Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation
Abstract
1. Introduction
2. Results and Discussion
2.1. Fruit Coloration during Different Developmental Stages
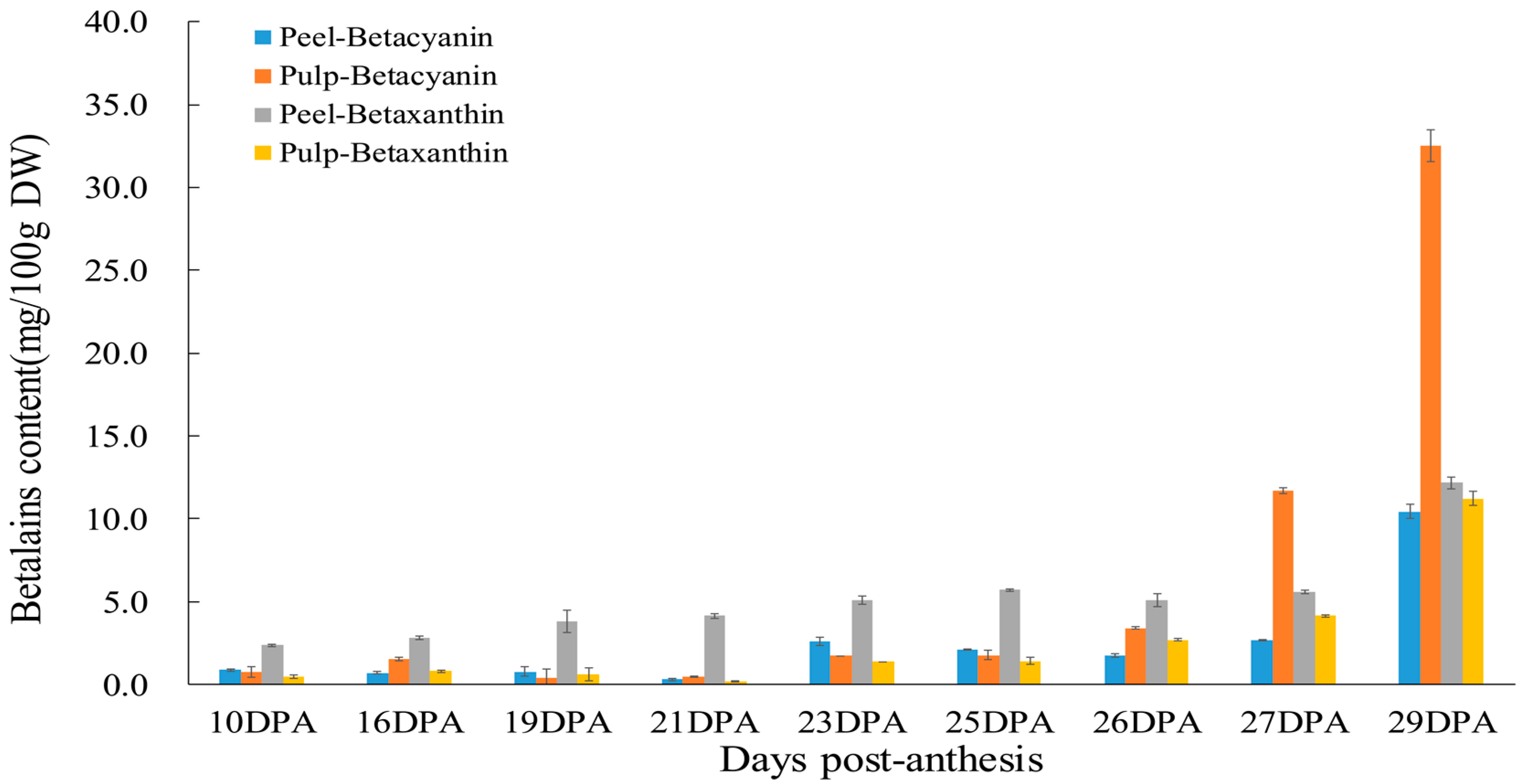
2.2. Betalain Contents
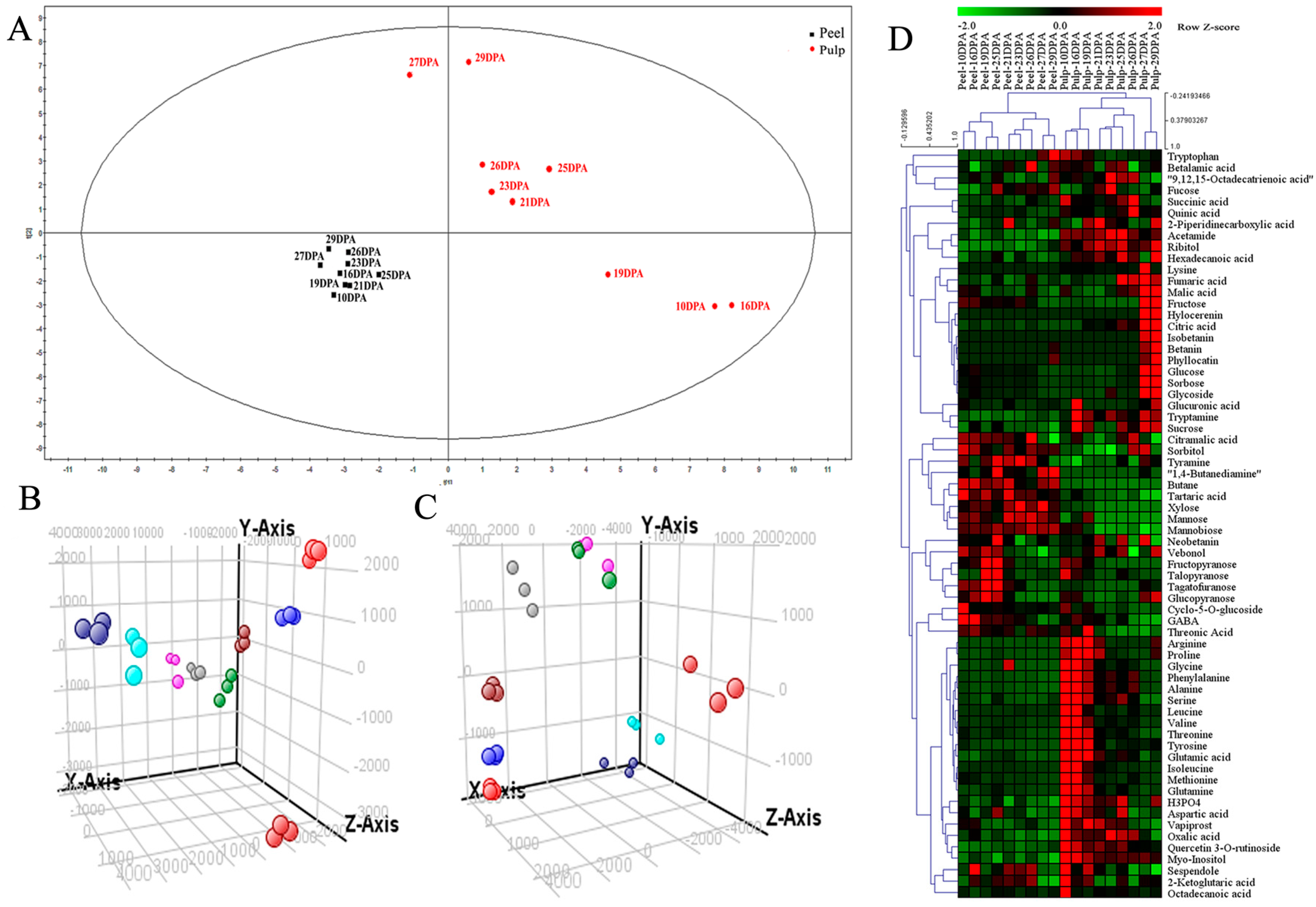
2.3. Compound Identification and Principal Component Analysis (PCA)
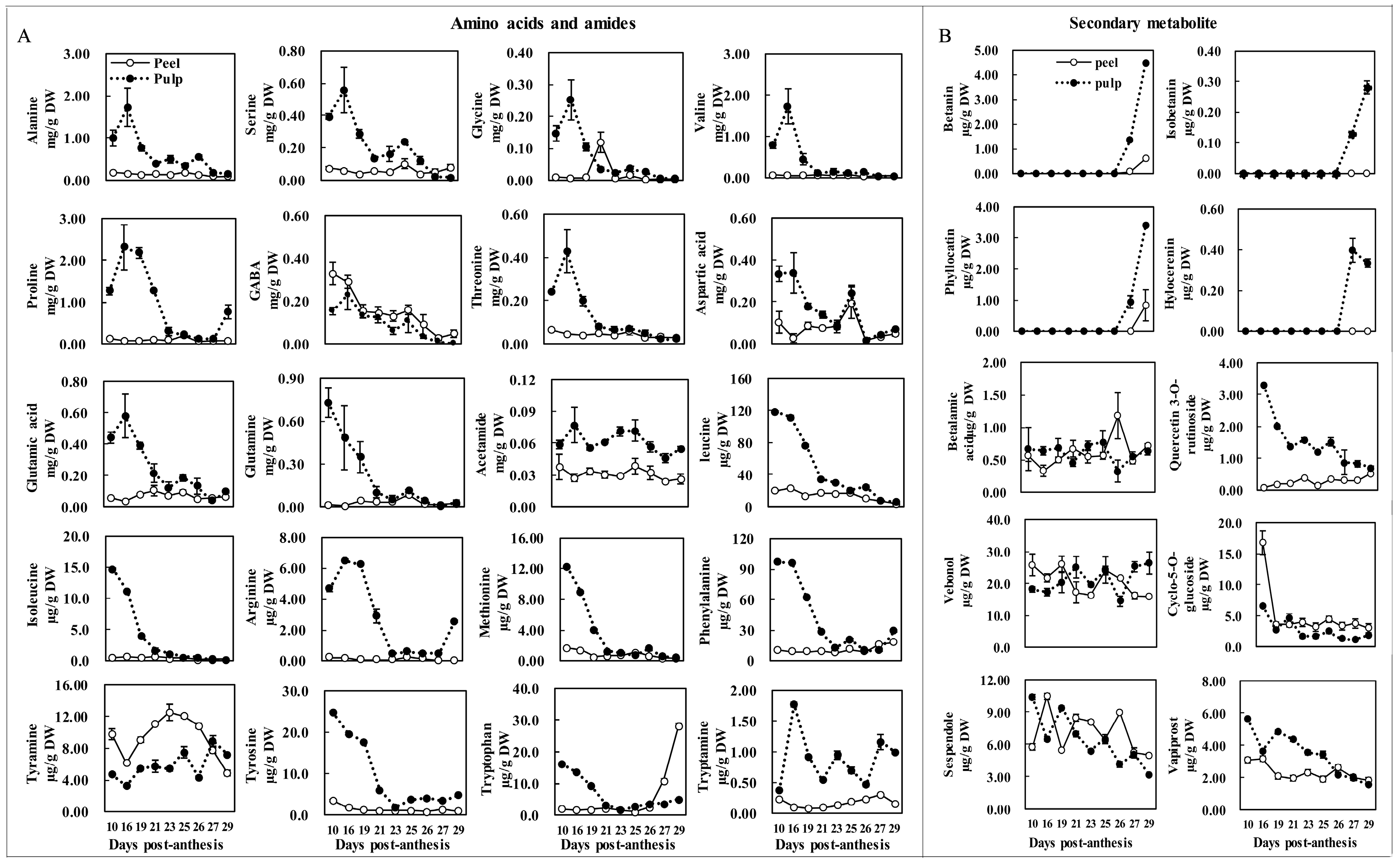
2.4. Major Amino Acid and Secondary Metabolite Changes during Fruit Maturation
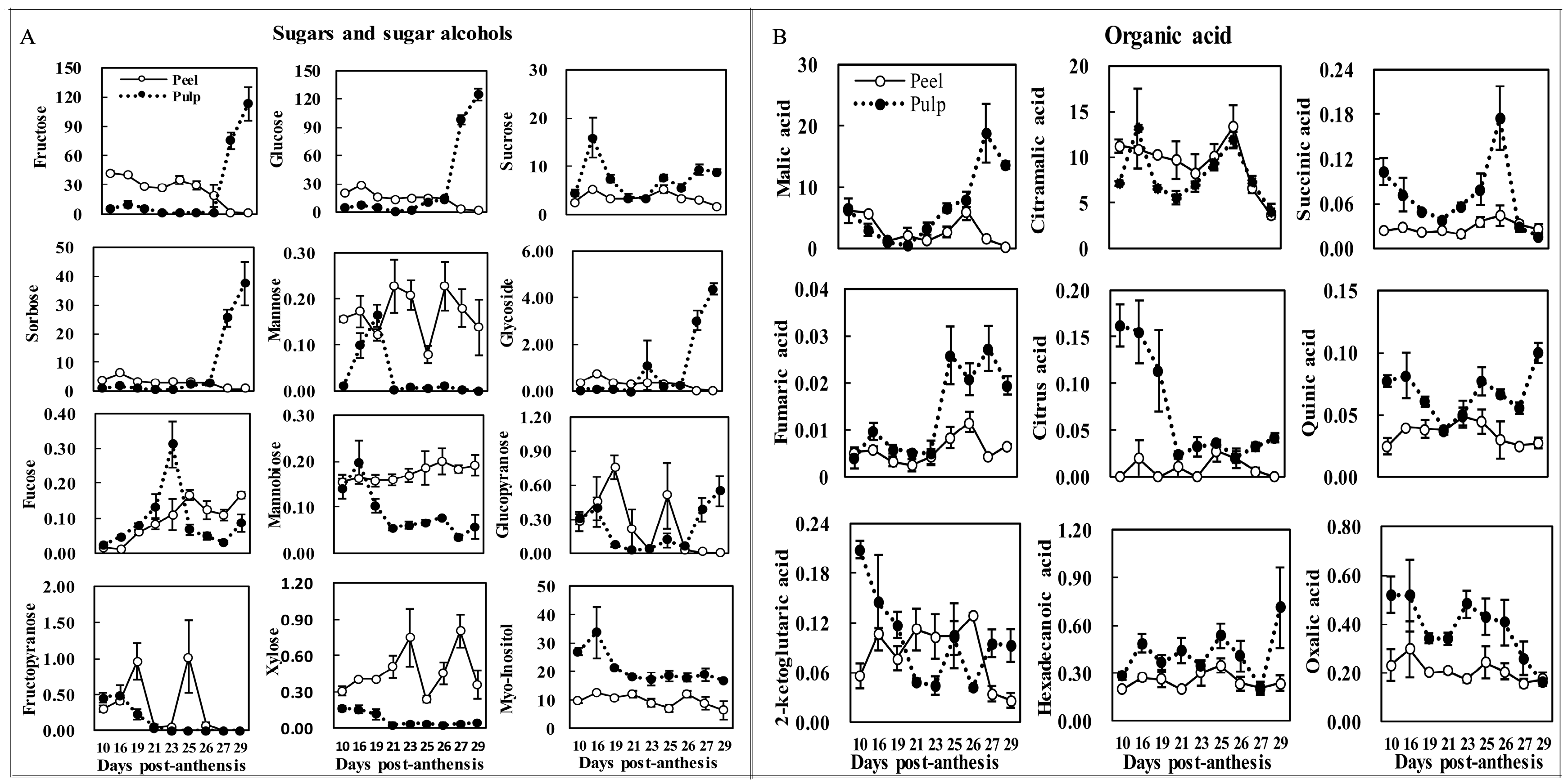
2.5. Changes of Major Sugars and Acids during Fruit Maturation
2.6. Analysis of Correlation Tests between Metabolites and Betalains
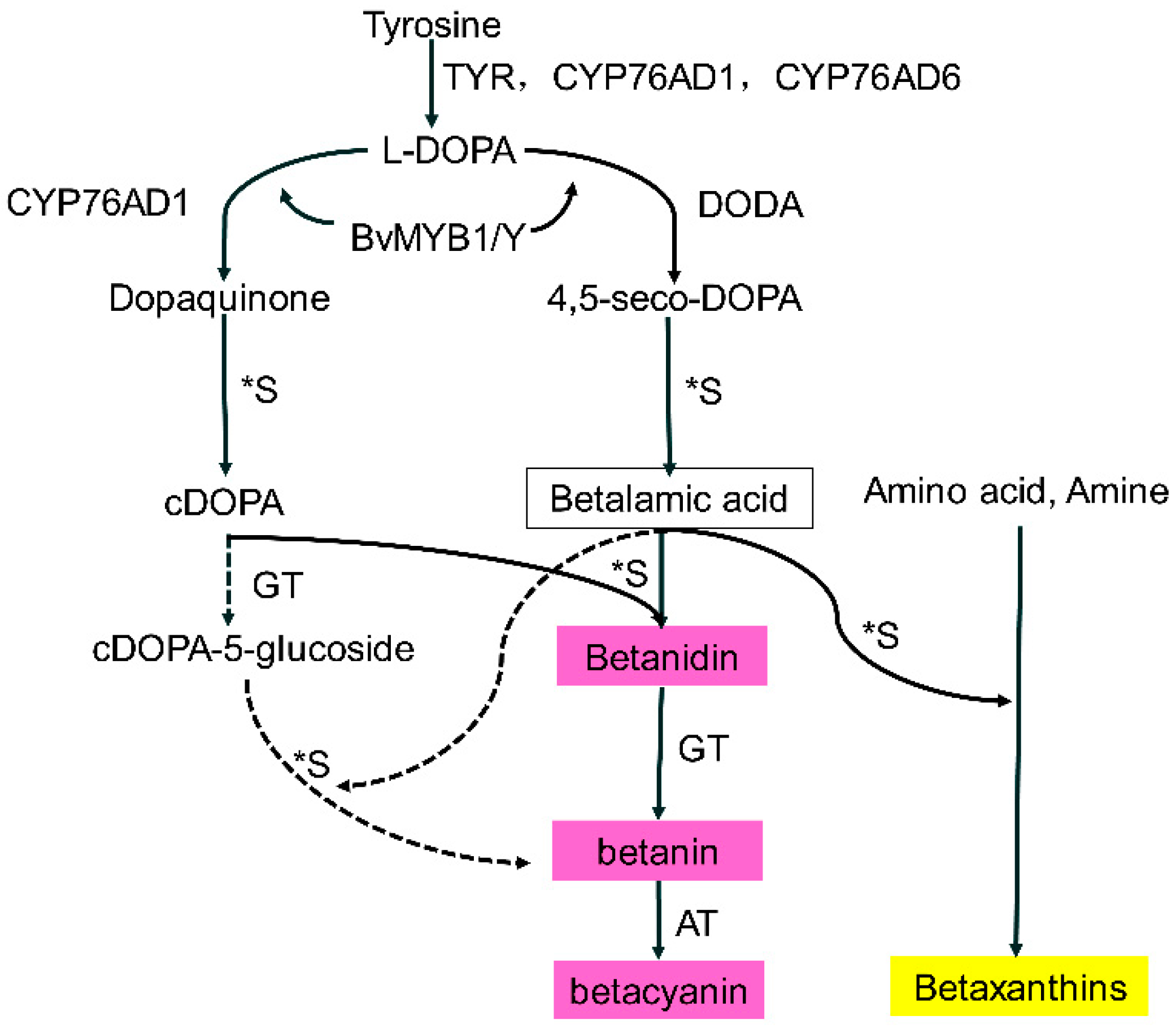
2.7. Identification of Representative Metabolites Related to Betalain Formation
3. Conclusions
4. Materials and Methods
4.1. Fruit Material
4.2. Determination of Color Rating
4.3. Determination of Total Betalain Content
4.4. Metabolic Profiling
4.5. Multivariate Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Suh, D.H.; Lee, S.; Heo, D.Y.; Kim, Y.S.; Cho, S.K.; Lee, S.; Lee, C.H. Metabolite profiling of red and white pitayas (Hylocereus polyrhizus and Hylocereus undatus) for comparing betalain biosynthesis and antioxidant activity. J. Agric. Food Chem. 2014, 62, 8764–8771. [Google Scholar] [CrossRef] [PubMed]
- García-Cruza, L.; Dueñas, M.; Santos-Buelgas, C.; Valle-Guadarrama, S.; Salinas-Moreno, Y. Betalains and phenolic compounds profiling and antioxidant capacity of pitaya (Stenocereus spp.) fruit from two species (S. pruinosus and S. stellatus). Food Chem. 2017, 234, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Polturak, G.; Heinig, U.; Grossman, N.; Battat, M.; Leshkowitz, D.; Malitsky, S.; Rogachev, I.; Aharoni, A. Transcriptome and Metabolic Profiling Provides Insights into Betalain Biosynthesis and Evolution in Mirabilis jalapa. Mol. Plant. 2018, 11, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional Properties of Anthocyanins and Betalains in Plants, Food, and in Human Nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Song, H.Z.; Chu, Q.; Xu, D.D.; Xu, Y.; Zheng, X.D. Purified betacyanins from Hylocereus undatus peel ameliorate obesity and insulin resistance in high-fat-diet-fed mice. J. Agric. Food Chem. 2016, 64, 236–244. [Google Scholar] [CrossRef]
- Khan, M.I. Plant betalains: Safety, antioxidant activity, clinical efficacy, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef]
- Polturak, G.; Aharoni, A. “La Vie en Rose”: Biosynthesis, Sources, and Applications of Betalain Pigments. Mol. Plant. 2017, 25, 1–16. [Google Scholar] [CrossRef]
- Hua, Q.Z.; Chen, C.B.; Zur, T.N.; Wanga, H.C.; Wu, J.Y.; Chen, J.Y.; Zhang, Z.K.; Zhao, J.T.; Hu, G.B.; Qin, Q.H. Metabolomic characterization of pitaya fruit from three red-skinned cultivars with different pulp colors. Plant Physiol. Biochem. 2018, 126, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Deloache, W.C.; Russ, Z.N.; Narcross, L.; Gonzales, A.M.; Martin, V.J.; Dueber, J.E. An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose. Nat. Chem. Biol. 2015, 11, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Gandia-Herrero, F.; Escribano, J.; Garcia-Carmona, F.; Jimenez-Atienzar, M. Fluorescent bioinspired protein labeling with betalamic acid. Derivatization and characterization of novel protein-betaxanthins. Dyes Pigments 2016, 133, 458–466. [Google Scholar] [CrossRef]
- Harris, N.N.; Javellana, J.; Davies, K.M.; Lewis, D.H.; Jameson, P.E.; Deroles, S.C.; Calcott, K.E.; Gould, K.S.; Schwinn, K.E. Betalain production is possible in anthocyanin-producing plant species given the presence of DOPA-dioxygenase and LDOPA. BMC Plant Biol. 2012, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Polturak, G.; Breitel, D.; Grossman, N.; Sarrion-Perdigones, A.; Weithorn, E.; Pliner, M.; Orzaez, D.; Granell, A.; Rogachev, I.; Aharoni, A. Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants. New Phytol. 2016, 210, 269–283. [Google Scholar] [CrossRef]
- Polturak, G.; Grossman, N.; Vela-Corcia, D.; Dong, Y.; Nudel, A.; Pliner, M.; Levy, M.; Rogachev, I.; Aharoni, A. Engineered gray mold resistance, antioxidant capacity and pigmentation in betalain-producing crops and ornamentals. Proc. Natl. Acad. Sci. USA 2017, 114, 9062–9067. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, X.; Yu, O.; Tang, J.J.; Gu, X.G.; Wan, X.C.; Fang, C.B. Metabolic profiling of strawberry (Fragaria×ananassa Duch.) during fruit development and maturation. J. Exp. Bot. 2011, 62, 1103–1118. [Google Scholar] [CrossRef]
- Den Ende, W.V.; Elesawe, S.K. Sucrose signaling pathways leading to fructan and anthocyanin accumulation: A dual function in abiotic and biotic stress responses? Environ. Exp. Bot. 2014, 108, 4–13. [Google Scholar] [CrossRef]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-Specific Induction of the Anthocyanin Biosynthetic Pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef]
- Dafnyyalin, M.; Glazer, I.; Barilan, I.; Kerem, Z.; Holland, D.; Amir, R. Color, Sugars and Organic Acids Composition in Aril Juices and Peel Homogenates Prepared from Different Pomegranate Accessions. J. Agric. Food Chem. 2010, 58, 4342–4352. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.G.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef]
- Jain, G.; Gould, K.S. Are betalain pigments the functional homologues of anthocyanins in plants? Environ. Exp. Bot. 2015, 119, 48–53. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef]
- Lee, S.; Jung, E.S.; Do, S.G.; Jung, G.; Song, G.; Song, J.; Lee, C.H. Correlation between species-specific metabolite profiles and bioactivities of blueberries (Vaccinium spp.). J. Agric. Food Chem. 2014, 62, 2126–2133. [Google Scholar] [CrossRef]
- Jang, Y.K.; Jung, S.E.; Hyun-Ah Lee, H.; Choi, D.; Lee, C.H. Metabolomic Characterization of Hot Pepper (Capsicum annuum “CM334”) during Fruit Development. J Agric. Food Chem. 2015, 63, 9452–9460. [Google Scholar] [CrossRef]
- Phebe, D.; Chew, M.K.; Suraini, A.A.; Lai, O.M.; Janna, O.A. Red-flshed pitaya (Hylocereus polyrhizus) fruit colour and betacyanin content depend on maturity. Int. Food Res. J. 2009, 16, 233–242. [Google Scholar]
- Hua, Q.Z.; Chen, C.J.; Chen, Z.C.; Chen, P.K.; Ma, Y.W.; Wu, J.Y.; Zheng, J.; Hu, G.B.; Zhao, J.T.; Qin, Y.H. Transcriptomic Analysis Reveals Key Genes Related to Betalain Biosynthesis in Pulp Coloration of Hylocereus polyrhizus. Front. Plant Sci. 2016, 6, 1179. [Google Scholar] [CrossRef]
- Harborne, J.B. Plant secondary metabolism. Phytochemistry 2000, 53, 132–155. [Google Scholar] [CrossRef]
- Lv, M.Y.; Sun, J.B.; Wang, M.; Fan, H.Y.; Zhang, Z.J.; Xu, F.G. Comparative analysis of volatile oils in the stems and roots of Ephedra sinica via GC-MS-based plant metabolomics. Chin. J. Nat. Med. 2016, 14, 133–140. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Rao, G.D.; Sui, J.K.; Zhang, J.G. Metabolomics reveals significant variations in metabolites and correlations regarding the maturation of walnuts (Juglans regia L.). Biol. Open 2016, 5, 829–836. [Google Scholar] [CrossRef]
- Atkins, C.A.; Pate, J.S.; Sharkey, P.J. Asparagine metabolism–key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975, 56, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Kugler, F.; Graneis, S.; Schreiter, P.P.-Y.; Stintzing, F.C.; Carle, R. Determination of free amino compounds in betalainic fruits and vegetables by gas chromatography with flame ionization and mass spectrometric detection. J. Agric. Food Chem. 2006, 54, 4311–4318. [Google Scholar] [CrossRef]
- Fait, A.; Hanhineva, K.; Beleggia, R.; Dai, N.; Rogachev, I.; Nikiforova, V.J.; Fernie, A.R.; Aharoni, A. Reconfiguration of the Achene and Receptacle Metabolic Networks during Strawberry Fruit Development. Plant Physiol. 2008, 148, 730–750. [Google Scholar] [CrossRef] [PubMed]
- Schauer, N.; Fernie, A.R. Plant metabolomics: Towards biological function and mechanism. Trends Plant Sci. 2006, 11, 508–516. [Google Scholar] [CrossRef]
- Smeekens, S. Sugar-induced signal transduction in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 49–81. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, I.F.; Castaño-Tostado, E.; Ramírez-de León, J.A.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chem. 2015, 172, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Nianlai Chen, N.L.; Zonghuan Ma, Z.H.; Che, F.; Mao, J.; Chen, B.H. The Changes in Color, Soluble Sugars, Organic Acids, Anthocyanins and Aroma Components in “Starkrimson” during the Ripening Period in China. Molecules 2016, 21, 812. [Google Scholar] [CrossRef]
- Saure, M.C. External control of anthocyan information in apple. Sci. Hortic. 1990, 42, 181–218. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analyis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Tian, L.; Liu, H.T.; Pan, Q.H.; Zhan, J.C.; Huang, W.D. Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 2009, 58, 251–260. [Google Scholar] [CrossRef]
- Moalem-Beno, D.; Tamari, G.; Leitner-Dagan, Y.; Borochov, A.; Weiss, D. Sugar-Dependent Gibberellin-Induced Chalcone Synthase Gene Expression in Petunia Corollas. Plant Physiol. 1997, 113, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Vitrac, X.; Larronde, F.; Krisa, S.; Decendit, A.; Deffieux, G.; Me’rillon, J.M. Sugar sensing and Ca2+-calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry 2000, 53, 659–665. [Google Scholar] [CrossRef]
- Hara, M.; Oki, K.; Hoshino, K.; Kuboi, T. Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyl. Plant Sci. 2003, 164, 259–265. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, P.M.; Cheng, L.L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Zhao, J.H.; Li, H.X.; Xi, W.P.; An, W.; Niu, L.L.; Cao, Y.L.; Wang, H.F.; Wang, Y.J.; Yin, Y. Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chem. 2015, 173, 718–724. [Google Scholar] [CrossRef]
- Léchaudel, M.; Joas, J.; Caro, Y.; Génard, M.; Jannoyer, M. Leaf: Fruit ratio and irrigation supply affect seasonal changes in minerals, organic acids and sugars of mango fruit. J. Agric. Food Chem. 2005, 85, 251–260. [Google Scholar] [CrossRef]
- Sugimoto, N.; Jones, A.D.; Beaudry, R.M. Changes in Free Amino Acid Content in ‘Jonagold’ Apple Fruit as Related to Branched-chain Ester Production, Ripening, and Senescence. J. Am. Soc. Hortic. Sci. 2011, 136, 429–440. [Google Scholar] [CrossRef]
- Noro, S.; Kudo, N.; Kitsuwa, T. Differences in sugars and organic acids between red and yellow apple cultivars at time of coloring, and effect of citramalic acid on development of anthocyanin. J. Jpn. Soc. Hortic. Sci. 1998, 57, 381–389. [Google Scholar] [CrossRef]
- Khorassani, R.; Hettwer, U.; Ratzinger, A.; Steingrobe, B.; Karlovsky, P.; Claassen, N. Citramalic acid and salicylic acid in sugar beet root exudates solubilize soil phosphorus. BMC Plant Biol. 2011, 11, 121. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Liu, Y.L.; Che, F.; Wang, L.X.; Meng, R.; Zhang, X.J.; Zhao, Z.Y. Fruit Coloration and Anthocyanin Biosynthesis after Bag Removal in Non-Red and Red Apples (Malus × domestica Borkh). Molecules 2013, 18, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Kugler, F.; Stintzing, F.; Carle, R. Identification of Betalains from Petioles of Differently Colored Swiss Chard (Beta vulgaris L. ssp. cicla [L.] Alef. Cv. Bright Lights) by High-Performance Liquid Chromatography-Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2004, 52, C2975–C2981. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.D.; Luo, Y.; Sun, X.H.; Wang, J.Q.; Luo, T.; Zeng, Y.L.; Xu, J.; Deng, X.X.; Cheng, Y.J. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar] [CrossRef]
- Tan, F.Q.; Tu, H.; Liang, W.J.; Long, J.M.; Wu, X.M.; Zhang, H.Y.; Guo, W.W. Comparative metabolic and transcriptional analysis of a doubled diploid and its diploid citrus root stock (C. junos cv. Ziyang xiangcheng) suggests its potential value for stress resistance improvement. BMC Plant Biol. 2015, 89. [Google Scholar] [CrossRef]
- Wang, S.C.; Tu, H.; Wan, J.; Chen, W.; Liu, X.Q.; Luo, J.; Xu, J.; Zhang, H.Y. Spatio-temporal distribution and natural variation of metabolites in citrus fruits. Food Chem. 2016, 199, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.Y.; Dykes, G.; Lee, S.M.; Choo, W.S. Comparative Study of Betacyanin Profile and Antimicrobial Activity of Red Pitahaya (Hylocereus polyrhizus) and Red Spinach (Amaranthus dubius). Plant Foods Hum. Nutr. 2017, 72, 41. [Google Scholar] [CrossRef] [PubMed]
- Abureidah, I.M.; Alishtayeh, M.S.; Jamous, R.M.; Arraezroman, D.; Seguracarretero, A. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]

| Tissue | Color Items | Harvest Date | ||||||
|---|---|---|---|---|---|---|---|---|
| 19 DPA | 21 DPA | 23 DPA | 25 DPA | 26 DPA | 27 DPA | 29 DPA | ||
| Peel | L* | 47.91 ± 2.89 | 47.73 ± 2.36 | 43.30 ± 1.08 | 42.98 ± 1.47 | 43.24 ± 1.13 | 45.19 ± 0.24 | 37.69 ± 0.92 |
| a* | −14.42 ± 0.49 | −15.33 ± 0.31 | −14.81 ± 0.31 | −13.78 ± 0.36 | −9.26 ± 0.42 | −4.46 ± 4.47 | 21.15 ± 3.19 | |
| b* | 22.36 ± 1.11 | 25.00 ± 1.12 | 22.34 ± 0.62 | 21.16 ± 0.98 | 21.33 ± 0.96 | 21.12 ± 0.89 | 10.38 ± 0.60 | |
| C* | 26.60 ± 1.20 | 29.33 ± 1.11 | 26.80 ± 0.67 | 25.25 ± 0.96 | 23.44 ± 1.02 | 22.45 ± 1.92 | 23.64 ± 2.94 | |
| h° | 122.86 ± 0.42 | 121.55 ± 0.68 | 123.55 ± 0.33 | 123.11 ± 0.42 | 115.66 ± 1.03 | 100.04 ± 11.19 | 26.83 ± 3.27 | |
| Pulp | L* | 78.25 ± 1.26 | 67.69 ± 0.84 | 66.54 ± 3.21 | 66.10 ± 1.04 | 51.32 ± 1.65 | 38.24 ± 0.43 | 32.64 ± 0.84 |
| a* | 2.15 ± 0.44 | 2.38 ± 0.23 | 0.53 ± 0.87 | −0.21 ± 0.96 | 14.38 ± 0.33 | 29.18 ± 0.68 | 27.41 ± 0.97 | |
| b* | 15.18 ± 1.16 | 10.32 ± 4.05 | 6.88 ± 2.94 | 4.40 ± 0.12 | −6.43 ± 0.14 | −3.22 ± 0.60 | 0.60 ± 0.52 | |
| C* | 15.35 ± 1.09 | 10.66 ± 3.96 | 6.96 ± 2.30 | 4.41 ± 0.12 | 16.68 ± 0.32 | 29.37 ± 0.73 | 27.42 ± 0.97 | |
| h° | 81.65 ± 2.14 | 73.91 ± 4.47 | 89.87 ± 5.04 | 92.82 ± 1.34 | 53.64 ± 2.15 | 6.26 ± 1.03 | 1.82 ± 0.44 | |
| Metabolites | Pulp | Peel | |||
|---|---|---|---|---|---|
| Betacyanin | Betaxanthin | Betacyanin | Betaxanthin | ||
| Amino Acids | Proline | −0.237 | −0.311 | −0.214 | −0.162 |
| Glycine | −0.456 | −0.502 | −0.298 | −0.172 | |
| Valine | −0.365 | −0.407 | −0.456 | −0.462 | |
| Serine | −0.631 | −0.657 | 0.321 | 0.297 | |
| Threonine | −0.474 | −0.522 | −0.499 | −0.568 | |
| Aspartic Acid | −0.446 | −0.504 | −0.151 | −0.095 | |
| Glutamic Acid | −0.505 | −0.553 | −0.103 | 0.075 | |
| Glutamine | −0.401 | −0.452 | −0.063 | 0.086 | |
| Organic Acids | Oxalic Acid | −0.803 | −0.770 | −0.446 | −0.508 |
| Citramalic Acid | −0.511 | −0.417 | −0.808 | −0.785 | |
| Malic Acid | 0.681 | 0.691 | −0.504 | −0.597 | |
| Citric Acid | 0.826 * | 0.822 * | −0.367 | −0.256 | |
| Sugars/Sugar Alcohols | Fructose | 0.952 ** | 0.923 ** | −0.681 | −0.769 |
| Glucose | 0.928 ** | 0.910 ** | −0.683 | −0.766 | |
| Sorbose | 0.954 ** | 0.934 ** | −0.655 | −0.740 | |
| Glycoside | 0.935 ** | 0.920 ** | −0.622 | −0.700 | |
| Myo-Inositol | −0.402 | −0.455 | −0.709 | −0.696 | |
| Sucrose | 0.375 | 0.356 | −0.560 | −0.501 | |
| Peel–29 DPA vs. Peel–27 DPA | Pulp–26 DPA vs. Pulp–25 DPA | Pulp–26 DPA vs. Pulp–27 DPA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Var ID (Primary) | VIP | P-Value | Var ID (Primary) | VIP | P-Value | Var ID (Primary) | VIP | P-Value | |
| 1 | Tryptophan | 1.696 | 0.0005 | Arginine | 1.476 | 0.0025 | Betanin | 1.374 | 0.0028 |
| 2 | Betanin | 1.670 | 0.0109 | Phenylalanine | 1.45 | 0.0488 | Isobetanin | 1.363 | 0.0066 |
| 3 | Malic Acid | 1.669 | 0.0026 | Methionine | 1.431 | 0.0208 | Propanoic Acid | 1.355 | 0.0005 |
| 4 | Quercetin | 1.663 | 0.0001 | Aspartic Acid | 1.413 | 0.0268 | Aspartic Acid | 1.353 | 0.0024 |
| 5 | Tryptamine * | 1.656 | 0.0154 | Vapiprost | 1.402 | 0.0143 | Fructose | 1.345 | 0.0135 |
| 6 | Citramalic Acid * | 1.620 | 0.0269 | Sespendole | 1.388 | 0.0183 | Methionine | 1.339 | 0.0168 |
| 7 | Tyramine * | 1.611 | 0.0046 | Tyramine * | 1.360 | 0.0482 | Alanine | 1.337 | 0.0045 |
| 8 | Tyrosine | 1.604 | 0.0120 | Serine | 1.353 | 0.0224 | Valine | 1.331 | 0.0055 |
| 9 | Fumaric Acid | 1.598 | 0.0060 | Tryptamine * | 1.326 | 0.0351 | Hylocerenin | 1.326 | 0.0208 |
| 10 | Isoleucine | 1.586 | 0.0147 | Alanine | 1.311 | 0.0269 | Tyramine * | 1.325 | 0.0210 |
| 11 | Sucrose | 1.523 | 0.0292 | Glutamine | 1.302 | 0.0369 | Glucose | 1.323 | 0.0235 |
| 12 | Betalamic Acid | 1.466 | 0.0439 | Tryptophan | 1.265 | 0.0393 | Glycoside | 1.322 | 0.0221 |
| 13 | Fucose | 1.448 | 0.0497 | Citramalic Acid * | 1.067 | 0.0306 | Isoleucine | 1.311 | 0.0272 |
| 14 | NA | NA | NA | NA | NA | NA | Tryptamine * | 1.298 | 0.0322 |
| 15 | NA | NA | NA | NA | NA | NA | Citric Acid | 1.295 | 0.0004 |
| 16 | NA | NA | NA | NA | NA | NA | Mannose | 1.283 | 0.0376 |
| 17 | NA | NA | NA | NA | NA | NA | Phyllocatin | 1.274 | 0.0414 |
| 18 | NA | NA | NA | NA | NA | NA | Citramalic Acid * | 1.239 | 0.0160 |
| 19 | NA | NA | NA | NA | NA | NA | GABA | 1.168 | 0.0342 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Xu, J.; He, Y.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules 2019, 24, 1114. https://doi.org/10.3390/molecules24061114
Wu Y, Xu J, He Y, Shi M, Han X, Li W, Zhang X, Wen X. Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules. 2019; 24(6):1114. https://doi.org/10.3390/molecules24061114
Chicago/Turabian StyleWu, Yawei, Juan Xu, Yizhong He, Meiyan Shi, Xiumei Han, Wenyun Li, Xingwu Zhang, and Xiaopeng Wen. 2019. "Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation" Molecules 24, no. 6: 1114. https://doi.org/10.3390/molecules24061114
APA StyleWu, Y., Xu, J., He, Y., Shi, M., Han, X., Li, W., Zhang, X., & Wen, X. (2019). Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules, 24(6), 1114. https://doi.org/10.3390/molecules24061114







