Antioxidative and Cardioprotective Effects of Schisandra chinensis Bee Pollen Extract on Isoprenaline-Induced Myocardial Infarction in Rats
Abstract
1. Introduction
2. Results
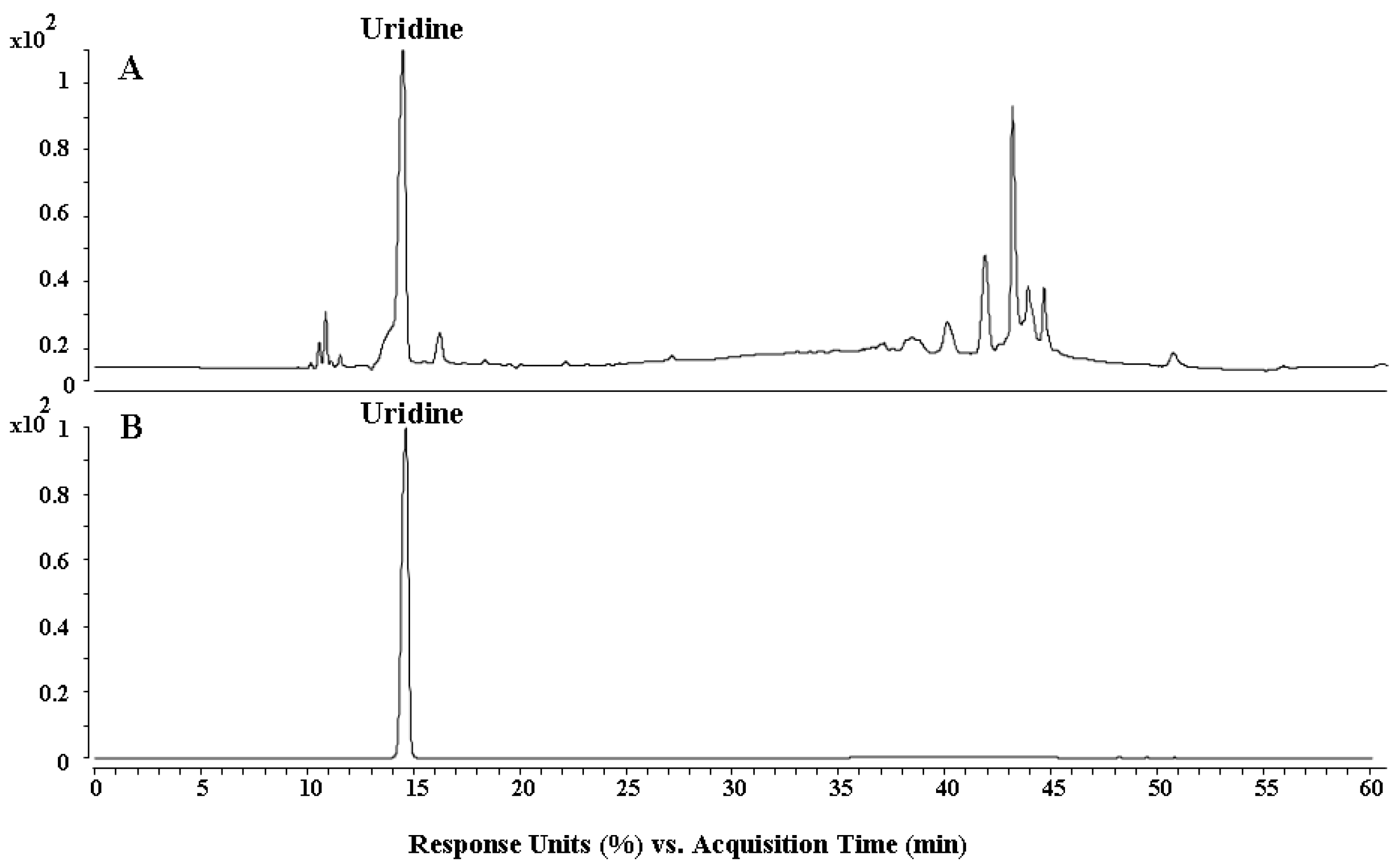
2.1. HPLC Analysis and Antioxidant Activity Analysis In Vitro
2.2. Effects of SCBPE on Serum Cardiac Enzymes in MI Rats Induced by ISO
2.3. Effects of SCBPE on Myocardial Antioxidant Enzyme Activities in MI Rats Induced by ISO
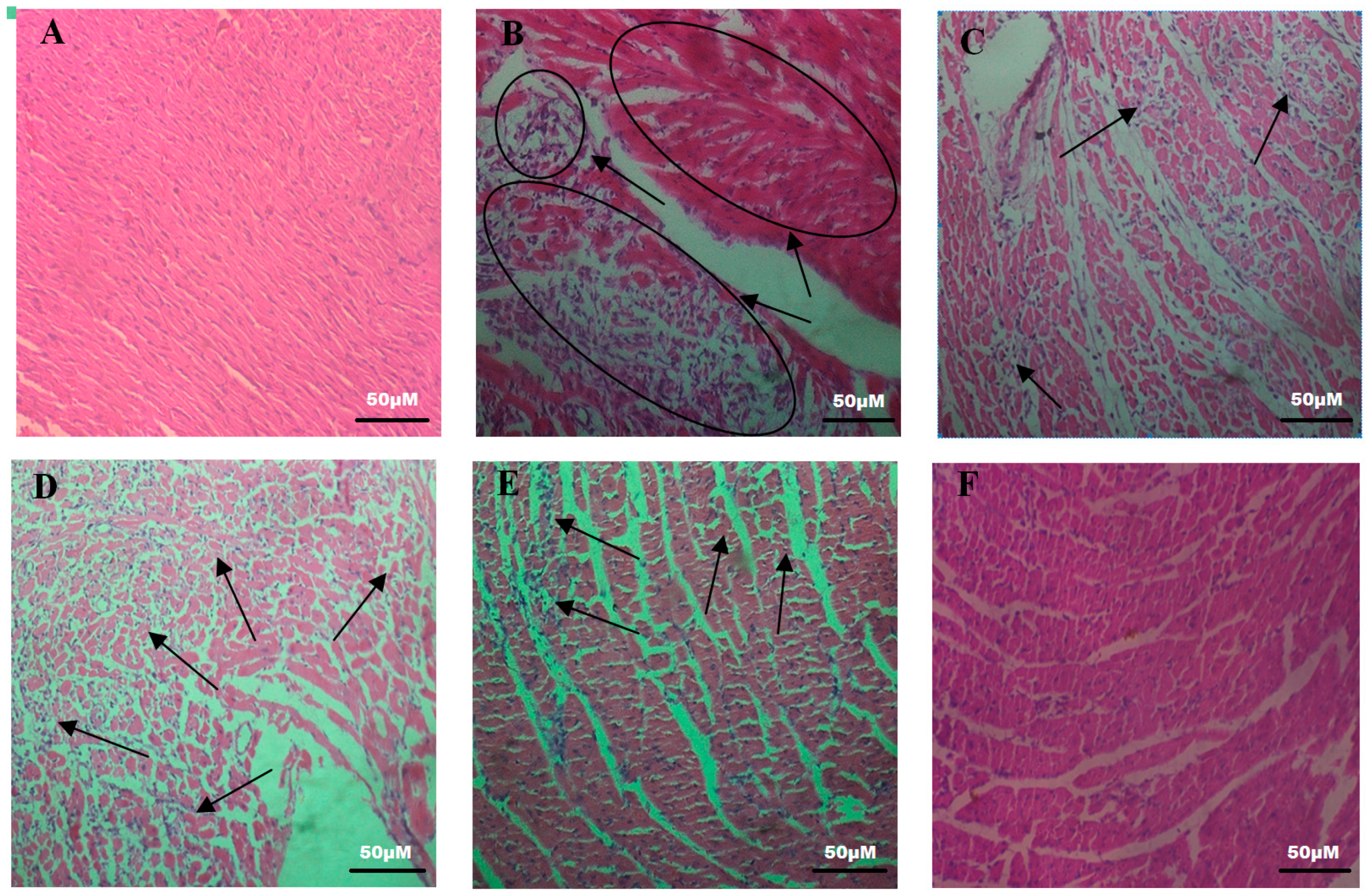
2.4. Effects of SCBPE on the Pathological Changes of Rat Hearts
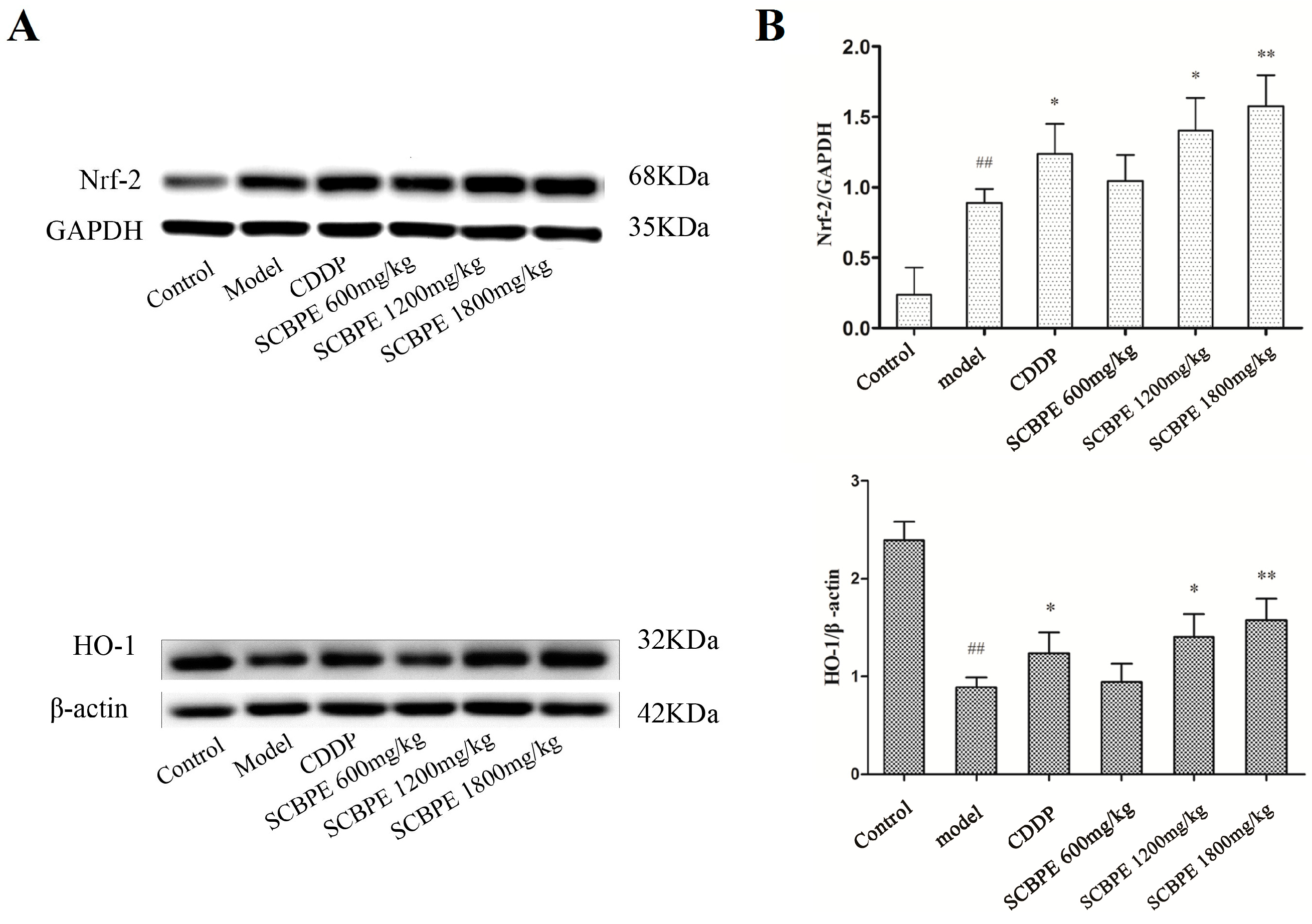
2.5. Effects of SCBPE on the Protein Expression of Nrf-2 and HO-1 in Heart Tissues of MI Rats Induced by ISO
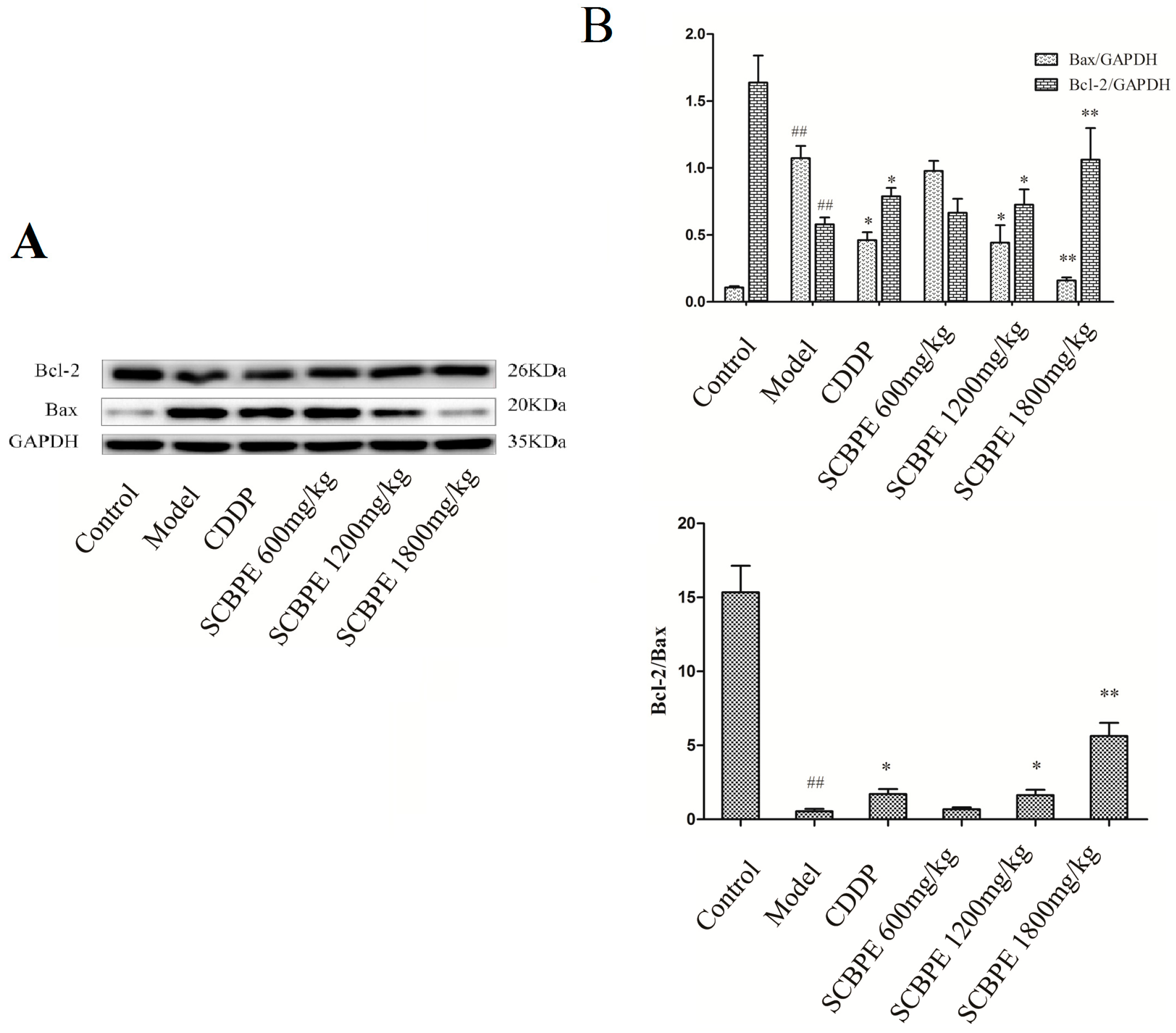
2.6. Effects of SCBPE on the Protein Expression of Bax and Bcl-2 in Heart Tissues of MI Rats Induced by ISO
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Preparation of SCBPE
4.3. HPLC Analysis
4.4. Analysis of Antioxidant Capacity
4.4.1. ABTS Radical Cation Scavenging Activity
4.4.2. FRAP Assay
4.5. Animal Experiments
4.5.1. Experimental Animals
4.5.2. Experimental Design
4.5.3. Biochemical Assay in the Serum
4.5.4. Determination of Antioxidant Parameters in Myocardial Tissues
4.5.5. Histopathological Examinations
4.5.6. Western Blotting Analyses
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third universal definition of myocardial infarction. Circulation 2012, 126, 2020–2035. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.K.; Sarwat, M.; Bhakuni, P.; Tuteja, R.; Tuteja, N. Oxidative stress and ischemic myocardial syndromes. Med. Sci. Monit. 2009, 15. [Google Scholar]
- Bagatini, M.D.; Martins, C.C.; Battisti, V.; Gasparetto, D.; da Rosa, C.S.; Spanevello, R.M.; Ahmed, M.; Schmatz, R.; Schetinger, M.R.; Morsch, V.M. Oxidative stress versus antioxidant defenses in patients with acute myocardial infarction. Heart Vessels 2011, 26, 55–63. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, O.A.; Allam, M.M. Effect of vitamin D on isoprenaline-induced myocardial infarction in rats: Possible role of peroxisome proliferator-activated receptor-γ. Can. J. Physiol. Pharmacol. 2017, 95, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Stanely Mainzen Prince, P.; Sathya, B. Protective effects of quercetin on mitochondrial oxidative stress in isoproterenol induced myocardial infarcted rats: An in vivo and in vitro study. Food Res. Int. 2012, 49, 233–241. [Google Scholar] [CrossRef]
- Upaganlawa, A.; Gandhi, H.; Balaraman, R. Isoproterenol induced myocardial infarction: Protective role of natural products. J. Pharmacol. Toxicol. 2011, 6, 1–17. [Google Scholar] [CrossRef]
- Ganesan, B.; Buddhan, S.; Anandan, R.; Sivakumar, R.; AnbinEzhilan, R. Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Mol. Biol. Rep. 2010, 37, 1319–1327. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Stanely Mainzen Prince, P.; Hidhayath Basha, R. Preventive effects of N-acetyl cysteine on lipids, lipoproteins and myocardial infarct size in isoproterenol induced myocardial infarcted rats: An in vivo and in vitro study. Eur. J. Pharmacol. 2012, 677, 116–122. [Google Scholar] [CrossRef]
- Long, J.; Gao, M.; Kong, Y.; Shen, X.; Du, X.; Son, Y.O.; Shi, X.; Liu, J.; Mo, X. Cardioprotective effect of total paeony glycosides against isoprenaline-induced myocardial ischemia in rats. Phytomedicine 2012, 19, 672–676. [Google Scholar] [CrossRef]
- Patel, K.J.; Panchasara, A.K.; Barvaliya, M.J.; Purohit, B.M.; Baxi, S.N.; Vadgama, V.K.; Tripathi, C.B. Evaluation of cardioprotective effect of aqueous extract of Garcinia indica Linn. fruit rinds on isoprenaline-induced myocardial injury in Wistar albino rats. Res. Pharm. Sci. 2015, 10, 388–396. [Google Scholar]
- Zhang, H.J.; Chen, R.C.; Sun, G.B.; Yang, L.P.; Zhu, Y.D.; Xu, X.D.; Sun, X.B. Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine 2018, 40, 88–97. [Google Scholar] [CrossRef]
- Liu, D.; Chen, L.; Zhao, J.; Cui, K. Cardioprotection activity and mechanism of Astragalus polysaccharide in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Suchal, K.; Bhatia, J.; Malik, S.; Malhotra, R.K.; Gamad, N.; Goyal, S.; Nag, T.C.; Arya, D.S.; Ojha, S. Seabuckthorn pulp oil protects against myocardial ischemia-reperfusion injury in rats through activation of Akt/eNOS. Front. Pharmacol. 2016, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Derbali, A.; Mnafgui, K.; Affes, M.; Derbali, F.; Hajji, R.; Gharsallah, N.; Allouche, N.; El Feki, A. Cardioprotective effect of linseed oil against isoproterenol-induced myocardial infarction in Wistar rats: A biochemical and electrocardiographic study. J. Physiol. Biochem. 2015, 71, 281–288. [Google Scholar] [CrossRef]
- Al-Taweel, A.M.; Raish, M.; Perveen, S.; Fawzy, G.A.; Ahmad, A.; Ansari, M.A.; Mudassar, S.; Ganaie, M.A. Nepeta deflersiana attenuates isoproterenol-induced myocardial injuries in rats: Possible involvement of oxidative stress, apoptosis, inflammation through nuclear factor (NF)-κB downregulation. Phytomedicine 2017, 34, 67–75. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Sakamoto, T.; Araki, Y.; Hara, H. Anti-inflammatory effect of bee pollen ethanol extract from Cistus sp. of Spanish on carrageenan-induced rat hind paw edema. BMC Complement. Altern. Med. 2010, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, G.; Kanbur, M.; Silici, S. Effect of carbaryl on some biochemical changes in rats: The ameliorative effect of bee pollen. Food Chem. Toxicol. 2009, 47, 86–91. [Google Scholar] [CrossRef]
- Wu, Y.D.; Lou, Y.J. A steroid fraction of chloroform extract from bee pollen of Brassica campestris induces apoptosis in human prostate cancer PC-3 cells. Phytother. Res. 2007, 21, 1087–1091. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R.; Suzuki, N.; Tanoue, Y.; Kai, N.; Nagashima, T. Antihypertensive activities of enzymatic hydrolysates from honeybee-collected pollen of Cistus ladaniferus. J. Food Agric. Environ. 2007, 5, 86–89. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wang, K.; Li, C. Antioxidant and tyrosinase inhibitory properties of aqueous ethanol extracts from monofloral bee pollen. J. Apic. Sci. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, J.; Ren, X.; Qin, J. Antioxidant activities of ethanol extracts from ten kinds of bee pollens. Food Sci. 2008, 10, 75–79. [Google Scholar] [CrossRef]
- Patel, V.; Upaganlawar, A.; Zalawadia, R.; Balaraman, R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: A biochemical, electrocardiographic and histoarchitectural evaluation. Eur. J. Pharmacol. 2010, 644, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Sabeena Farvin, K.H.; Anandan, R.; Kumar, S.H.; Shiny, K.S.; Sankar, T.V.; Thankappan, T.K. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol. Res. 2004, 50, 231–236. [Google Scholar] [CrossRef]
- Anandan, R.; Ganesan, B.; Obulesu, T.; Mathew, S.; Kumar, R.S.; Lakshmanan, P.T.; Zynudheen, A.A. Dietary chitosan supplementation attenuates isoprenaline-induced oxidative stress in rat myocardium. Int. J. Biol. Macromol. 2012, 51, 783–787. [Google Scholar] [CrossRef]
- Amran, A.Z.; Jantan, I.; Dianita, R.; Buang, F. Protective effects of the standardized extract of Zingiber officinale on myocardium against isoproterenol-induced biochemical and histopathological alterations in rats. Pharm. Biol. 2015, 53, 1795–1802. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Bai, B.R.; Devaraj, S.N. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. Int. J. Cardiol. 2007, 115, 326–333. [Google Scholar] [CrossRef]
- Selvaraj, P.; Pugalendi, K.V. Hesperidin, a flavanone glycoside, on lipid peroxidation and antioxidant status in experimental myocardial ischemic rats. Redox. Rep. 2010, 15, 217–223. [Google Scholar] [CrossRef]
- Sahu, B.D.; Putcha, U.K.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Carnosic acid promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress and apoptosis in mice. Mol. Cell. Biochem. 2014, 394, 163–176. [Google Scholar] [CrossRef]
- Barančík, M.; Grešová, L.; Barteková, M.; Dovinová, I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol. Res. 2016, 65, S1–S10. [Google Scholar]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef]
- Lakkisto, P.; Palojoki, E.; Bäcklund, T.; Saraste, A.; Tikkanen, I.; Voipio-Pulkki, L.M.; Pulkki, K. Expression of heme oxygenase-1 in response to myocardial infarction in rats. J. Mol. Cell. Cardiol. 2002, 34, 1357–1365. [Google Scholar] [CrossRef]
- Novo, G.; Cappello, F.; Rizzo, M.; Fazio, G.; Zambuto, S.; Tortorici, E.; Marino Gammazza, A.; Corrao, S.; Zummo, G.; De Macario, E.C.; et al. Hsp60 and heme oxygenase-1 (Hsp32) in acute myocardial infarction. Transl. Res. 2011, 157, 285–292. [Google Scholar] [CrossRef]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S.; et al. Graminex pollen: Phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules 2018, 23, 1145. [Google Scholar] [CrossRef]
- Hegstad, A.C.; Ytrehus, K.; Myklebust, R.; Jørgensen, L. Ultrastructural changes in the myocardial myocytic mitochondria: Crucial step in the development of oxygen radical-induced damage in isolated rat hearts? Basic Res. Cardiol. 1994, 89, 128–138. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Kumar, D.; Jugdutt, B.I. Apoptosis and oxidants in the heart. J. Lab. Clin. Med. 2003, 142, 288–297. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: A sample of the uridine is available from the authors. |
| Group | AST (U/L) a | LDH (U/L) a | CK (U/L) a |
|---|---|---|---|
| Control group | 87.00 ± 8.10 | 423.33 ± 129.04 | 382.83 ± 121.11 |
| Model group | 284.30 ± 79.28 ## | 1468.40 ± 416.06 ## | 954.20 ± 353.05 ## |
| CDDP group | 145.50 ± 60.26 * | 856.50 ± 282.77 * | 580.67 ± 218.88 * |
| SCBPE (600 mg/kg) group | 254.80 ± 96.90 | 919.60 ± 314.29 | 757.20 ± 279.17 |
| SCBPE (1200 mg/kg) group | 163.80 ± 73.09 * | 771.33 ± 166.13 * | 604.17 ± 191.42 * |
| SCBPE (1800 mg/kg) group | 146.3 ± 63.13 ** | 560.00 ± 187.45 ** | 409.00 ± 128.36 ** |
| Group | SOD (U/mg prot) a | GSH-Px (U/mg prot) a | CAT (U/mg prot) a |
|---|---|---|---|
| Control group | 263.92 ± 15.69 | 123.72 ± 13.02 | 13.77 ± 1.04 |
| Model group | 155.53 ± 17.71 ## | 78.74 ± 8.78 ## | 7.87 ± 1.59 ## |
| CDDP group | 203.39 ± 19.48 ** | 100.62 ± 4.95 ** | 10.62 ± 1.34 * |
| SCBPE (600 mg/kg) group | 175.25 ± 30.97 | 94.96 ± 8.68 * | 8.59 ± 1.56 |
| SCBPE (1200 mg/kg) group | 221.82 ± 13.92 ** | 98.02 ± 3.87 ** | 10.83 ± 1.61 * |
| SCBPE (1800 mg/kg) group | 226.58 ± 16.64 ** | 105.96 ± 4.66 ** | 12.22 ± 1.89 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Geng, Q.; Huang, H.; Yao, H.; Du, T.; Chen, L.; Wu, Z.; Miao, X.; Shi, P. Antioxidative and Cardioprotective Effects of Schisandra chinensis Bee Pollen Extract on Isoprenaline-Induced Myocardial Infarction in Rats. Molecules 2019, 24, 1090. https://doi.org/10.3390/molecules24061090
Shen Z, Geng Q, Huang H, Yao H, Du T, Chen L, Wu Z, Miao X, Shi P. Antioxidative and Cardioprotective Effects of Schisandra chinensis Bee Pollen Extract on Isoprenaline-Induced Myocardial Infarction in Rats. Molecules. 2019; 24(6):1090. https://doi.org/10.3390/molecules24061090
Chicago/Turabian StyleShen, Zhenhuang, Qianqian Geng, Haibo Huang, Hong Yao, Tianyu Du, Lifu Chen, Zhenhong Wu, Xiaoqing Miao, and Peiying Shi. 2019. "Antioxidative and Cardioprotective Effects of Schisandra chinensis Bee Pollen Extract on Isoprenaline-Induced Myocardial Infarction in Rats" Molecules 24, no. 6: 1090. https://doi.org/10.3390/molecules24061090
APA StyleShen, Z., Geng, Q., Huang, H., Yao, H., Du, T., Chen, L., Wu, Z., Miao, X., & Shi, P. (2019). Antioxidative and Cardioprotective Effects of Schisandra chinensis Bee Pollen Extract on Isoprenaline-Induced Myocardial Infarction in Rats. Molecules, 24(6), 1090. https://doi.org/10.3390/molecules24061090