Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds
Abstract
1. Introduction
2. Results and Discussion
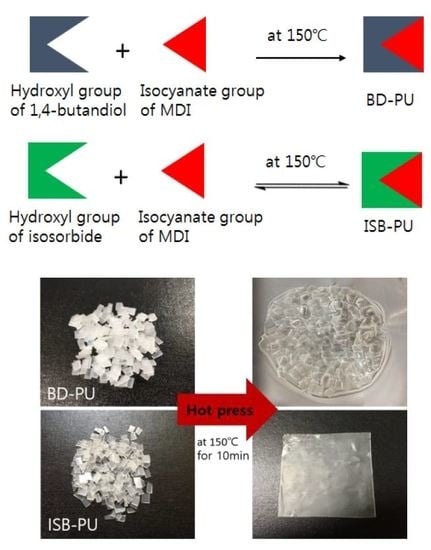
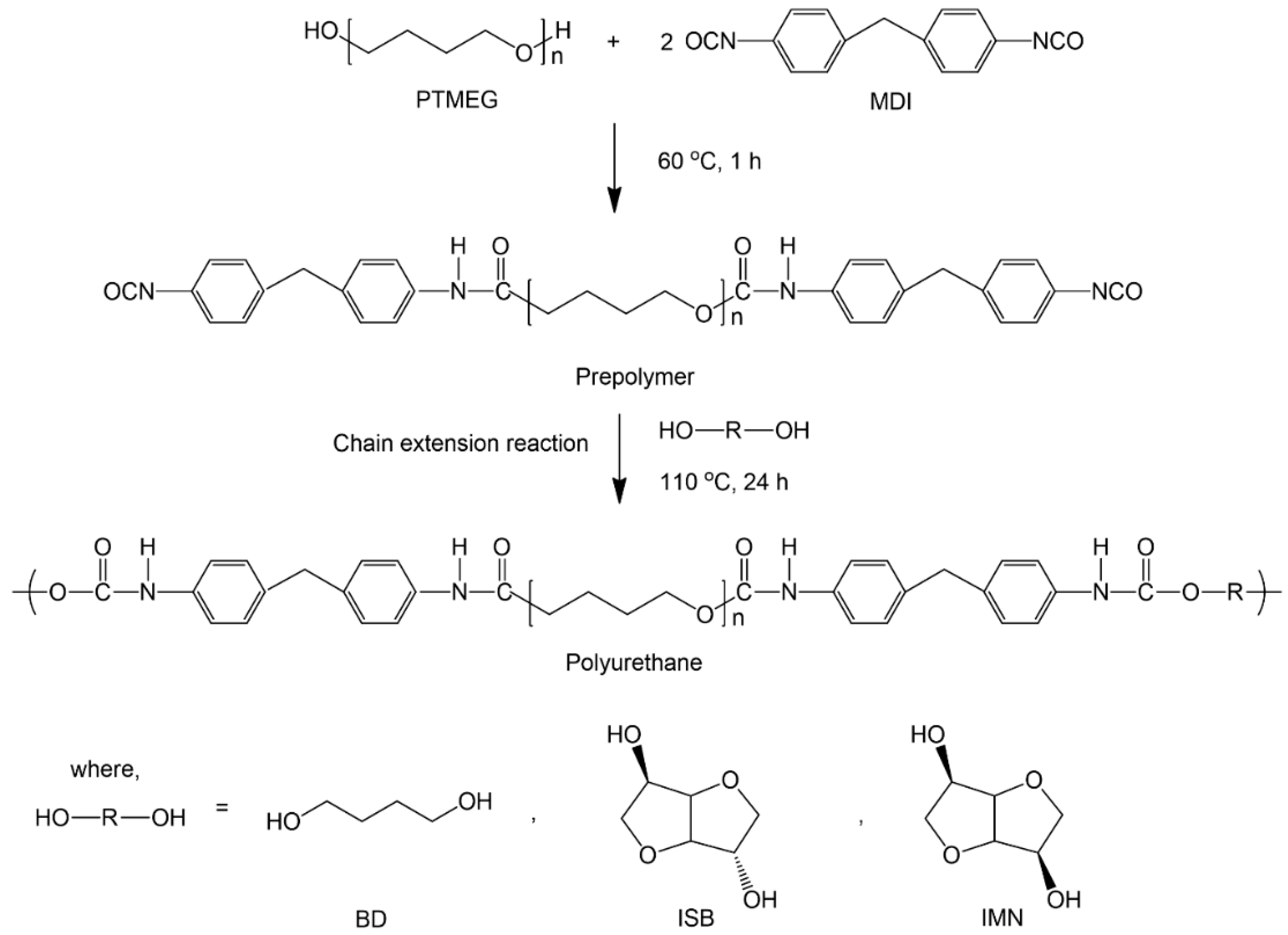
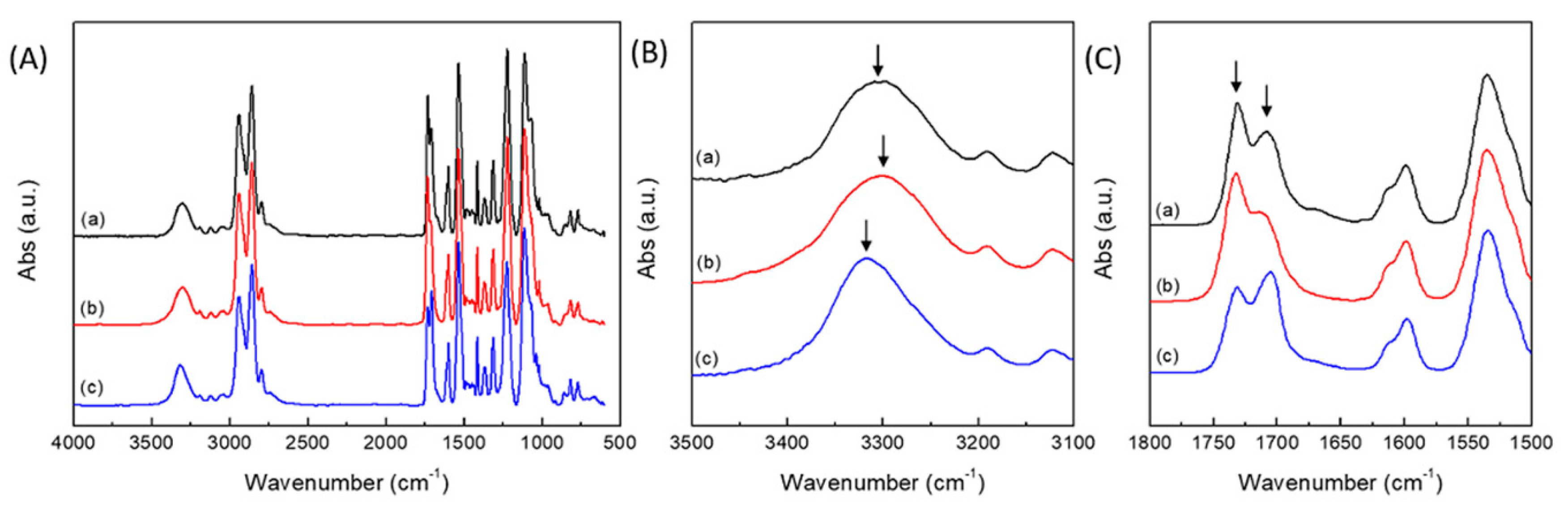
2.1. Synthesis and Characterization of PUEs
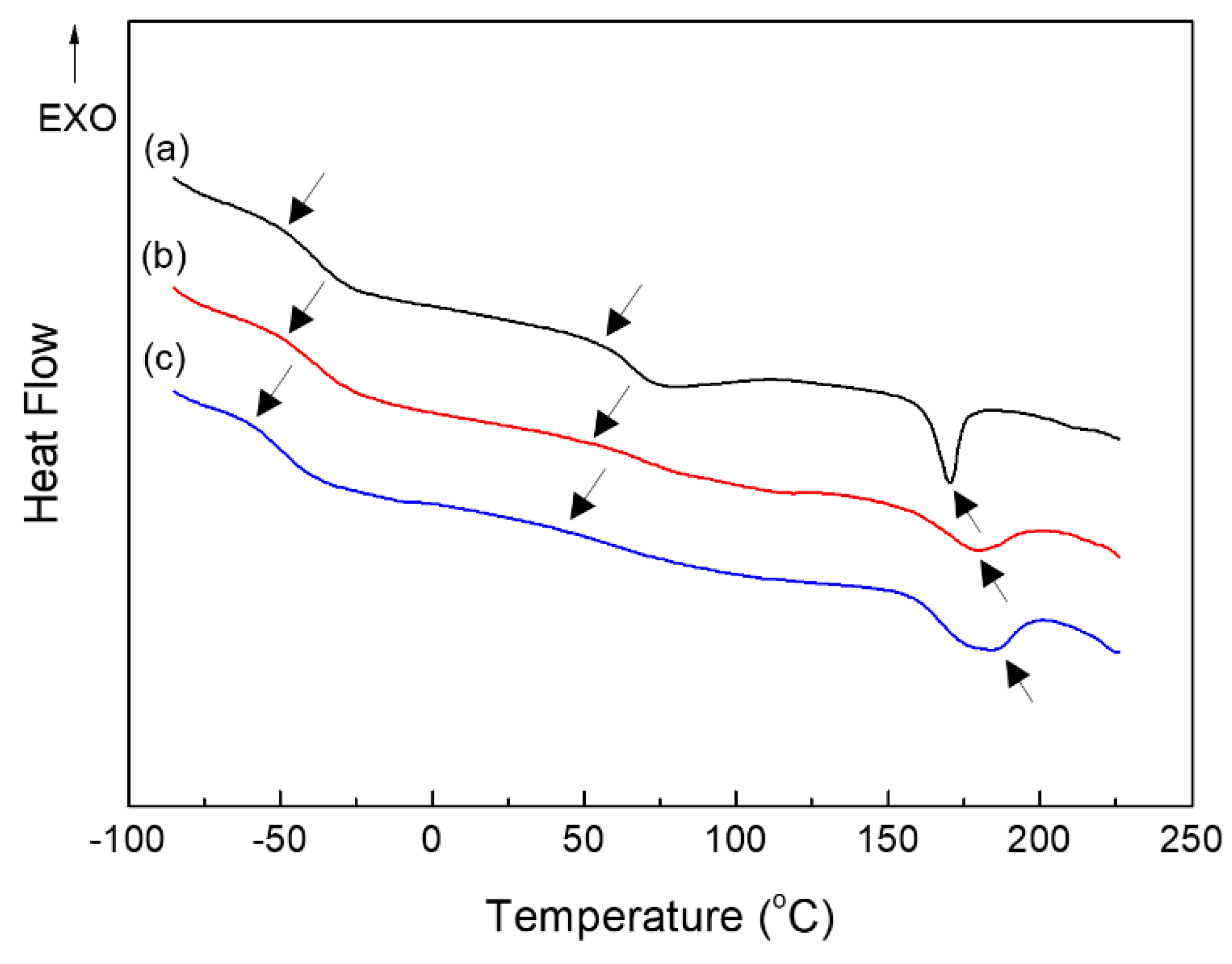
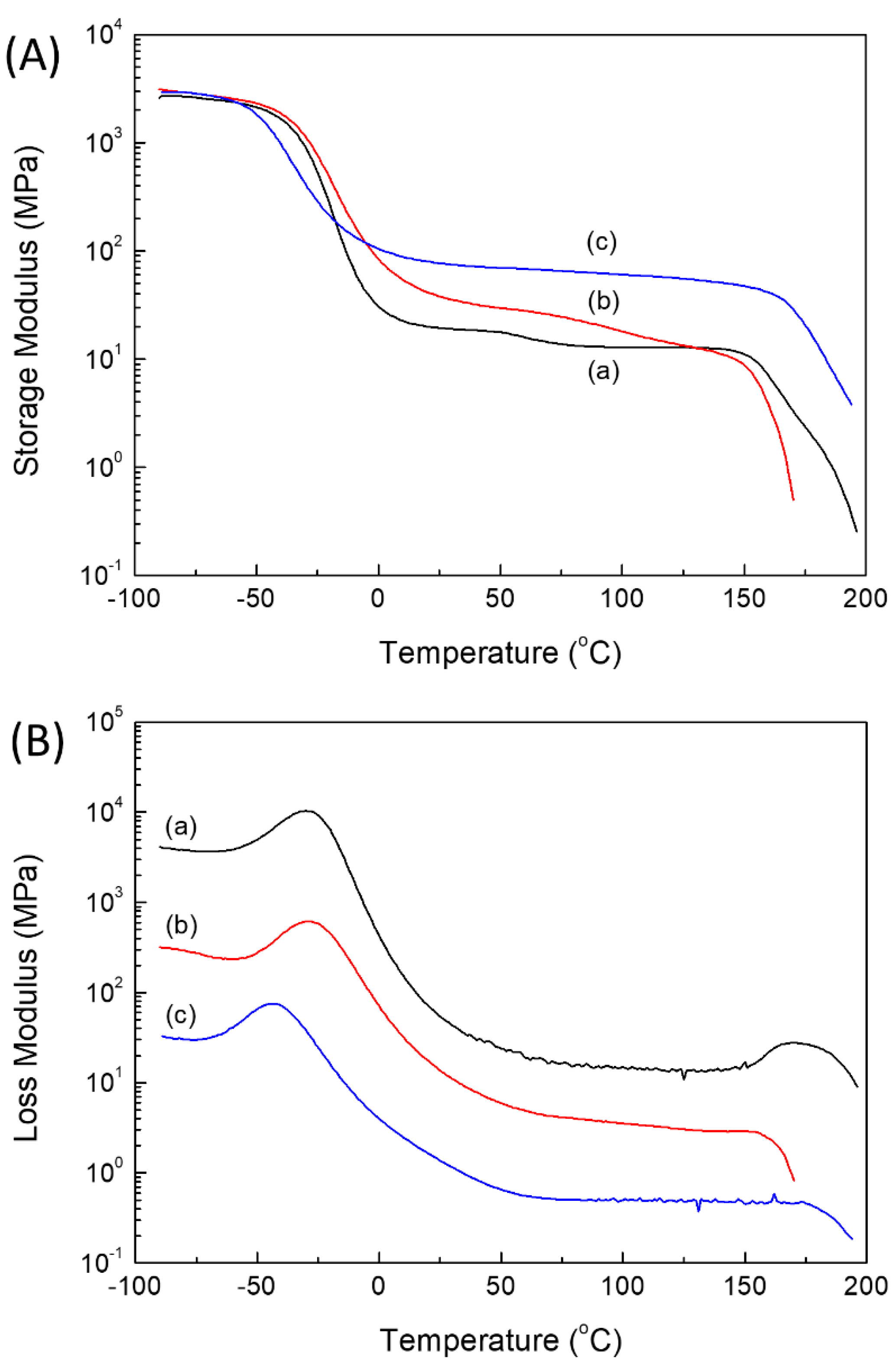
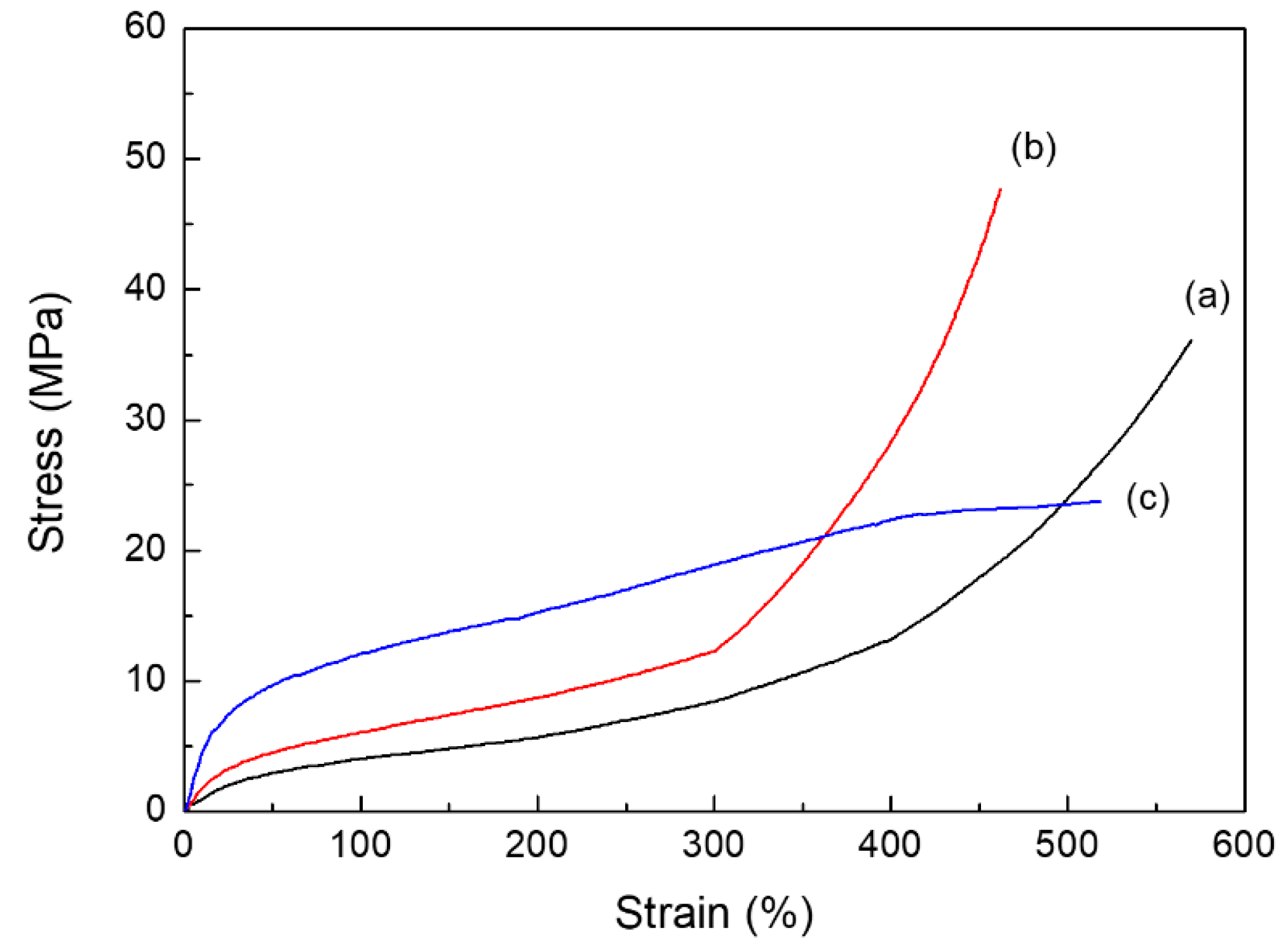
2.2. Thermal and Mechanical Properties of PUEs
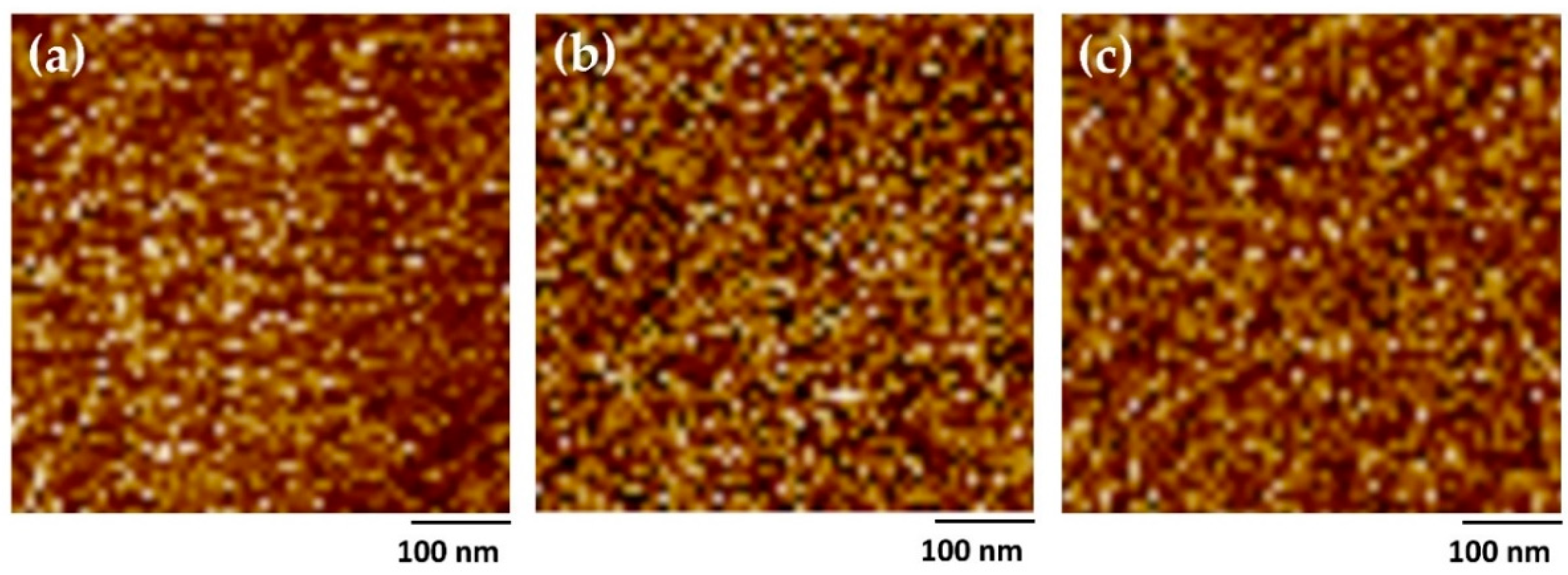
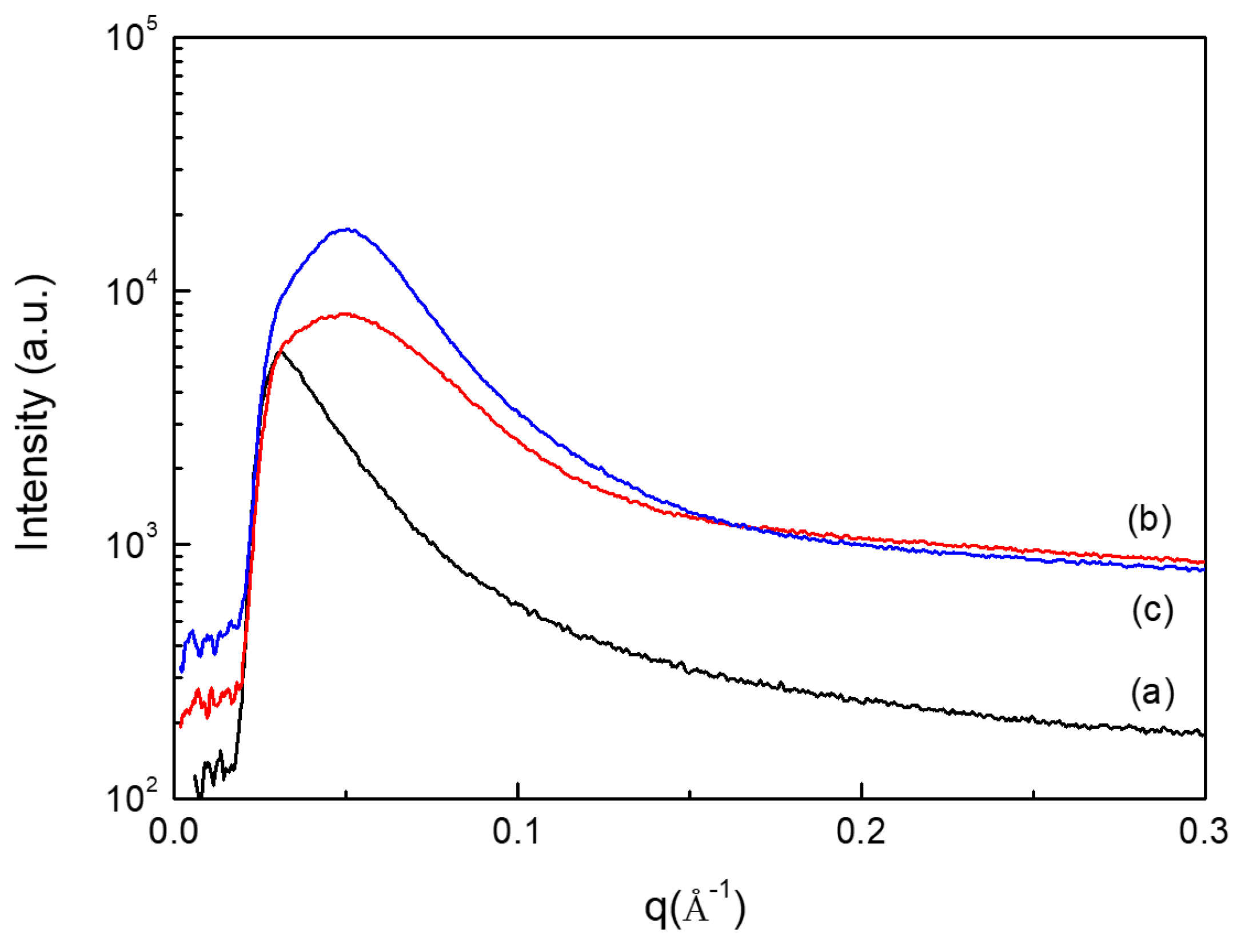
2.3. Morphologies of PUEs
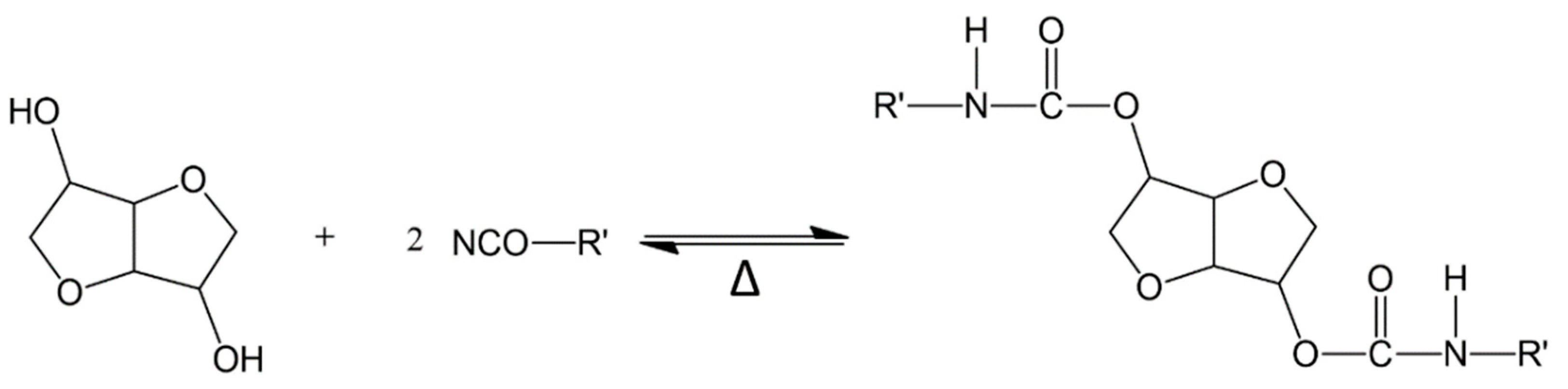
2.4. Reversibility of PUEs
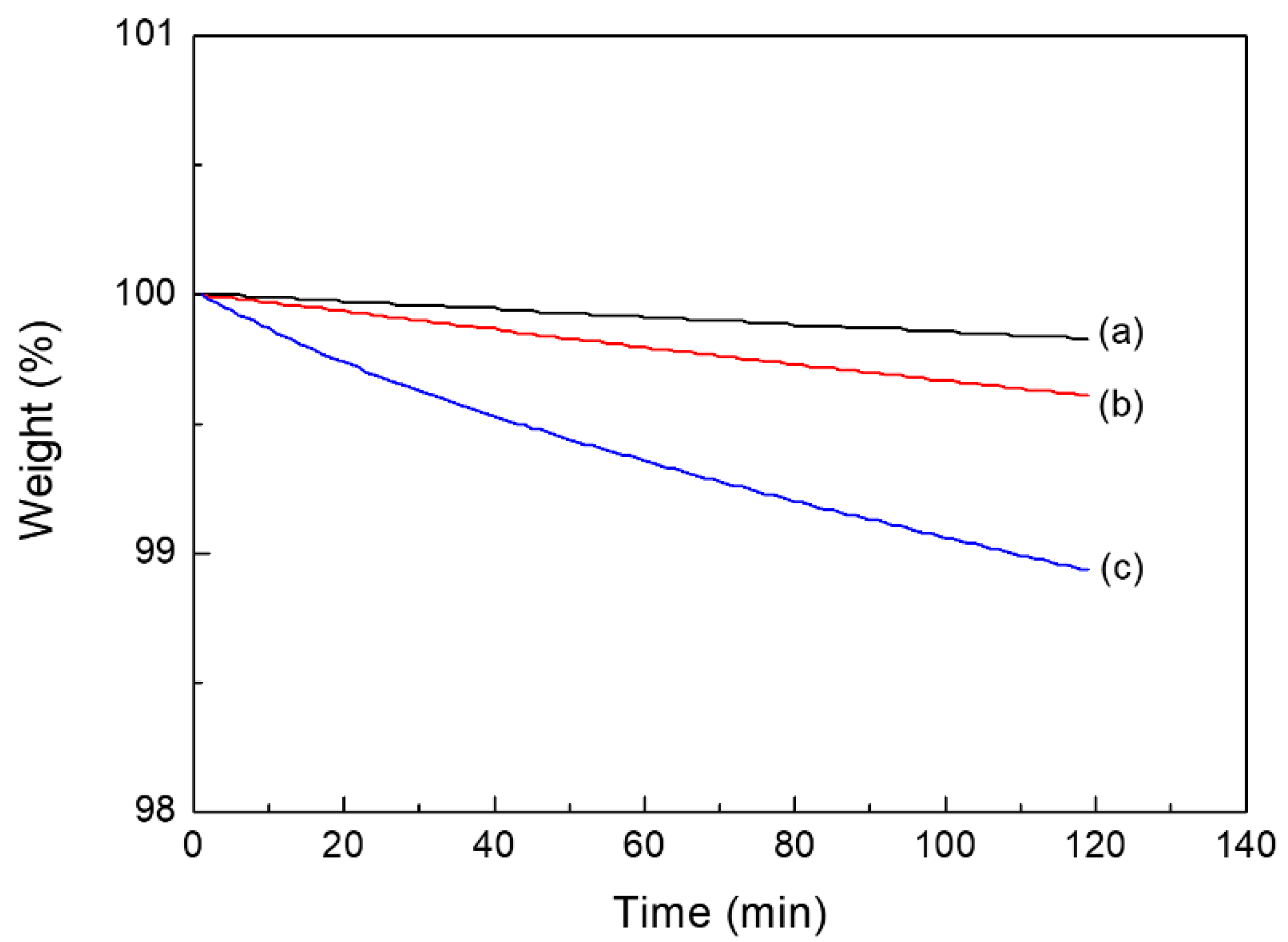
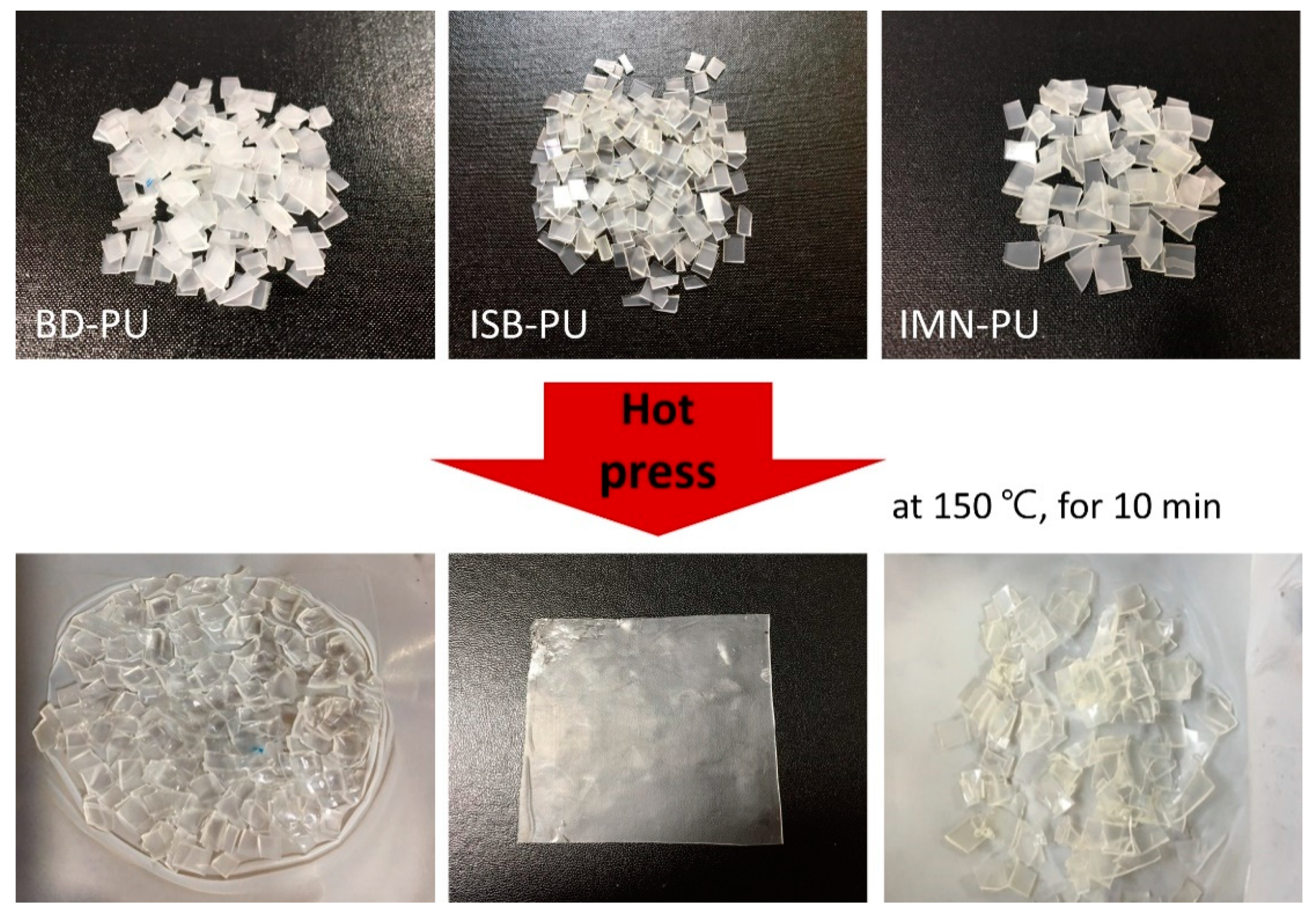
2.5. Reprocessability of ISB-Based PU
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PUEs
4.3. Characterization
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Biesmans, G. Polyurethane Technology & Applications; Lee, S., Ed.; Huntsman International: Everberg, Belgium, 2002. [Google Scholar]
- Hepburn, C. Polyurethane Elastomers, 2nd ed.; Elsevier Applied Science Publishers: London, UK; New York, NY, USA, 1992; ISBN 1581665897. [Google Scholar]
- Eceiza, A.; De La Caba, K.; Kortaberria, G.; Gabilondo, N.; Marieta, C.; Corcuera, M.A.; Mondragon, I. Influence of molecular weight and chemical structure of soft segment in reaction kinetics of polycarbonate diols with 4,4′-diphenylmethane diisocyanate. Eur. Polym. J. 2005, 41, 3051–3059. [Google Scholar] [CrossRef]
- Gisselfält, K.; Helgee, B. Effect of soft segment length and chain extender structure on phase separation and morphology in poly(urethane urea)s. Macromol. Mater. Eng. 2003, 288, 265–271. [Google Scholar] [CrossRef]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Bruchmann, B.; Lehn, J.M. Dynamers: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [Google Scholar] [CrossRef] [PubMed]
- Wicks, Z.W. Bloked isocyanates. Prog. Org. Coat. 1975, 3, 73–99. [Google Scholar] [CrossRef]
- Rolph, M.S.; Markowska, A.L.J.; Warriner, C.N.; O’Reilly, R.K. Blocked isocyanates: From analytical and experimental considerations to non-polyurethane applications. Polym. Chem. 2016, 7, 7351–7364. [Google Scholar] [CrossRef]
- Sankar, G.; Sultan Nasar, A. Effect of isocyanate structure on deblocking and cure reaction of N-methylaniline-blocked diisocyanates and polyisocyanates. Eur. Polym. J. 2009, 45, 911–922. [Google Scholar] [CrossRef]
- Subramani, S.; Park, Y.J.; Lee, Y.S.; Kim, J.H. New development of polyurethane dispersion derived from blocked aromatic diisocyanate. Prog. Org. Coat. 2003, 48, 71–79. [Google Scholar] [CrossRef]
- Cao, S.; Li, S.; Li, M.; Xu, L.; Ding, H.; Xia, J.; Zhang, M.; Huang, K. A thermal self-healing polyurethane thermoset based on phenolic urethane. Polym. J. 2017, 49, 775–781. [Google Scholar] [CrossRef]
- Erice, A.; Ruiz de Luzuriaga, A.; Matxain, J.M.; Ruipérez, F.; Asua, J.M.; Grande, H.J.; Rekondo, A. Reprocessable and recyclable crosslinked poly(urea-urethane)s based on dynamic amine/urea exchange. Polymer 2018, 145, 127–136. [Google Scholar] [CrossRef]
- Zheng, K.; Tian, Y.; Fan, M.; Zhang, J.; Cheng, J. Recyclable, shape-memory, and self-healing soy oil-based polyurethane crosslinked by a thermoreversible Diels–Alder reaction. J. Appl. Polym. Sci. 2018, 135, 1–10. [Google Scholar] [CrossRef]
- Lee, A.; Deng, Y. Green polyurethane from lignin and soybean oil through non-isocyanate reactions. Eur. Polym. J. 2015, 63, 67–73. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Biobased Thermosets Prepared from Rigid Isosorbide and Flexible Soybean Oil Derivatives. ACS Sustain. Chem. Eng. 2017, 5, 774–783. [Google Scholar] [CrossRef]
- Blache, H.; Méchin, F.; Rousseau, A.; Fleury, É.; Pascault, J.P.; Alcouffe, P.; Jacquel, N.; Saint-Loup, R. New bio-based thermoplastic polyurethane elastomers from isosorbide and rapeseed oil derivatives. Ind. Crops Prod. 2018, 121, 303–312. [Google Scholar] [CrossRef]
- Li, C.; Dai, J.; Liu, X.; Jiang, Y.; Ma, S.; Zhu, J. Green Synthesis of a Bio-Based Epoxy Curing Agent from Isosorbide in Aqueous Condition and Shape Memory Properties Investigation of the Cured Resin. Macromol. Chem. Phys. 2016, 217, 1439–1447. [Google Scholar] [CrossRef]
- Charlon, M.; Heinrich, B.; Matter, Y.; Couzigné, E.; Donnio, B.; Avérous, L. Synthesis, structure and properties of fully biobased thermoplastic polyurethanes, obtained from a diisocyanate based on modified dimer fatty acids, and different renewable diols. Eur. Polym. J. 2014, 61, 197–205. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Yokoe, M.; Keigo, A.O.I.; Okada, M. Biodegradable polymers based on renewable resources. VII. Novel random and alternating copolycarbonates from 1,4:3,6-dianhydrohexitols and aliphatic diols. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2312–2321. [Google Scholar] [CrossRef]
- Chatti, S.; Bortolussi, M.; Bogdal, D.; Blais, J.C.; Loupy, A. Microwave-assisted polycondensation of aliphatic diols of isosorbide with aliphatic disulphonylesters via phase-transfer catalysis. Eur. Polym. J. 2004, 40, 561–577. [Google Scholar] [CrossRef]
- Wu, J.; Eduard, P.; Thiyagarajan, S.; Noordover, B.A.J.; Van Es, D.S.; Koning, C.E. Semi-aromatic polyesters based on a carbohydrate-derived rigid diol for engineering plastics. ChemSusChem 2015, 8, 67–72. [Google Scholar] [CrossRef]
- Yoon, W.J.; Hwang, S.Y.; Koo, J.M.; Lee, Y.J.; Lee, S.U.; Im, S.S. Synthesis and characteristics of a biobased high-T g terpolyester of isosorbide, ethylene glycol, and 1,4-cyclohexane dimethanol: Effect of ethylene glycol as a chain linker on polymerization. Macromolecules 2013, 46, 7219–7231. [Google Scholar] [CrossRef]
- Varkey, E.C.; Sreekumar, K. Isosorbide based chiral polyurethanes: Optical and thermal studies. J. Mater. Sci. 2010, 45, 1912–1920. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Kasmi, N.; Tsanaktsis, V.; Doulakas, N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, G.Z. Synthesis and characterization of bio-based polyesters: Poly(2-methyl-1,3-propylene-2,5-furanoate), Poly(isosorbide-2,5-furanoate), Poly(1,4-cyclohexanedimethylene-2,5-furanoate). Materials 2017, 10, 801. [Google Scholar] [CrossRef]
- Gorna, K.; Gogolewski, S. The effect of gamma radiation on molecular stability and mechanical properties of biodegradable polyurethanes for medical applications. Polym. Degrad. Stab. 2003, 79, 465–474. [Google Scholar] [CrossRef]
- Kang, H.; Li, M.; Tang, Z.; Xue, J.; Hu, X.; Zhang, L.; Guo, B. Synthesis and characterization of biobased isosorbide-containing copolyesters as shape memory polymers for biomedical applications. J. Mater. Chem. B 2014, 2, 7877–7886. [Google Scholar] [CrossRef]
- Gorna, K.; Gogolewski, S. Biodegradable porous polyurethane scaffolds for tissue repair and regeneration. Biomed. Mater. Res. Part A 2006, 79, 128–138. [Google Scholar] [CrossRef]
- Dirlikov, S.K.; Schneider, C.J. Polyurethane based on 1;4-3:6 dianhydrohexitol. U.S. Patent 4,443,563A, 17 April 1984. [Google Scholar]
- Javni, I.; Bilić, O.; Bilić, N.; Petrović, Z.S.; Eastwood, E.A.; Zhang, F.; Ilavský, J. Thermoplastic polyurethanes with isosorbide chain extender. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Javni, I.; Bilić, O.; Bilić, N.; Petrović, Z.S.; Eastwood, E.A.; Zhang, F.; Ilavský, J. Thermoplastic polyurethanes with controlled morphology based on methylenediphenyldiisocyanate/isosorbide/butanediol hard segments. Polym. Int. 2015, 64, 1607–1616. [Google Scholar] [CrossRef]
- Cognet-Georjon, E.; Méchin, F.; Pascault, J.P. New polyurethanes based on 4,4′-diphenylmethane diisocyanate and 1,4:3,6 dianhydrosorbitol, 2. Synthesis and properties of segmented polyurethane elastomers. Macromol. Chem. Phys. 1996, 197, 3593–3612. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Luo, M.; Xing, J.; Wu, J.; Pan, H.; Ruan, C.; Luo, Y. Incorporating isosorbide as the chain extender improves mechanical properties of linear biodegradable polyurethanes as potential bone regeneration materials. RSC Adv. 2017, 7, 13886–13895. [Google Scholar] [CrossRef]
- Lim, D.I.; Park, H.S.; Park, J.H.; Knowles, J.C.; Gong, M.S. Application of high-strength biodegradable polyurethanes containing different ratios of biobased isomannide and poly (ε-caprolactone) diol. J. Bioact. Compat. Polym. 2013, 28, 274–288. [Google Scholar] [CrossRef]
- Brunette, C.M.; Hsu, S.L.; MacKnight, W.J. Hydrogen-Bonding Properties of Hard-Segment Model Compounds in Polyurethane Block Copolymers. Macromolecules 1982, 77, 71–77. [Google Scholar] [CrossRef]
- Coleman, M.M.; Skrovanek, D.J.; Hu, J. Hydrogen Bonding in Polymer Blends. 1. FTIR Studies of Urethane-Ether Blends. Macromolecules 1988, 21, 59–65. [Google Scholar] [CrossRef]
- Wang, C.; Feve, M.; Lam, T.M.Y.; Pascault, J.P. FTIR Analysis of Hydrogen Bonding in Amorphous Linear Aromatic Polyurethanes. I. Influence of Temperature. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 1305–1313. [Google Scholar] [CrossRef]
- Wong, C.S.; Badri, K.H. Chemical Analyses of Palm Kernel Oil-Based Polyurethane Prepolymer. Mater. Sci. Appl. 2012, 3, 78–86. [Google Scholar] [CrossRef]
- Tien, Y.I.; Wei, K.H. Hydrogen bonding and mechanical properties in segmented montmorillonite/polyurethane nanocomposites of different hard segment ratios. J. Polym. Sci. 2001, 42, 3213–3221. [Google Scholar] [CrossRef]
- Hanna, J.G.; Siggia, S.; Chemical, O.M. Primary and Secondary Hydroxyl Group Content of Polypropylene Glycols. J. Polym. Sci. 1962, 56, 297–304. [Google Scholar] [CrossRef]
- Kojio, K.; Nakashima, S.; Furukawa, M. Microphase-separated structure and mechanical properties of norbornane diisocyanate-based polyurethanes. Polymer 2007, 48, 997–1004. [Google Scholar] [CrossRef]
- Frick, A.; Rochman, A. Characterization of TPU-elastomers by thermal analysis (DSC). Polym. Test. 2004, 23, 413–417. [Google Scholar] [CrossRef]
- Hossieny, N.; Shaayegan, V.; Ameli, A.; Saniei, M.; Park, C.B. Characterization of hard-segment crystalline phase of thermoplastic polyurethane in the presence of butane and glycerol monosterate and its impact on mechanical property and microcellular morphology. Polymer 2017, 112, 208–218. [Google Scholar] [CrossRef]
- Beniah, G.; Heath, W.H.; Jeon, J.; Torkelson, J.M. Tuning the properties of segmented polyhydroxyurethanes via chain extender structure. J. Appl. Polym. Sci. 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Beniah, G.; Uno, B.E.; Lan, T.; Jeon, J.; Heath, W.H.; Scheidt, K.A.; Torkelson, J.M. Tuning nanophase separation behavior in segmented polyhydroxyurethane via judicious choice of soft segment. Polymer 2017, 110, 218–227. [Google Scholar] [CrossRef]
- Pedrazzoli, D. Understanding phase separation and morphology in thermoplastic polyurethanes nanocomposites. Polymer 2016, 90, 256–263. [Google Scholar] [CrossRef]
- Schön, P.; Bagdi, K.; Molnár, K.; Markus, P.; Pukánszky, B.; Vancso, G.J. Quantitative mapping of elastic moduli at the nanoscale in phase separated polyurethanes by AFM. Eur. Polym. J. 2011, 47, 692–698. [Google Scholar] [CrossRef]
- Leung, L.M.; Koberstein, J.T. Small-angle scattering analysis of hard-microdomain structure and microphase mixing in polyurethane elastomers. J. Polym. Sci. Polym. Phys. Ed. 1985, 23, 1883–1913. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Galembos, A.F.; Leung, L.M. Compression-Molded Polyurethane Block Copolymers. 1. Microdomain Morphology and Thermomechanical Properties. Macromolecules 1992, 25, 6195–6204. [Google Scholar] [CrossRef]
- Nasar, A.S.; Kalaimani, S. Synthesis and studies on forward and reverse reactions of phenol-blocked polyisocyanates: an insight into blocked isocyanates. RSC Adv. 2016, 6, 76802–76812. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Sample Code | Composition (Molar Ratio) | Average Molecular Weight | ||||||
|---|---|---|---|---|---|---|---|---|
| Prepolymer | Chain Extender | Mn | Mw | Ð | ||||
| PTMEG | MDI | BD | ISB | IMN | (g/mol) | (g/mol) | ||
| BD–PU | 1 | 2 | 1 | - | - | 27,000 | 45,000 | 1.67 |
| ISB–PU | 1 | 2 | - | 1 | - | 23,000 | 44,000 | 1.91 |
| IMN–PU | 1 | 2 | - | - | 1 | 16,000 | 34,000 | 2.12 |
| Sample Code | DSC | DMA | ||||
|---|---|---|---|---|---|---|
| Tgs (°C) | Tgh (°C) | Tmh (°C) | ΔHmh (J/g) | Tgs (°C) | Tflow (°C) | |
| BD–PU | −50 | 58 | 151–183 | 4.7 | −30 | 151 |
| ISB–PU | −51 | 57 | 147–198 | 4.6 | −29 | 145 |
| IMN–PU | −60 | 47 | 151–199 | 7.2 | −44 | 168 |
| Sample Code | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| BD–PU | 8 (±0.3) | 36 (±2.1) | 570 (±30.1) |
| ISB–PU | 14 (±0.8) | 48 (±3.4) | 462 (±49.0) |
| IMN–PU | 39 (±1.2) | 24 (±2.1)) | 518 (±21.1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-N.; Lee, D.-W.; Ryu, H.; Song, G.-S.; Lee, D.-S. Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds. Molecules 2019, 24, 1061. https://doi.org/10.3390/molecules24061061
Kim H-N, Lee D-W, Ryu H, Song G-S, Lee D-S. Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds. Molecules. 2019; 24(6):1061. https://doi.org/10.3390/molecules24061061
Chicago/Turabian StyleKim, Han-Na, Dae-Woo Lee, Hoon Ryu, Gwang-Seok Song, and Dai-Soo Lee. 2019. "Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds" Molecules 24, no. 6: 1061. https://doi.org/10.3390/molecules24061061
APA StyleKim, H.-N., Lee, D.-W., Ryu, H., Song, G.-S., & Lee, D.-S. (2019). Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds. Molecules, 24(6), 1061. https://doi.org/10.3390/molecules24061061