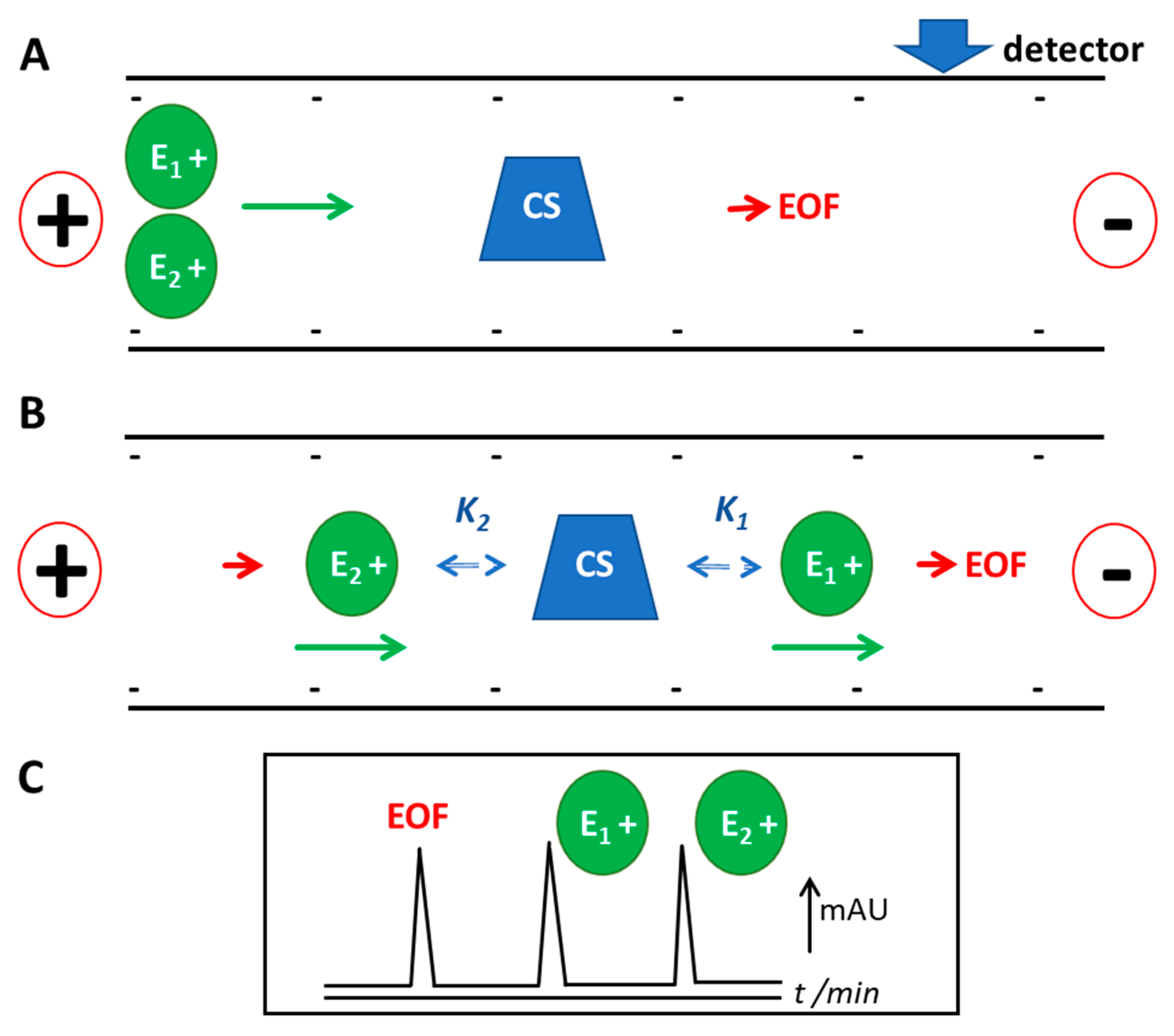
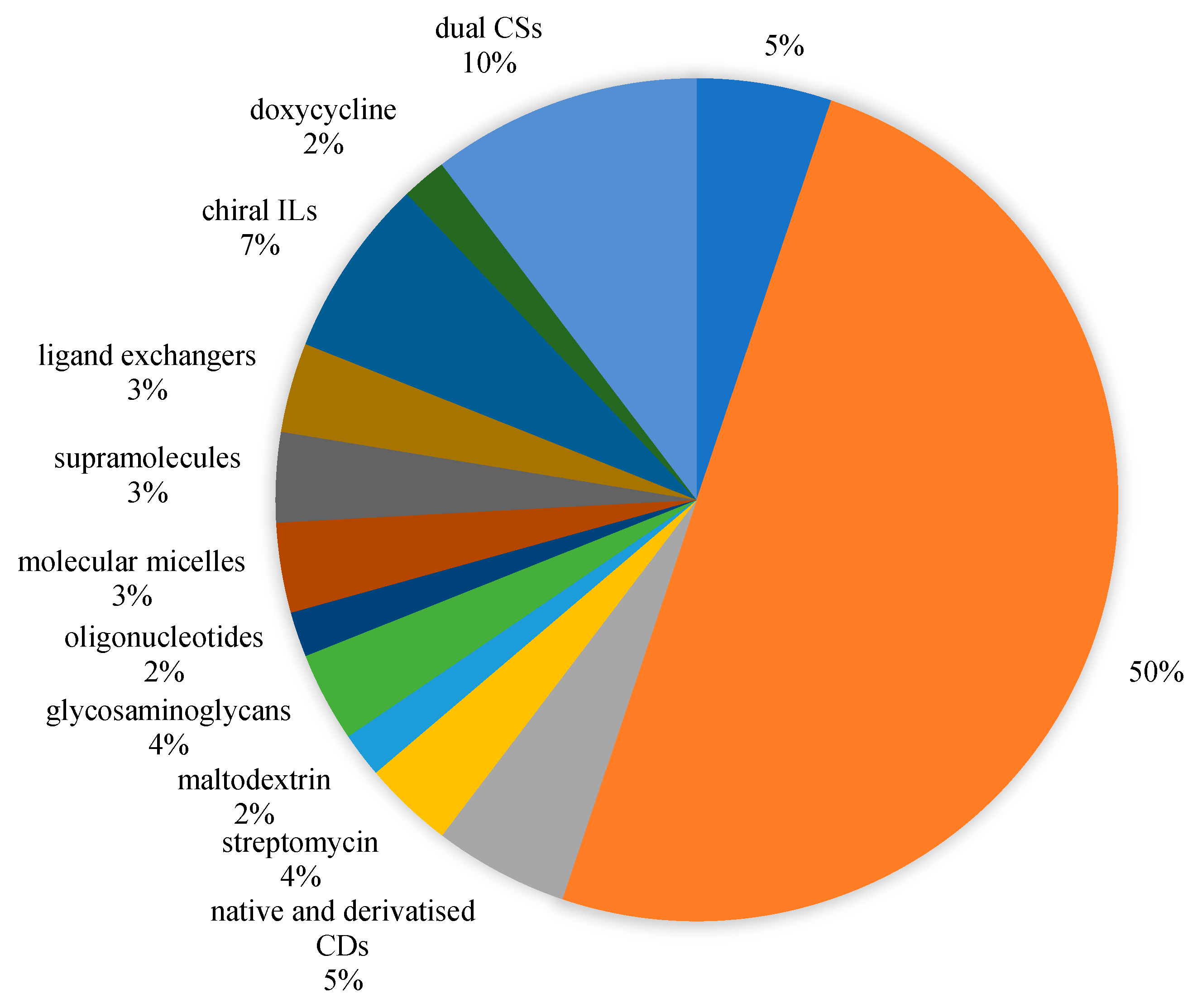
:1. Introduction
2. Macromolecules
2.1. Sugar-Based Macromolecules
2.1.1. Cyclodextrins
Native CDs
Modified CDs
2.1.2. Streptomycin
2.1.3. Polysaccharides
Maltodextrin
Glycosaminoglycans
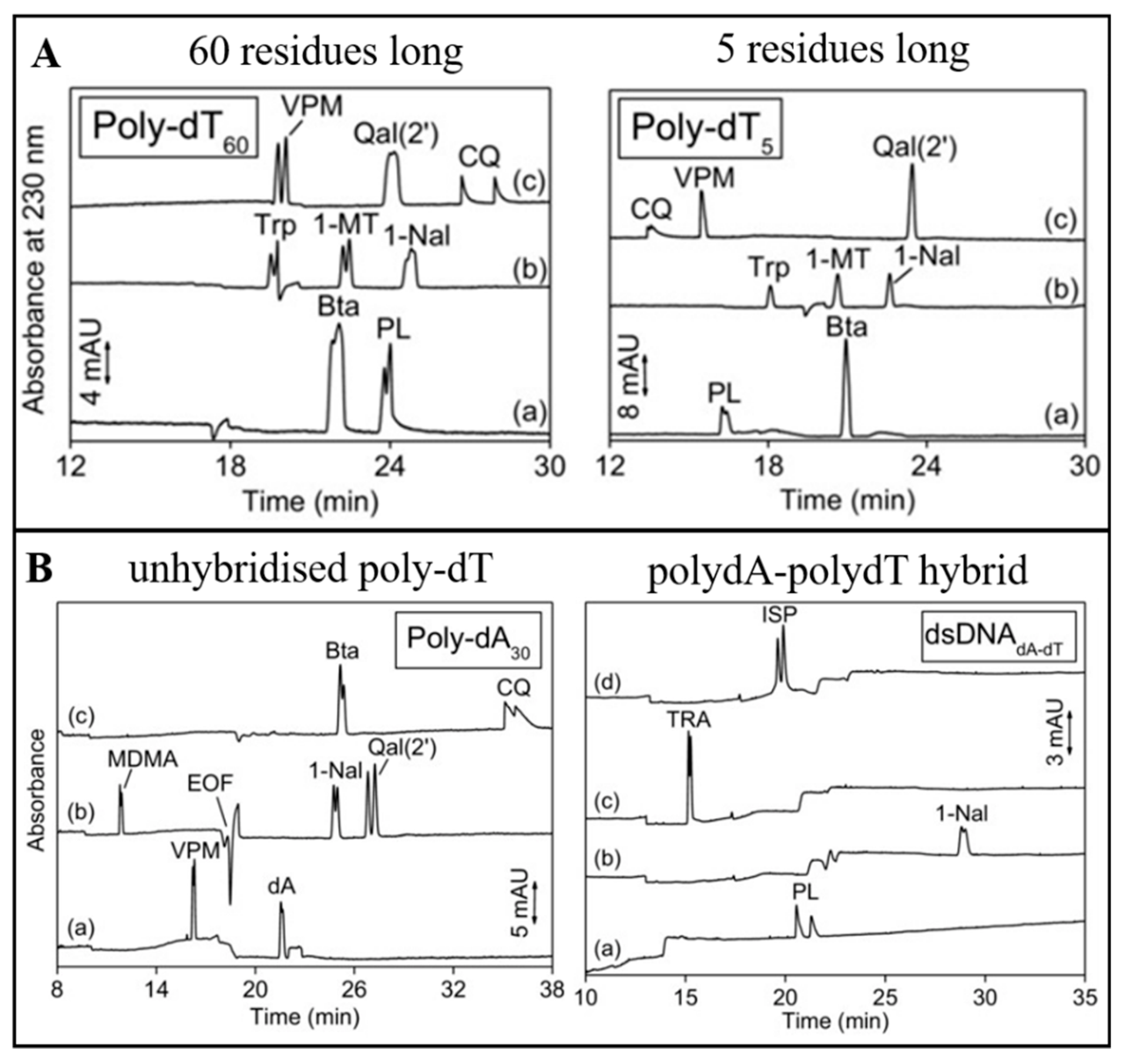
2.2. Chiral Selectors Based on Nucleotides as Basic Units
2.3. Molecular Micelles
3. Supramolecules
4. Low Molecular Weight Compounds
4.1. CSs with a Central Ion
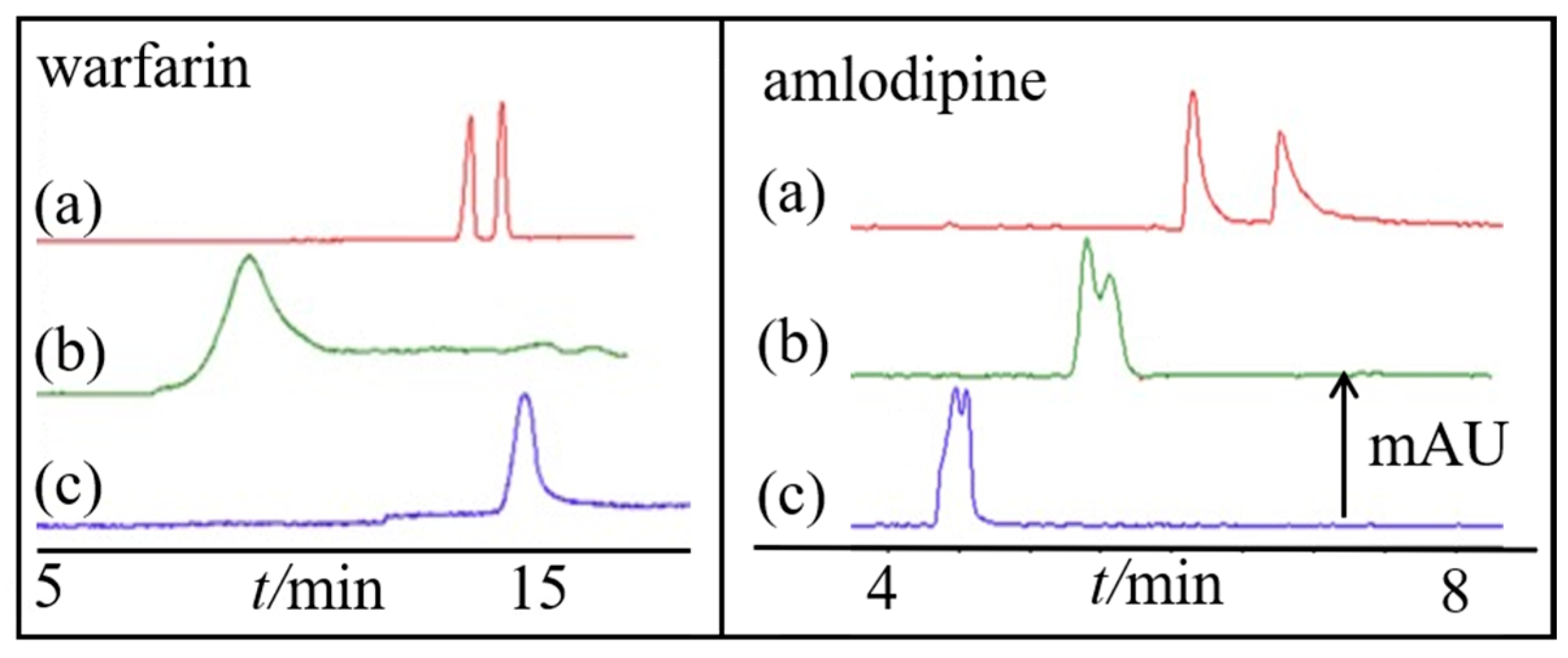
4.2. ILs
4.3. Small Molecule Antiobiotic
5. Molecular Modelling and NMR Studies
6. Applications to Real Samples
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Uwai, Y. Enantioselective drug recognition by drug transporters. Molecules 2018, 23, 3062. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lin, X.; Yin, S.; Zhao, L.; Liu, Y.; Liu, K.; Li, F.; Yang, F.; Liu, W. Enantioselectivity in biotransformation and bioaccumulation processes of typical chiral contaminants. Environ. Pollut. 2018, 243, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Padró, J.M.; Keunchkarian, S. State-of-the-art and recent developments of immobilized polysaccharide-based chiral stationary phases for enantioseparations by high-performance liquid chromatography (2013–2017). Microchem. J. 2018, 140, 142–157. [Google Scholar] [CrossRef]
- Ribeiro, C.; Santos, C.; Gonçalves, V.; Ramos, A.; Afonso, C.; Tiritan, M.E. Chiral drug analysis in forensic chemistry: An overview. Molecules 2018, 23, 262. [Google Scholar] [CrossRef] [PubMed]
- Tarongoy, F.M.; Haddad, P.R.; Boysen, R.I.; Hearn, M.T.W.; Quirino, J.P. Open tubular-capillary electrochromatography: Developments and applications from 2013 to 2015. Electrophoresis 2016, 37, 66–85. [Google Scholar] [CrossRef]
- Saz, J.M.; Marina, M.L. Recent advances on the use of cyclodextrins in the chiral analysis of drugs by capillary electrophoresis. J. Chromatogr. A 2016, 1467, 79–94. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, G.; Fanali, C.; Asensio-Ramos, M.; Fanali, S. Chiral separations in food analysis. Trends Anal. Chem. 2017, 96, 151–171. [Google Scholar] [CrossRef]
- Wuethrich, A.; Haddad, P.R.; Quirino, J.P. Online sample concentration in partial-filling chiral electrokinetic chromatography—Mass spectrometry. Chirality 2014, 26, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Terabe, S. Enantiomer separation of drugs by micellar electrokinetic chromatography using chiral surfactants. J. Chromatogr. A 2000, 875, 163–178. [Google Scholar] [CrossRef]
- Wuethrich, A.; Quirino, J.P. Capillary Electrophoresis as a Green Alternative Separation Technique. In The Application of Green Solvents in Separation Processes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 517–532. [Google Scholar]
- Wuethrich, A.; Haddad, P.R.; Quirino, J.P. Chiral capillary electromigration techniques-mass spectrometry-hope and promise. Electrophoresis 2014, 35, 2–11. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. International Union of Pure and Applied Chemistry. In Compendium of Chemical Terminology: IUPAC Recommendations/Compiled by Alan D. McNaught and Andrew Wilkinson, 2nd ed.; Blackwell Science: Oxford, UK; Boston, MA, USA, 1997; 450p. [Google Scholar]
- Chankvetadze, B. Contemporary theory of enantioseparations in capillary electrophoresis. J. Chromatogr. A 2018, 1567, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.J.; Agathokleous, E.A.; Kapnissi-Christodoulou, C.P. Chiral selectors in CE: Recent development and applications (mid-2014 to mid-2016). Electrophoresis 2017, 38, 786–819. [Google Scholar] [CrossRef] [PubMed]
- Greño, M.; Marina, M.L.; Castro-Puyana, M. Enantioseparation by Capillary Electrophoresis Using Ionic Liquids as Chiral Selectors. Crit. Rev. Anal. Chem. 2018, 48, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Ionic liquids in capillary electrophoresis for enantioseparation. Trends Anal. Chem. 2018, 100, 145–154. [Google Scholar] [CrossRef]
- Greño, M.; Castro-Puyana, M.; García, M.Á.; Marina, M.L. Analysis of antibiotics by CE and CEC and their use as chiral selectors: An update. Electrophoresis 2018, 39, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Crego, A.L.; Mateos, M.; Nozal, L. Recent contributions for improving sensitivity in chiral CE. Electrophoresis 2018, 39, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, J.; Xue, S.; Rui, M.; Gao, B.; Li, A.; Bai, J.; Yin, Z.; Anochie, E.M. Enhanced enantioselectivity of native α-cyclodextrins by the synergy of chiral ionic liquids in capillary electrophoresis. J. Sep. Sci. 2018, 41, 4525–4532. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Ghulam, M.; Chen, H.; Qu, F. Predefine resolution of enantiomers in partial filling capillary electrophoresis and two discontinuous function plugs coupling in-capillary. Electrophoresis 2018, 39, 2391–2397. [Google Scholar] [CrossRef]
- Adamusová, H.; Novotná, N.; Bosáková, Z.; Douša, M. Enantiomeric Separation of (R,S)-Aclidinium Bromide with Negatively Charged Gamma-Cyclodextrin by CE. Chromatographia 2017, 80, 559–563. [Google Scholar] [CrossRef]
- Cecilio Fonseca, M.; Santos da Silva, R.C.; Nascimento, C.S., Jr.; Bastos Borges, K. Computational contribution to the electrophoretic enantiomer separation mechanism and migration order using modified β-cyclodextrins. Electrophoresis 2017, 38, 1860–1868. [Google Scholar] [CrossRef]
- Chalavi, S.; Fakhari, A.R.; Nojavan, S. Development of a modified partial filling method in capillary electrophoresis using two chiral plugs for the simultaneous enantioseparation of chiral drugs: Comparison with mixed chiral selector capillary electrophoresis. J. Chromatogr. A 2018, 1567, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Du, Y.; Hu, X.; Luo, L.; Guo, X.; Guo, X.; Yu, J. Carboxymethyl β-cyclodextrin as chiral selector in capillary electrophoresis: Enantioseparation of 16 basic chiral drugs and its chiral recognition mechanism associated with drugs’ structural features. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Fiori, J.; Pasquini, B.; Caprini, C.; Orlandini, S.; Furlanetto, S.; Gotti, R. Chiral analysis of theanine and catechin in characterization of green tea by cyclodextrin-modified micellar electrokinetic chromatography and high performance liquid chromatography. J. Chromatogr. A 2018, 1562, 115–122. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Zhang, H.; Yu, L.; Xu, W. Use of various β-cyclodextrin derivatives as chiral selectors for the enantiomeric separation of higenamine by capillary electrophoresis. Microchem. J. 2017, 134, 289–294. [Google Scholar] [CrossRef]
- Michalska, K.; Gruba, E.; Bocian, W.; Cielecka-Piontek, J. Enantioselective recognition of radezolid by cyclodextrin modified capillary electrokinetic chromatography and electronic circular dichroism. J. Pharm. Biomed. Anal. 2017, 139, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Olesek, K.; Woźniakiewicz, M.; Kościelniak, P. Simultaneous enantioseparation of methcathinone and two isomeric methylmethcathinones using capillary electrophoresis assisted by 2-hydroxyethyl-β-cyclodextrin. Electrophoresis 2018, 39, 2406–2409. [Google Scholar] [CrossRef] [PubMed]
- Řezanková, K.; Kohoutová, R.; Kuchař, M.; Král, V.; Řezanka, P. Enantioseparation of novel psychoactive chiral amines and their mixture by capillary electrophoresis using cyclodextrins as chiral selectors. Chem. Pap. 2018, 72, 2737–2743. [Google Scholar] [CrossRef]
- Szabó, Z.I.; Foroughbakhshfasaei, M.; Gál, R.; Horváth, P.; Komjáti, B.; Noszál, B.; Tóth, G. Chiral separation of lenalidomide by liquid chromatography on polysaccharide-type stationary phases and by capillary electrophoresis using cyclodextrin selectors. J. Sep. Sci. 2018, 41, 1414–1423. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Chen, M.; Zhang, G.; Chang, R.; Chen, A. Separation and determination of corynoxine and corynoxine B using chiral ionic liquid and hydroxypropyl-β-cyclodextrin as additives by field-amplified sample stacking in capillary electrophoresis. Electrophoresis 2018, 39, 2195–2201. [Google Scholar] [CrossRef]
- Yao, Y.; Song, P.; Wen, X.; Deng, M.; Wang, J.; Guo, X. Chiral separation of 12 pairs of enantiomers by capillary electrophoresis using heptakis-(2,3-diacetyl-6-sulfato)-β-cyclodextrin as the chiral selector and the elucidation of the chiral recognition mechanism by computational methods. J. Sep. Sci. 2017, 40, 2999–3007. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, S.; Guo, X.; Wei, L.; Yu, J.; Wang, T. Use of various β-cyclodextrin derivatives as chiral selectors for the enantiomeric separation of ofloxacin and its five related substances by capillary electrophoresis. J. Sep. Sci. 2017, 40, 1784–1795. [Google Scholar] [CrossRef]
- Pasquini, B.; Orlandini, S.; Villar-Navarro, M.; Caprini, C.; Del Bubba, M.; Douša, M.; Giuffrida, A.; Gotti, R.; Furlanetto, S. Chiral capillary zone electrophoresis in enantioseparation and analysis of cinacalcet impurities: Use of Quality by Design principles in method development. J. Chromatogr. A 2018, 1568, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.; Tatunashvili, E.; Gogolashvili, A.; Chankvetadze, B.; Gago, F. Structural rationale for the chiral separation and migration order reversal of clenpenterol enantiomers in capillary electrophoresis using two different β-cyclodextrins. Phys. Chem. Chem. Phys. 2017, 19, 27935–27939. [Google Scholar] [CrossRef]
- Gogolashvili, A.; Tatunashvili, E.; Chankvetadze, L.; Sohajda, T.; Szeman, J.; Gumustas, M.; Ozkan, S.A.; Salgado, A.; Chankvetadze, B. Separation of terbutaline enantiomers in capillary electrophoresis with cyclodextrin-type chiral selectors and investigation of structure of selector-selectand complexes. J. Chromatogr. A 2018, 1571, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Valimaña-Traverso, J.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; García, M.Á.; Sierra, I.; Marina, M.L. Cationic amine-bridged periodic mesoporous organosilica materials for off-line solid-phase extraction of phenoxy acid herbicides from water samples prior to their simultaneous enantiomeric determination by capillary electrophoresis. J. Chromatogr. A 2018, 1566, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Q.; Luo, L.; Sun, T.; Guo, X. Comparison of three S-β-CDs with different degrees of substitution for the chiral separation of 12 drugs in capillary electrophoresis. Chirality 2017, 29, 558–565. [Google Scholar] [CrossRef]
- Malanga, M.; Fejős, I.; Varga, E.; Benkovics, G.; Darcsi, A.; Szemán, J.; Béni, S. Synthesis, analytical characterization and capillary electrophoretic use of the single-isomer heptakis-(6-O-sulfobutyl)-beta-cyclodextrin. J. Chromatogr. A 2017, 1514, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, C.; Ma, Y.; Zhou, S.; Li, Z.; Sun, T. Effectively enhancing the enantioseparation ability of β-cyclodextrin derivatives by: De novo design and molecular modeling. Analyst 2017, 142, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Kanizsová, L.; Ansorge, M.; Zusková, I.; Dubský, P. Using single-isomer octa(6-O-sulfo)-γ-cyclodextrin for fast capillary zone electrophoretic enantioseparation of pindolol: Determination of complexation constants, software-assisted optimization, and method validation. J. Chromatogr. A 2018, 1568, 214–221. [Google Scholar] [CrossRef]
- Cucinotta, V.; Messina, M.; Contino, A.; Maccarrone, G.; Orlandini, S.; Giuffrida, A. Chiral separation of terbutaline and non-steroidal anti-inflammatory drugs by using a new lysine–bridged hemispherodextrin in capillary electrophoresis. J. Pharm. Biomed. Anal. 2017, 145, 734–741. [Google Scholar] [CrossRef]
- Silva, M.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Marina, M.L.; Sierra, I. Preconcentration of β-blockers using functionalized ordered mesoporous silica as sorbent for SPE and their determination in waters by chiral CE. Electrophoresis 2017, 38, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, T.; Xu, G.; Du, Y.; Liu, Z.; Feng, Z.; Yang, X.; Xi, Y.; Liu, J. Synthesis and application of ionic liquid functionalized β-cyclodextrin, mono-6-deoxy-6-(4-amino-1,2,4-triazolium)-β-cyclodextrin chloride, as chiral selector in capillary electrophoresis. J. Chromatogr. A 2018, 1559, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Deng, J.; Shi, G.; Zhou, T. β-cyclodextrin-ionic liquid polymer based dynamically coating for simultaneous determination of tetracyclines by capillary electrophoresis. Electrophoresis 2017, 38, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.V.; Kawai, T.; Wang, L.; Rubakhin, S.S.; Sweedler, J.V. Chiral Measurement of Aspartate and Glutamate in Single Neurons by Large-Volume Sample Stacking Capillary Electrophoresis. Anal. Chem. 2017, 89, 12375–12382. [Google Scholar] [CrossRef]
- De Cock, B.; Mangelings, D.; Vander Heyden, Y. Interinstrumental Transfer of a Chiral Capillary Electrophoretic Method: The Use of Robustness Test Information to Overcome Differences in Detector and Data-Handling Specifications. Chromatographia 2018, 81, 335–348. [Google Scholar] [CrossRef]
- Hamidi, S.; Khoubnasabjafari, M.; Ansarin, K.; Jouyban-Gharamaleki, V.; Jouyban, A. Chiral separation of methadone in exhaled breath condensate using capillary electrophoresis. Anal. Methods 2017, 9, 2342–2350. [Google Scholar] [CrossRef]
- Šesták, J.; Theurillat, R.; Sandbaumhüter, F.A.; Thormann, W. Fundamental aspects of field-amplified electrokinetic injection of cations for enantioselective capillary electrophoresis with sulfated cyclodextrins as selectors. J. Chromatogr. A 2018, 1558, 85–95. [Google Scholar] [CrossRef]
- Budău, M.; Hancu, G.; Szabó, Z.I.; Kelemen, H.; Rusu, A.; Muntean, D.L.; Cârje, A.G. Captisol® as chiral selector in capillary electrophoresis of non-acidic drugs. J. Chil. Chem. Soc. 2017, 62, 3566–3571. [Google Scholar] [CrossRef]
- Li, S.; Purdy, W.C. Circular dichroism, ultraviolet, and proton nuclear magnetic resonance spectroscopic studies of the chiral recognition mechanism of beta-cyclodextrin. Anal. Chem. 1992, 64, 1405–1412. [Google Scholar] [CrossRef]
- Quirino, J.P. A cationic β-cyclodextrin as a dynamic coating for the separation of proteins in capillary electrophoresis. J. Sep. Sci. 2017, 40, 4835–4838. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, Q.; Wang, W.; Liu, L.; Wang, L. Enantioseparation of amino acids by micellar capillary electrophoresis using binary chiral selectors and determination of D-glutamic acid and D-aspartic acid in rice wine. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 783–789. [Google Scholar] [CrossRef]
- Li, X.; Du, Y.; Feng, Z.; Sun, X.; Huang, Z. A novel enantioseparation approach based on liposome electrokinetic capillary chromatography. J. Pharm. Biomed. Anal. 2017, 145, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qi, S.; Liu, C.; Zhao, X. Enantiomeric separation of five acidic drugs via capillary electrophoresis using streptomycin as chiral selector. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1063, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, J.; Zhang, X.; Zhao, L.; Li, S. Enantiomeric separation of adrenaline, noradrenaline, and isoprenaline by capillary electrophoresis using streptomycin-modified gold nanoparticles. Microchim. Acta 2018, 185, 227. [Google Scholar] [CrossRef] [PubMed]
- Dziomba, S.; Ciura, K.; Kocialkowska, P.; Prahl, A.; Wielgomas, B. Gold nanoparticles dispersion stability under dynamic coating conditions in capillary zone electrophoresis. J. Chromatogr. A 2018, 1550, 63–67. [Google Scholar] [CrossRef]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef]
- Naghdi, E.; Fakhari, A.R. Simultaneous chiral separation of tramadol and methadone in tablets, human urine, and plasma by capillary electrophoresis using maltodextrin as the chiral selector. Chirality 2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, Y.; Feng, Z.; Liu, Z.; Li, J. Establishment and molecular modeling study of maltodextrin-based synergistic enantioseparation systems with two new hydroxy acid chiral ionic liquids as additives in capillary electrophoresis. J. Chromatogr. A 2018, 1559, 170–177. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, Y.; Chen, J.; Xu, G.; Yu, T.; Hua, X.; Zhang, J. Investigation of chondroitin sulfate D and chondroitin sulfate E as novel chiral selectors in capillary electrophoresis. Anal. Bioanal. Chem. 2014, 406, 1557–1566. [Google Scholar] [CrossRef]
- Yang, X.; Yan, Z.; Yu, T.; Du, Y.; Chen, J.; Liu, Z.; Xi, Y. Study of the enantioselectivity and recognition mechanism of chiral dual system based on chondroitin sulfate D in capillary electrophoresis. Anal. Bioanal. Chem. 2018, 410, 5889–5898. [Google Scholar] [CrossRef]
- Stalcup, A.M.; Agyei, N.M. Heparin: A Chiral Mobile-Phase Additive for Capillary Zone Electrophoresis. Anal. Chem. 1994, 66, 3054–3059. [Google Scholar] [CrossRef]
- Liu, Y.; Sombra, L.L.; Stege, P.W. Enantiomeric separation of β-blockers and tryptophan using heparin as stationary and pseudostationary phases in capillary electrophoresis. Chirality 2018, 30, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Corradini, R.; Sforza, S.; Tedeschi, T.; Marchelli, R. Chirality as a tool in nucleic acid recognition: Principles and relevance in biotechnology and in medicinal chemistry. Chirality 2007, 19, 269–294. [Google Scholar] [CrossRef] [PubMed]
- Tohala, L.; Oukacine, F.; Ravelet, C.; Peyrin, E. Sequence requirements of oligonucleotide chiral selectors for the capillary electrophoresis resolution of low-affinity DNA binders. Electrophoresis 2017, 38, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Tohala, L.; Oukacine, F.; Ravelet, C.; Peyrin, E. Chiral Resolution Capabilities of DNA Oligonucleotides. Anal. Chem. 2015, 87, 5491–5495. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, A.; Mamada, M.; Dobashi, Y.; Yamaguchi, J. Enantiomeric Separation with Sodium Dodecanoyl-L-Amino Acidate Micelles and Poly(Sodium (10-Undecenoyl)-L-Valinate) by Electrokinetic Chromatography. Anal. Chem. 1995, 67, 3011–3017. [Google Scholar] [CrossRef]
- Terabe, S.; Ozaki, H.; Tanaka, Y. New Pseudostationary Phases for Electrokinetic Chromatography: A High-Molecular Surfactant and Proteins. J. Chin. Chem. Soc. 1994, 41, 251–257. [Google Scholar] [CrossRef]
- Wang, J.; Warner, I.M. Chiral Separations Using Micellar Electrokinetic Capillary Chromatography and a Polymerized Chiral Micelle. Anal. Chem. 1994, 66, 3773–3776. [Google Scholar] [CrossRef]
- Shamsi, S.A.; Warner, I.M. Monomeric and polymeric chiral surfactants as pseudo-stationary phases for chiral separations. Electrophoresis 1997, 18, 853–872. [Google Scholar] [CrossRef]
- Morris, K.F.; Billiot, E.J.; Billiot, F.H.; Ingle, J.A.; Krause, K.B.; Lewis, C.R.; Lipkowitz, K.B.; Southerland, W.M.; Fang, Y. Using molecular dynamics simulations to identify the key factors responsible for chiral recognition by an amino acid-based molecular micelle. J. Dispers. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Morris, K.F.; Billiot, E.J.; Billiot, F.H.; Ingle, J.A.; Zack, S.R.; Krause, K.B.; Lipkowitz, K.B.; Southerland, W.M.; Fang, Y. Investigation of chiral recognition by molecular micelles with molecular dynamics simulations. J. Dispers. Sci. Technol. 2018, 39, 45–54. [Google Scholar] [CrossRef]
- Terabe, S.; Shibata, M.; Miyashita, Y. chiral separation by electronkinetic chromatography while bile salt micelles. J. Chromatogr. A 1989, 480, 403–411. [Google Scholar] [CrossRef]
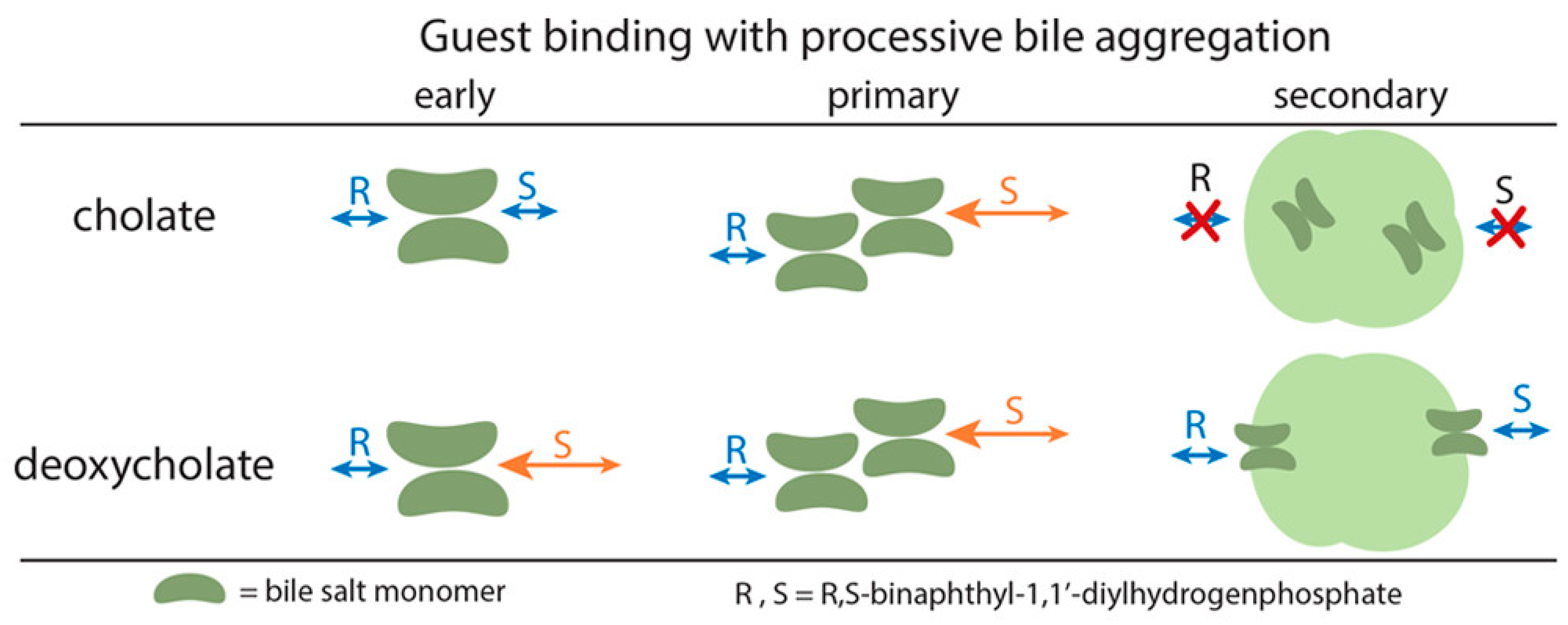
- Meier, A.R.; Yehl, J.B.; Eckenroad, K.W.; Manley, G.A.; Strein, T.G.; Rovnyak, D. Stepwise Aggregation of Cholate and Deoxycholate Dictates the Formation and Loss of Surface-Available Chirally Selective Binding Sites. Langmuir 2018, 34, 6489–6501. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, Q.; Hu, S.; Guo, W.; Yang, Z.; Zhang, Y. Thermodynamic models to elucidate the enantioseparation of drugs with two stereogenic centers by micellar electrokinetic chromatography. J. Chromatogr. A 2017, 1512, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Quirino, J.P.; Tarongoy, F.M. Liquid chromatography with micelles in open-tube capillaries. Green Chem. 2018, 20, 2486–2493. [Google Scholar] [CrossRef]
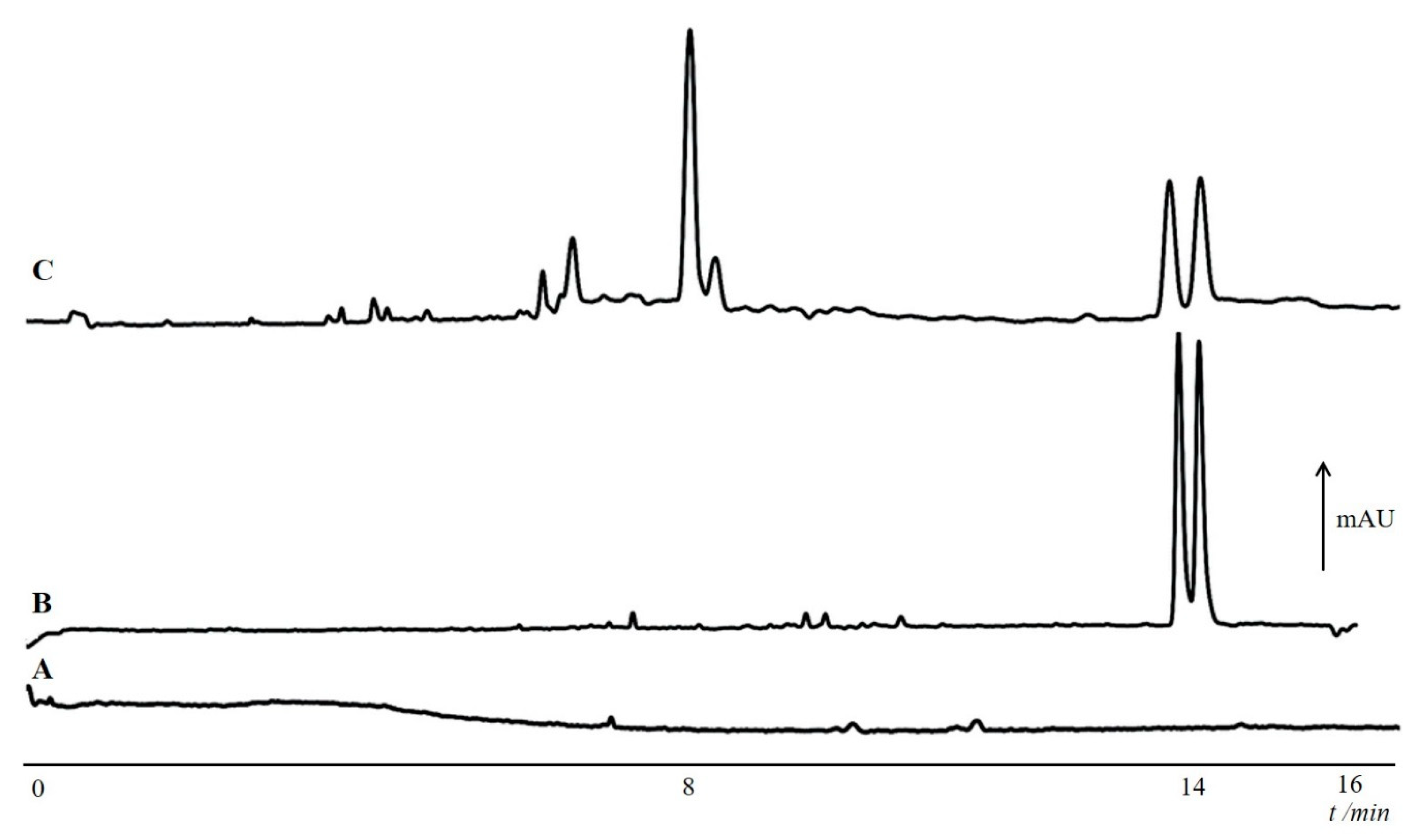
- Liu, Y.; Wang, X. Enantioseparation of ofloxacin and its four related substances with ligand exchange–micellar electrokinetic chromatography using copper(II)-L-isoleucine complex as chiral selector. Chirality 2017, 29, 422–429. [Google Scholar] [CrossRef]
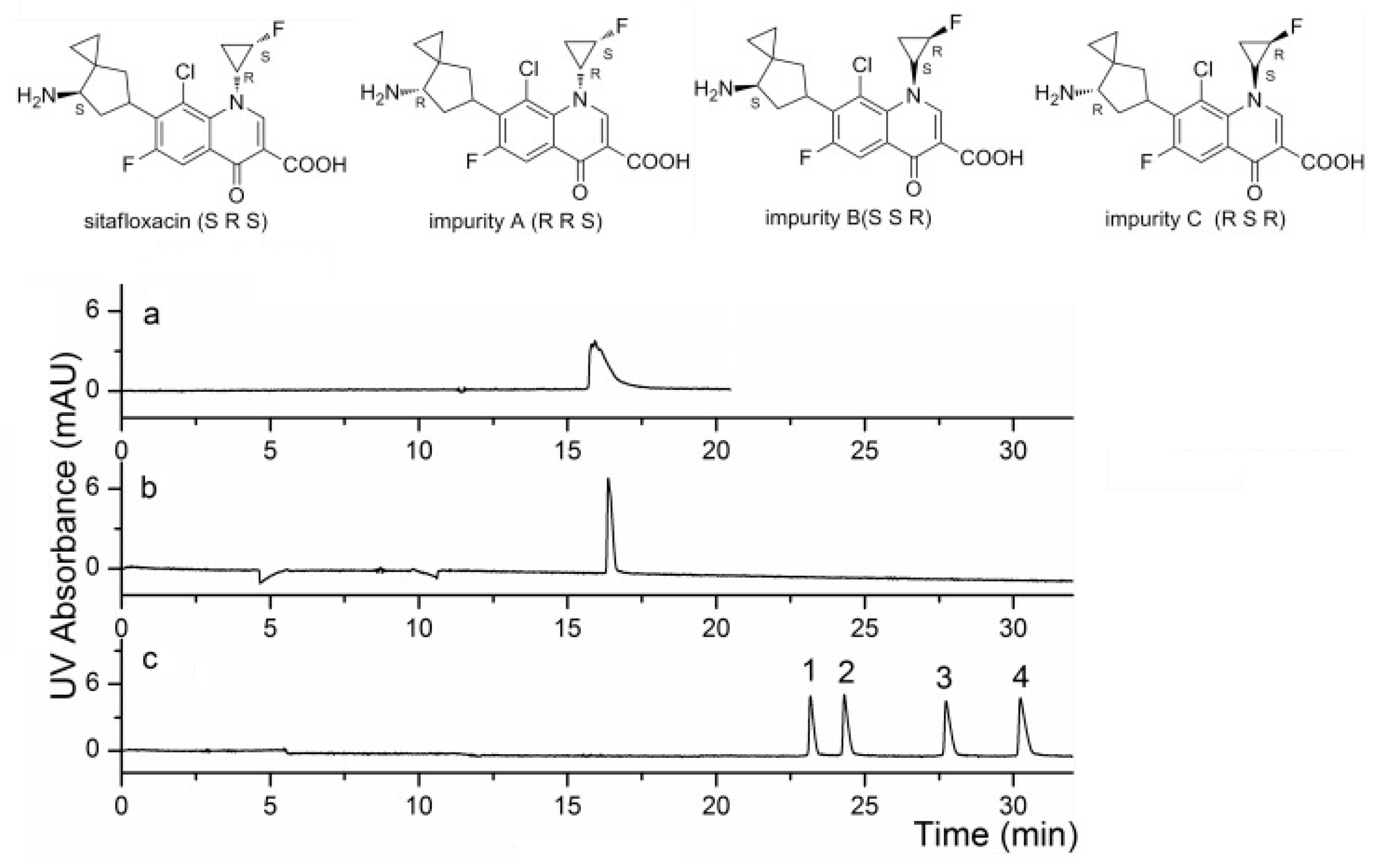
- Meng, R.; Kang, J. Determination of the stereoisomeric impurities of sitafloxacin by capillary electrophoresis with dual chiral additives. J. Chromatogr. A 2017, 1506, 120–127. [Google Scholar] [CrossRef]
- Lv, L.; Wang, L.; Li, J.; Jiao, Y.; Gao, S.; Wang, J.; Yan, H. Enantiomeric separation of seven β-agonists by NACE—Study of chiral selectivity with diacetone-D-mannitol–boric acid complex. J. Pharm. Biomed. Anal. 2017, 145, 399–405. [Google Scholar] [CrossRef]
- Alopina, E.; Dobryakov, Y.; Safonova, E.; Smirnova, N.; Kolobova, E.; Kartsova, L. Densities, refractive indices and conductivities of aqueous [C n mim][Pro] solutions (n = 4, 8, 12); micellization and the capillary electrophoresis data at 298.15 K. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 137–143. [Google Scholar] [CrossRef]
- Wahl, J.; Holzgrabe, U. Capillary electrophoresis separation of phenethylamine enantiomers using amino acid based ionic liquids. J. Pharm. Biomed. Anal. 2018, 148, 245–250. [Google Scholar] [CrossRef]
- Jang, M.G.; Jang, M.D.; Park, J.H. Doxycycline as a new chiral selector in capillary electrophoresis. J. Chromatogr. A 2017, 1508, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, E.; Kuo, J.E.; Zare, R.N. Electrokinetic separation of chiral compounds. Science 1985, 230, 813–814. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, L.; Mao, L.; Chen, Y. Chiral separation using capillary electromigration techniques based on ligand exchange principle. J. Sep. Sci. 2012, 35, 1236–1248. [Google Scholar] [CrossRef]
- Quirino, J.P.; Terabe, S. Electrokinetic chromatography. J. Chromatogr. A 1999, 856, 465–482. [Google Scholar] [CrossRef]
- Wang, L.J.; Liu, X.F.; Lu, Q.N.; Yang, G.L.; Chen, X.G. An ion-pair principle for enantioseparations of basic analytes by nonaqueous capillary electrophoresis using the di-n-butyl l-tartrate-boric acid complex as chiral selector. J. Chromatogr. A 2013, 1284, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Bjørnsdottir, I.; HonoréHansen, S.; Terabe, S. Chiral separation in non-aqueous media by capillary electrophoresis using the ion-pair principle. J. Chromatogr. A 1996, 745, 37–44. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Shamsi, S.A. Synthesis, characterization, and application of chiral ionic liquids and their polymers in micellar electrokinetic chromatography. Anal. Chem. 2006, 78, 7061–7069. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Sun, X. Investigation of maltodextrin-based synergistic system with amino acid chiral ionic liquid as additive for enantioseparation in capillary electrophoresis. Chirality 2017, 29, 824–835. [Google Scholar] [CrossRef]
- Greño, M.; Marina, M.L.; Castro-Puyana, M. Effect of the combined use of γ-cyclodextrin and a chiral ionic liquid on the enantiomeric separation of homocysteine by capillary electrophoresis. J. Chromatogr. A 2018, 1568, 222–228. [Google Scholar] [CrossRef]
- Kolobova, E.A.; Kartsova, L.A.; Alopina, E.V.; Smirnova, N.A. Separation of amino acids and β-blockers enantiomers by capillary electrophoresis with 1-butyl-3-methylimidazolium L-prolinate [C4MIm][L-Pro] as a chiral selector. Analitika i Kontrol 2018, 22, 51–60. [Google Scholar] [CrossRef]
- Stănescu, M.; Monciu, C.M.; Nițulescu, M.; Drăghici, C.; Doicin, I.; Lupașcu, G.; Lupu, A.; Aramă, C.C. Amino acids based chiral ionic liquids for enantiomer separation by capillary electrophoresis. Farmacia 2017, 65, 46–55. [Google Scholar]
- Krait, S.; Salgado, A.; Chankvetadze, B.; Gago, F.; Scriba, G.K.E. Investigation of the complexation between cyclodextrins and medetomidine enantiomers by capillary electrophoresis, NMR spectroscopy and molecular modeling. J. Chromatogr. A 2018, 1567, 198–210. [Google Scholar] [CrossRef]
- Abdel-Megied, A.M.; Hanafi, R.S.; Aboul-Enein, H.Y. A chiral enantioseparation generic strategy for anti-Alzheimer and antifungal drugs by short end injection capillary electrophoresis using an experimental design approach. Chirality 2018, 30, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.; Chankvetadze, B. Applications of nuclear magnetic resonance spectroscopy for the understanding of enantiomer separation mechanisms in capillary electrophoresis. J. Chromatogr. A 2016, 1467, 95–144. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, B.; Melani, F.; Caprini, C.; Del Bubba, M.; Pinzauti, S.; Orlandini, S.; Furlanetto, S. Combined approach using capillary electrophoresis, NMR and molecular modeling for ambrisentan related substances analysis: Investigation of intermolecular affinities, complexation and separation mechanism. J. Pharm. Biomed. Anal. 2017, 144, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Quirino, J.P.; Terabe, S. Large volume sample stacking of positively chargeable analytes in capillary zone electrophoresis without polarity switching: Use of low reversed electroosmotic flow induced by a cationic surfactant at acidic pH. Electrophoresis 2000, 21, 355–359. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.B.; Quirino, J.P. Chiral Selectors in Capillary Electrophoresis: Trends during 2017–2018. Molecules 2019, 24, 1135. https://doi.org/10.3390/molecules24061135
Yu RB, Quirino JP. Chiral Selectors in Capillary Electrophoresis: Trends during 2017–2018. Molecules. 2019; 24(6):1135. https://doi.org/10.3390/molecules24061135
Chicago/Turabian StyleYu, Raymond B., and Joselito P. Quirino. 2019. "Chiral Selectors in Capillary Electrophoresis: Trends during 2017–2018" Molecules 24, no. 6: 1135. https://doi.org/10.3390/molecules24061135
APA StyleYu, R. B., & Quirino, J. P. (2019). Chiral Selectors in Capillary Electrophoresis: Trends during 2017–2018. Molecules, 24(6), 1135. https://doi.org/10.3390/molecules24061135





