A Comparative Study on the Effects of Different Parts of Panax ginseng on the Immune Activity of Cyclophosphamide-Induced Immunosuppressed Mice
Abstract
1. Introduction
2. Results
2.1. Effect of Different Parts of Ginseng on Body Weight and Immune Organ Indices of Mice
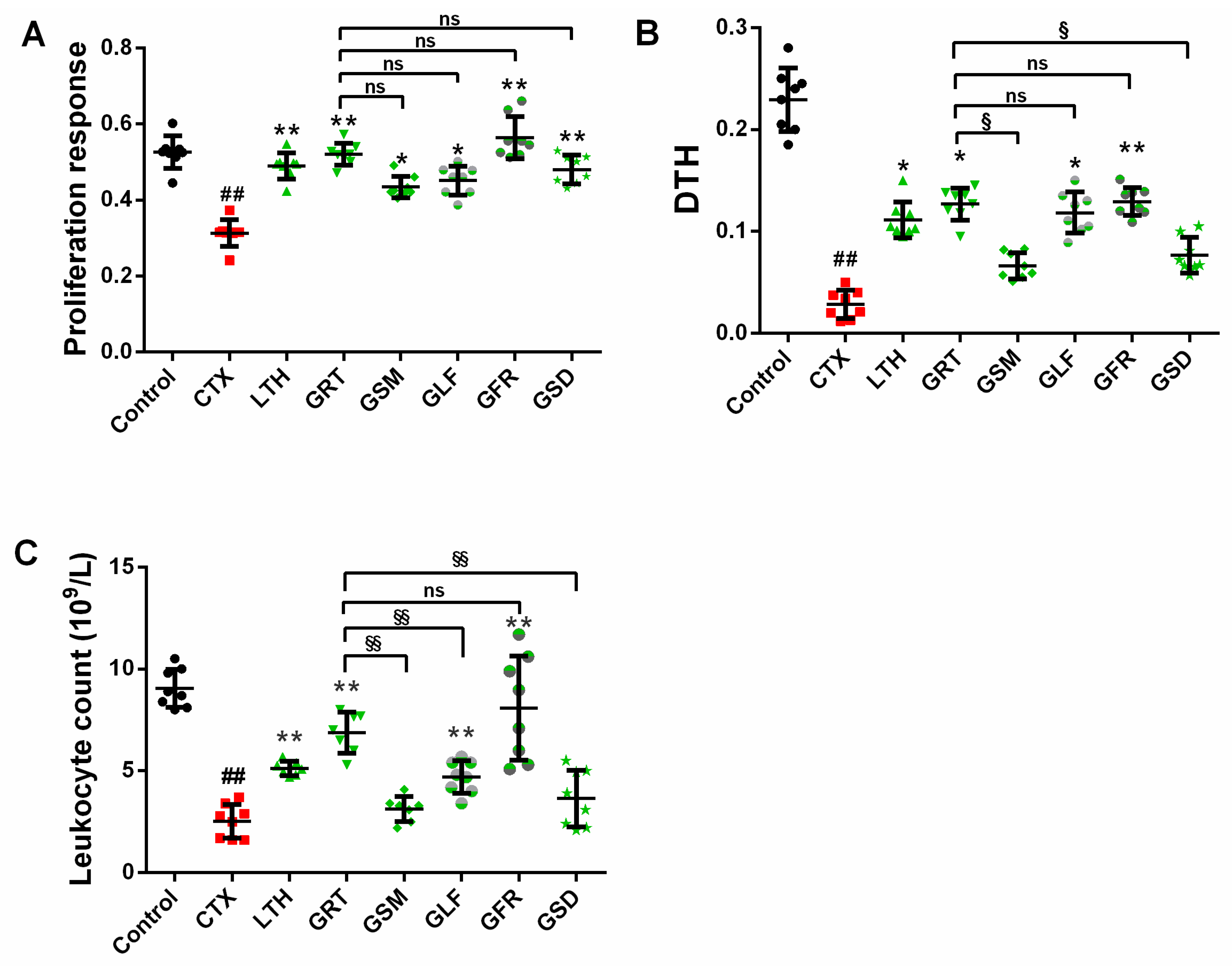
2.2. Effect of Different Parts of Ginseng on Cellular Immunity
2.2.1. Effect of Different Parts of Ginseng on the Concanavalin A (ConA)-Induced Splenocyte Proliferation
2.2.2. Effect of Different Parts of Ginseng on Delayed-Type Hypersensitivity (DTH) to Sheep Red Blood Cells (SRBC)
2.3. Effect of Different Parts of Ginseng on Leukocyte Count
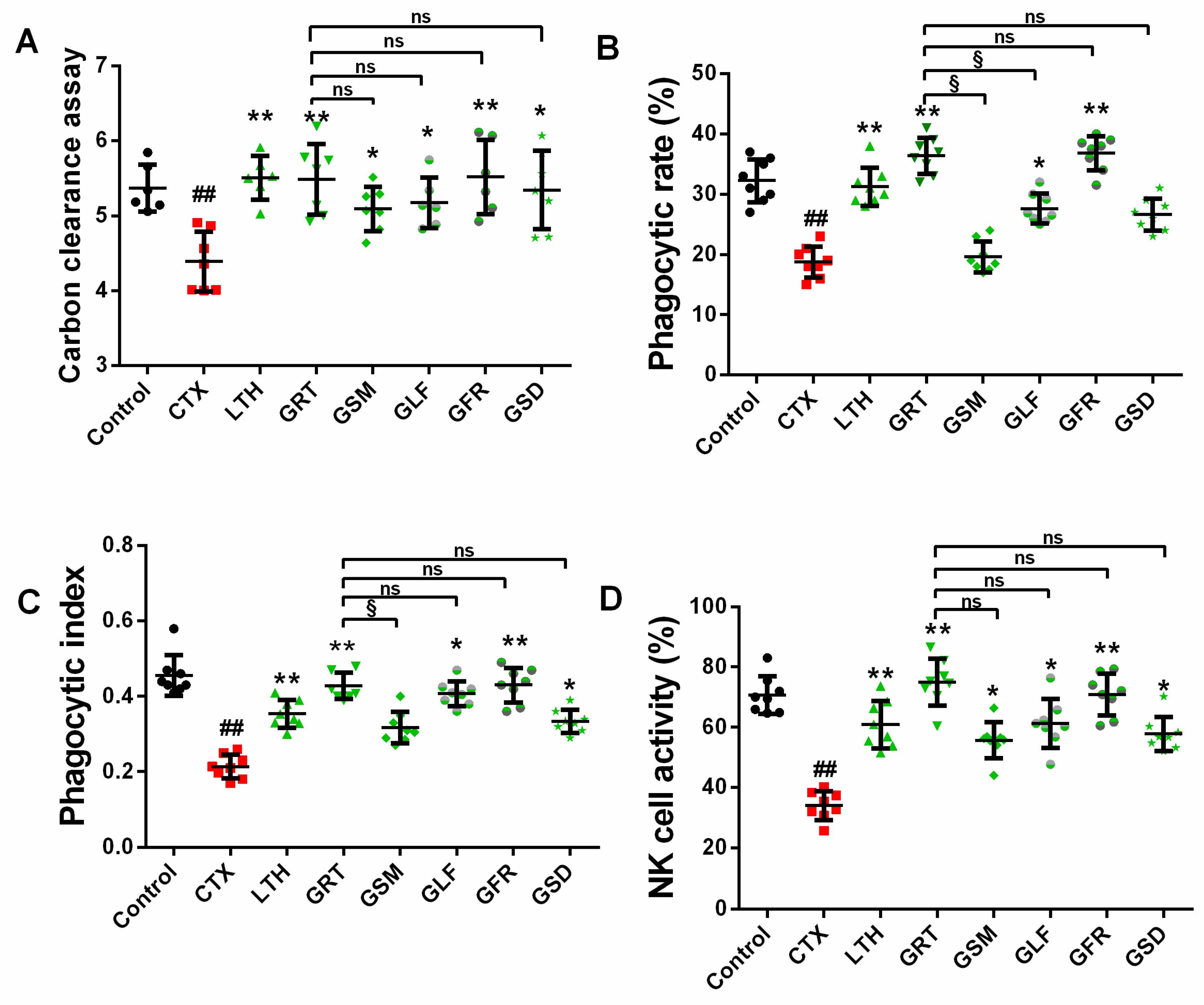
2.4. Effect of Different Parts of Ginseng on Macrophage Phagocytosis
2.4.1. Effect of Different Parts of Ginseng on Carbon Granular Clearance Assay
2.4.2. Effect of Different Parts of Ginseng on the Phagocytic Capacity of Macrophage Cells
2.5. Effects of Different Parts of Ginseng on Splenic NK Cell Activity
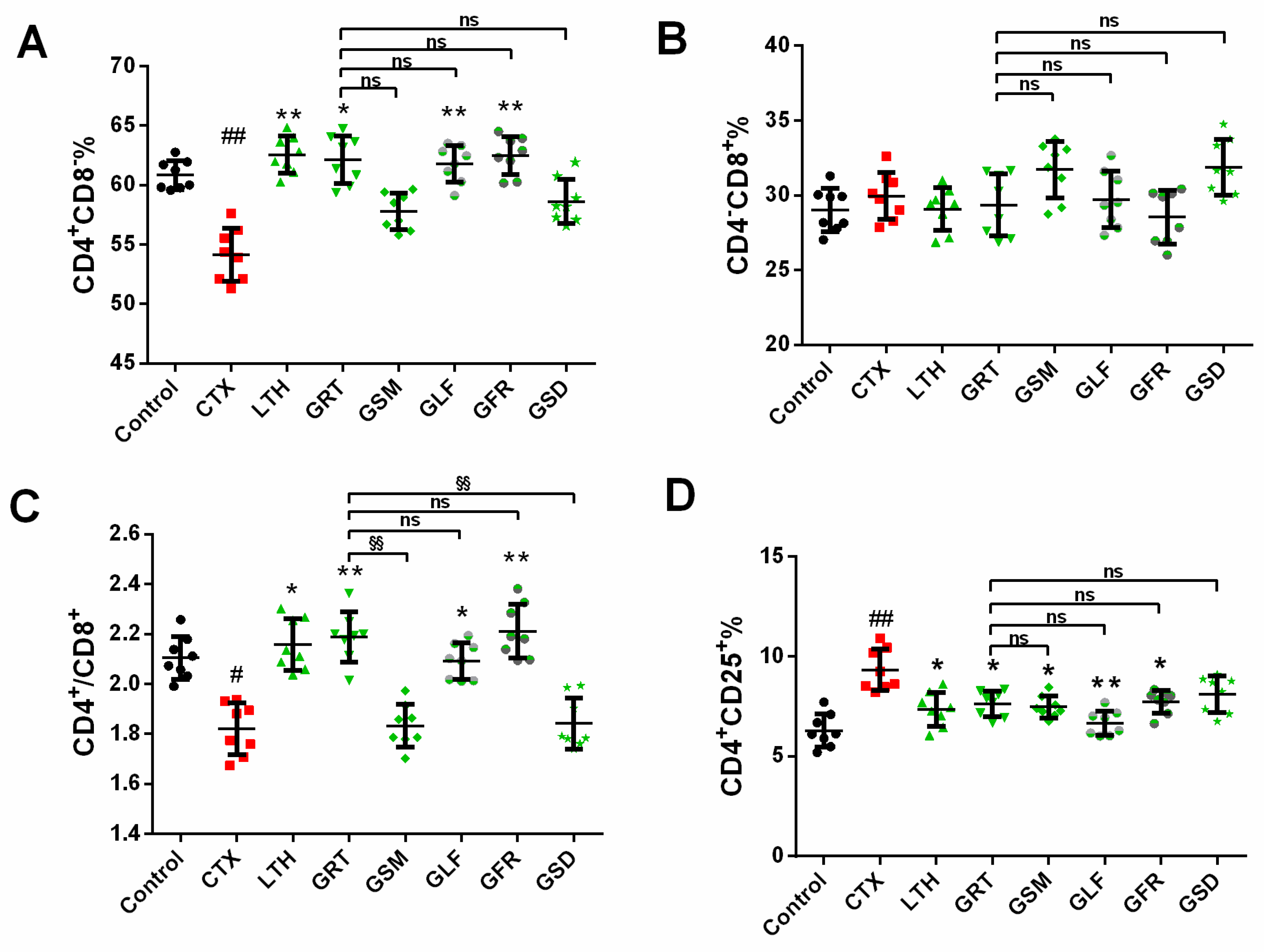
2.6. Effect of Different Parts of Ginseng on Splenic T-Lymphocyte Subpopulations
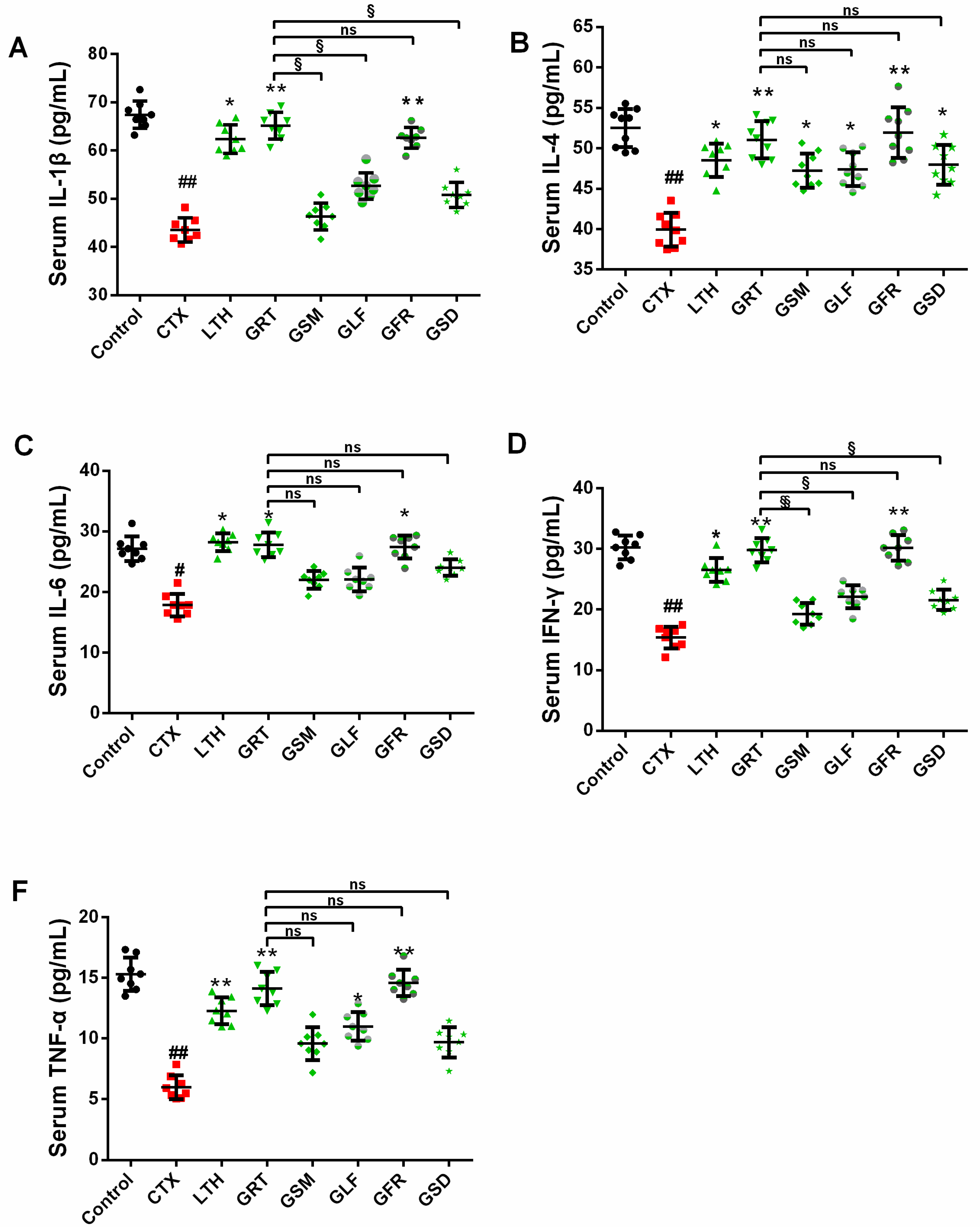
2.7. Effects of Different Parts of Ginseng on Cytokine Concentrations in Serum
2.8. Effects of Different Parts of Ginseng on the Nrf2, HO-1, NQO1, SOD1, SOD2, CAT Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Ginseng Extracts of Different Parts
4.3. Experimental Animal and Treatment
4.4. Statistical Analysis
4.5. Experimental Assays
4.5.1. SRBC-Induced DTH
4.5.2. Carbon Granular Clearance Assay
4.5.3. Phagocytic Function of Peritoneal Macrophage
4.5.4. Splenocyte Proliferation Assay and Splenic NK Cell Activity Assay
4.5.5. Mouse Leukocyte Assay and Splenic T-Lymphocyte Subpopulations Assay
4.5.6. The Determination of Cytokines in Serum by ELISA
4.5.7. Western Blotting Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kang, S.; Min, H. Ginseng, the ‘immunity boost’: The effects of Panax ginseng on immune system. J. Ginseng Res. 2012, 36, 354–368. [Google Scholar] [CrossRef]
- Helms, S. Cancer prevention and therapeutics: Panax ginseng. Altern. Med. Rev. 2004, 9, 259–274. [Google Scholar]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharm. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Tang, Y.; Zhao, Z.; Yi, T.; Chen, H. Preparation-related structural diversity and medical potential in the treatment of diabetes mellitus with ginseng pectins. Ann. N. Y. Acad. Sci. 2017, 1401, 75–89. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, Y.; Xu, L.; Qin, M.; Yi, T.; Chen, H.; Zhao, Z. Localization of ginsenosides in the rhizome and root of Panax ginseng by laser microdissection and liquid chromatography–quadrupole/time of flight-mass spectrometry. J. Pharm. Biomed. Anal. 2015, 105, 121–133. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Chen, H.; Brand, E.; Tao, Y.; Qin, M.; Liang, Z. Determination of ginsenosides in Asian and American ginsengs by liquid chromatography–quadrupole/time-of-flight MS: Assessing variations based on morphological characteristics. J. Ginseng Res. 2017, 41, 10–22. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, Z.; Zhou, B.; Fu, S.; Hong, T.; Li, P.; Liu, J. A new ocotillol-type ginsenoside from stems and leaves of Panax quinquefolium L. and its anti-oxidative effect on hydrogen peroxide exposed A549 cells. Nat. Prod. Res. 2018, 1–8. [Google Scholar] [CrossRef]
- Heo, S.B.; Lim, S.W.; Jhun, J.Y.; La Cho, M.; Chung, B.H.; Yang, C.W. Immunological benefits by ginseng through reciprocal regulation of Th17 and Treg cells during cyclosporine-induced immunosuppression. J. Ginseng Res. 2016, 40, 18–27. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L.; Li, Z.; Shao, Z.; Qi, Y.; Gao, K.; Liu, S.; Sun, Y.; Li, P.; Liu, J. Immunomodulatory Effects of (24R)-Pseudo-Ginsenoside HQ and (24S)-Pseudo-Ginsenoside HQ on Cyclophosphamide-Induced Immunosuppression and Their Anti-Tumor Effects Study. Int. J. Mol. Sci. 2019, 20, 836. [Google Scholar] [CrossRef]
- Wong, A.S.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Gu, J.; Meng, Z.J.; Zhao, L.C.; Zheng, Y.N.; Chen, L.; Yang, G.L. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on Type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia 2012, 83, 192–198. [Google Scholar] [CrossRef]
- Seo, E.; Kim, S.; Lee, S.J.; Oh, B.C.; Jun, H.S. Ginseng berry extract supplementation improves age-related decline of insulin signaling in mice. Nutrients 2015, 7, 3038–3053. [Google Scholar] [CrossRef]
- Qi, Z.; Li, W.; Tan, J.; Wang, C.; Lin, H.; Zhou, B.; Liu, J.; Li, P. Effect of ginsenoside Rh2 on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine 2019, 152862, 1–32. [Google Scholar] [CrossRef]
- Chen, L.; Dai, J.; Wang, Z.; Zhang, H.; Huang, Y.; Zhao, Y. The Antidepressant Effects of Ginseng Total Saponins in Male C57BL/6 N Mice by Enhancing Hippocampal Inhibitory Phosphorylation of GSK-3β. Phytother. Res. 2014, 28, 1102–1106. [Google Scholar] [CrossRef]
- Murphy, L.L.; LEE, T.J.F. Ginseng, sex behavior, and nitric oxide. Ann. N. Y. Acad. Sci. 2002, 962, 372–377. [Google Scholar] [CrossRef]
- Caso, A.M.; Vargas, R.R.; Salas, A.V.; Begoña, C.I. Double-blind study of a multivitamin complex supplemented with ginseng extract. Drug Exp. Clin. Res. 1996, 22, 323–329. [Google Scholar]
- Kim, M.K.; Lee, J.W.; Lee, K.Y.; Yang, D.C. Microbial conversion of major ginsenoside Rb1 to pharmaceutically active minor ginsenoside Rd. J. Microbiol. 2005, 43, 456–462. [Google Scholar]
- Lu, J.M.; Yao, Q.; Chen, C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, S.H.; Yook, H.S. Effect of flower-buds of Panax ginseng fermented by various microorganisms on the activation of T cell immune responses. Food Sci. Biotechnol. 2015, 24, 1061–1067. [Google Scholar] [CrossRef]
- Xie, J.T.; Wang, C.Z.; Wang, A.B.; Wu, J.; Basila, D.; Yuan, C.S. Antihyperglycemic effects of total ginsenosides from leaves and stem of Panax ginseng. Acta Pharm. Sin. 2005, 26, 1104–1110. [Google Scholar] [CrossRef]
- Wang, H.; Peng, D.; Xie, J. Ginseng leaf-stem: Bioactive constituents and pharmacological functions. Chin. Med.-UK 2009, 4, 20. [Google Scholar] [CrossRef]
- Zhai, L.; Li, Y.; Wang, W.; Wang, Y.; Hu, S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine 2011, 29, 5007–5014. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Wu, C.F.; Duan, L.; Yang, J.Y. Protective effects of total saponins from stem and leaf of Panax ginseng against cyclophosphamide-induced genotoxicity and apoptosis in mouse bone marrow cells and peripheral lymphocyte cells. Food Chem. Toxicol. 2008, 46, 293–302. [Google Scholar] [CrossRef]
- Pyo, M.K.; Choi, S.H.; Shin, T.J.; Hwang, S.H.; Lee, B.H.; Kang, J.; Kim, H.J.; Lee, S.H.; Nah, S.Y. A simple method for the preparation of crude gintonin from ginseng root, stem, and leaf. J. Ginseng Res. 2011, 35, 209–218. [Google Scholar] [CrossRef]
- Choi, J.E.; Li, X.; Han, Y.H.; Lee, K.T. Changes of saponin contents of leaves, stems and flower-buds of Panax ginseng CA Meyer by harvesting days. Korean J. Med. Crop Sci. 2009, 17, 251–256. [Google Scholar]
- Kim, G.W.; Jo, H.K.; Chung, S.H. Ginseng seed oil ameliorates hepatic lipid accumulation in vitro and in vivo. J. Ginseng Res. 2018, 42, 419–428. [Google Scholar] [CrossRef]
- Than, N.N.; Newsome, P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis 2015, 239, 192–202. [Google Scholar] [CrossRef]
- Quan, F.S.; Compans, R.W.; Cho, Y.K.; Kang, S.M. Ginseng and Salviae herbs play a role as immune activators and modulate immune responses during influenza virus infection. Vaccine 2007, 25, 272–282. [Google Scholar] [CrossRef]
- Turk, J.; Parker, D. Effect of cyclophosphamide on immunological control mechanisms. Immunol. Rev. 1982, 65, 99–113. [Google Scholar] [CrossRef]
- Cho, C.W.; Han, C.J.; Rhee, Y.K.; Lee, Y.C.; Shin, K.S.; Shin, J.S.; Lee, K.T.; Hong, H.D. Cheonggukjang polysaccharides enhance immune activities and prevent cyclophosphamide-induced immunosuppression. Int. J. Biol. Macromol. 2015, 72, 519–525. [Google Scholar] [CrossRef]
- Lin, X.; Yin, L.; Gao, R.; Liu, Q.; Xu, W.; Jiang, X.; Chong, B.H. The effects of panaxadiol saponins on megakaryocytic maturation and immune function in a mouse model of immune thrombocytopenia. Exp. Hematol. 2015, 43, 364–373. [Google Scholar] [CrossRef]
- Azike, C.G.; Charpentier, P.A.; Hou, J.; Pei, H.; Lui, E.M.K. The Yin and Yang actions of North American ginseng root in modulating the immune function of macrophages. Chin. Med.-UK 2011, 6, 2–11. [Google Scholar] [CrossRef]
- Yin, J.; Jin, H.; Yang, F.; Ding, Z.; Huang, C.; Zhu, Q.; Wang, B. Synergistic effects of adjuvants interferon-γ and levamisole on DNA vaccination against infection with Newcastle disease virus. Viral Immunol. 2007, 20, 288–299. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, T.; Xiu, L.; Shi, X.; Bian, Z.; Zhang, Y.; Ruhan, A.; Wang, X. Levamisole promotes murine bone marrow derived dendritic cell activation and drives Th1 immune response in vitro and in vivo. Int. Immunopharm. 2016, 31, 57–65. [Google Scholar] [CrossRef]
- Ourives, S.; Dos Santos, D.; Alem, L.; Rios-Santos, F.; Fontes, C.; Damazo, A. Analysis of Parasitological and Haematological Parameters and of CD4+ and CD8+ Cell Number in Patients with Plasmodium vivax Malaria. Res. J. Parasitol. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Humeau, C.; Darrouzain, F.; Gouilleux-Gruart, V. T lymphocyte immunophenotyping in bronchoalveolar lavage on AQUIOS CL® cytometer. J. Immunol. Methods 2018, 461, 91–99. [Google Scholar] [CrossRef]
- Huang, M.; Yang, D.; Xiang, M.; Wang, J. Role of interleukin-6 in regulation of immune responses to remodeling after myocardial infarction. Heart Fail. Rev. 2015, 20, 25–38. [Google Scholar] [CrossRef]
- Almughamsi, H.; Whalen, M.M. Hexabromocyclododecane and tetrabromobisphenol A alter secretion of interferon gamma (IFN-γ) from human immune cells. Arch. Toxicol. 2016, 90, 1695–1707. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Yi, R.; Li, G.; Sun, P.; Qian, Y.; Zhao, X. Immunomodulatory effect of tremella polysaccharides against cyclophosphamide-induced immunosuppression in mice. Molecules 2018, 23, 239. [Google Scholar] [CrossRef]
- Kuwahara, M.; Ise, W.; Ochi, M.; Suzuki, J.; Kometani, K.; Maruyama, S.; Izumoto, M.; Matsumoto, A.; Takemori, N.; Takemori, A. Bach2–Batf interactions control Th2-type immune response by regulating the IL-4 amplification loop. Nat. Commun. 2016, 7, 12596. [Google Scholar] [CrossRef]
- Kaushal, P.; Lu, Z.; Rajesh, S.; Fuhler, G.M.; J Jasper, D.; Tjasso, B.; Anouk, R.; Kuipers, E.J.; Weersma, R.K.; Nuij, V.J. Suppression of p21Rac signaling and increased innate immunity mediate remission in Crohn’s disease. Sci. Transl. Med. 2014, 6, 233ra53. [Google Scholar]
- Tschurtschenthaler, M.; Adolph, T.E.; Ashcroft, J.W.; Niederreiter, L.; Bharti, R.; Saveljeva, S.; Bhattacharyya, J.; Flak, M.B.; Shih, D.Q.; Fuhler, G.M.; et al. Defective ATG16L1-mediated removal of IRE1alpha drives Crohn’s disease-like ileitis. J. Exp. Med. 2017, 214, 401–422. [Google Scholar] [CrossRef]
- Sun, J.; Hu, S.; Song, X. Adjuvant effects of protopanaxadiol and protopanaxatriol saponins from ginseng roots on the immune responses to ovalbumin in mice. Vaccine 2007, 25, 1114–1120. [Google Scholar] [CrossRef]
- He, L.X.; Ren, J.W.; Liu, R.; Chen, Q.H.; Zhao, J.; Wu, X.; Zhang, Z.F.; Wang, J.B.; Pettinato, G.; Li, Y. Ginseng (Panax ginseng Meyer) oligopeptides regulate innate and adaptive immune responses in mice via increased macrophage phagocytosis capacity, NK cell activity and Th cells secretion. Food Funct. 2017, 8, 3523–3532. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Hsu, W.H.; Lee, B.H.; Huang, Y.C.; Hsu, Y.W.; Pan, T.M. Ankaflavin, a novel Nrf-2 activator for attenuating allergic airway inflammation. Free Radic. Biol. Med. 2012, 53, 1643–1651. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, S.H.; Chae, S.W.; Soung, N.K.; Oh, M.J.; Jin, S.K.; Kim, Y.O.; Chae, H.J. Aqueous ginseng extract has a preventive role in RANKL-induced osteoclast differentiation and estrogen deficiency-induced osteoporosis. J. Funct. Foods 2015, 13, 192–203. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds ginseng root, leaf, stem, flower and seed are available from the authors. |

| Group | Initial Body Weight (g) | Final Body Weight (g) | Spleen Index (mg/g) | Thymus Index (mg/g) |
|---|---|---|---|---|
| (n = 10) | (n = 10) | (n = 10) | (n = 10) | |
| Control | 19.84 ± 0.66 | 27.00 ± 1.41 | 4.16 ± 0.34 | 1.86 ± 0.12 |
| CTX | 22.07 ± 0.82 | 24.33 ± 1.28 # | 1.79 ± 0.21 ## | 0.29 ± 0.05 ## |
| LTH | 22.62 ± 1.22 | 26.29 ± 1.00 * | 2.11 ± 0.30 | 0.33 ± 0.12 |
| GRT | 22.27 ± 1.38 | 24.92 ± 2.04 | 2.29 ± 0.26 ** | 0.49 ± 0.17 * |
| GSM | 20.15 ± 1.51 | 23.12 ± 0.88 | 2.01 ± 0.18 | 0.29 ± 0.08 |
| GLF | 22.53 ± 2.23 | 24.62 ± 3.53 | 2.14 ± 0.21 * | 0.39 ± 0.06 * |
| GFR | 21.33 ± 1.76 | 23.58 ± 3.38 | 2.31 ± 0.23 * * | 0.49 ± 0.23 * |
| GSD | 23.98 ± 1.81 | 27.36 ± 2.70 * | 2.03 ± 0.25 | 0.45 ± 0.12 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-x.; Qi, Y.-l.; Qi, Z.; Gao, K.; Gong, R.-z.; Shao, Z.-j.; Liu, S.-x.; Li, S.-s.; Sun, Y.-s. A Comparative Study on the Effects of Different Parts of Panax ginseng on the Immune Activity of Cyclophosphamide-Induced Immunosuppressed Mice. Molecules 2019, 24, 1096. https://doi.org/10.3390/molecules24061096
Chen L-x, Qi Y-l, Qi Z, Gao K, Gong R-z, Shao Z-j, Liu S-x, Li S-s, Sun Y-s. A Comparative Study on the Effects of Different Parts of Panax ginseng on the Immune Activity of Cyclophosphamide-Induced Immunosuppressed Mice. Molecules. 2019; 24(6):1096. https://doi.org/10.3390/molecules24061096
Chicago/Turabian StyleChen, Li-xue, Yu-li Qi, Zeng Qi, Kun Gao, Rui-ze Gong, Zi-jun Shao, Song-xin Liu, Shan-shan Li, and Yin-shi Sun. 2019. "A Comparative Study on the Effects of Different Parts of Panax ginseng on the Immune Activity of Cyclophosphamide-Induced Immunosuppressed Mice" Molecules 24, no. 6: 1096. https://doi.org/10.3390/molecules24061096
APA StyleChen, L.-x., Qi, Y.-l., Qi, Z., Gao, K., Gong, R.-z., Shao, Z.-j., Liu, S.-x., Li, S.-s., & Sun, Y.-s. (2019). A Comparative Study on the Effects of Different Parts of Panax ginseng on the Immune Activity of Cyclophosphamide-Induced Immunosuppressed Mice. Molecules, 24(6), 1096. https://doi.org/10.3390/molecules24061096





