The Studies on Structure and Stability of CaBn Clusters
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussions
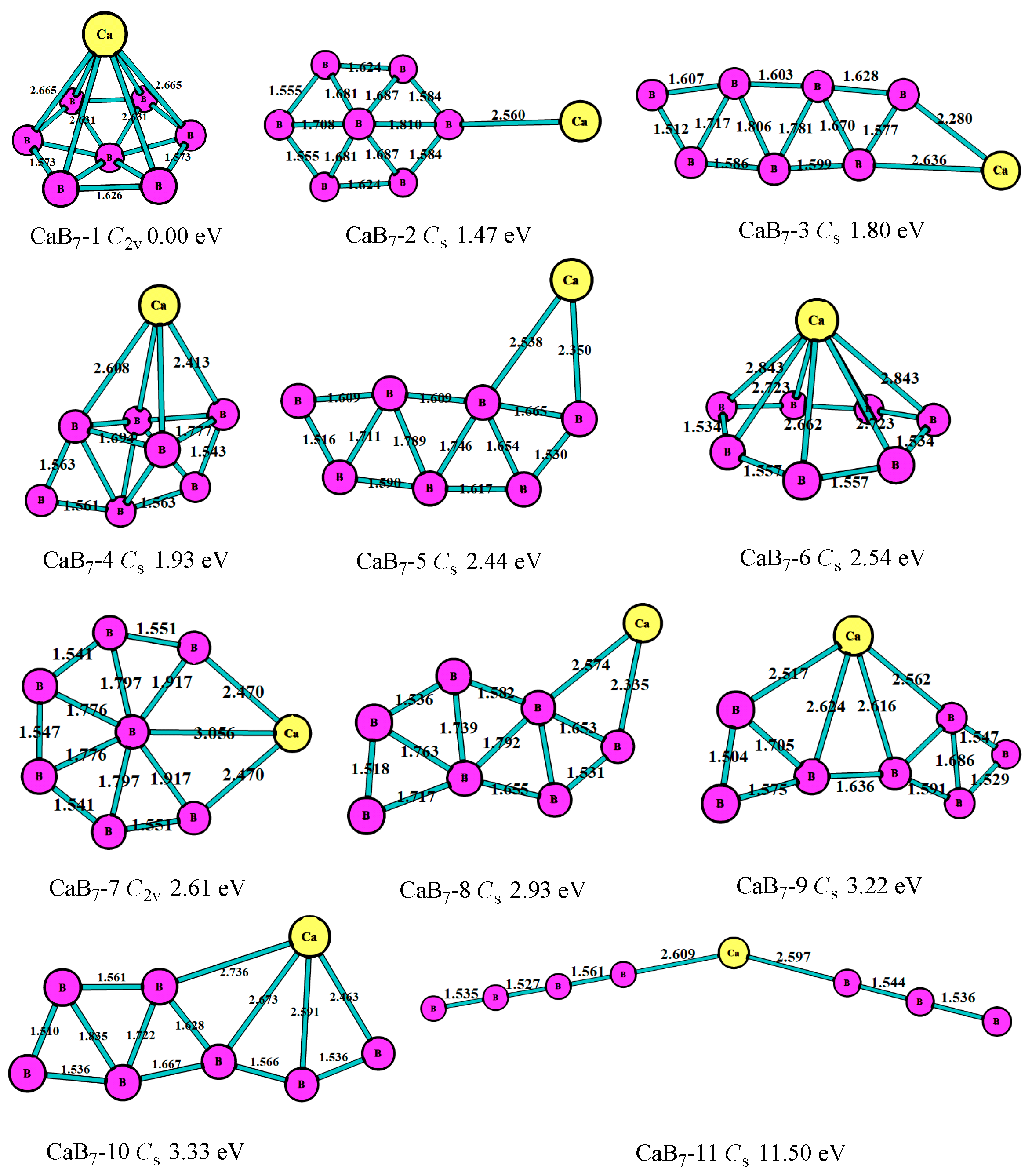
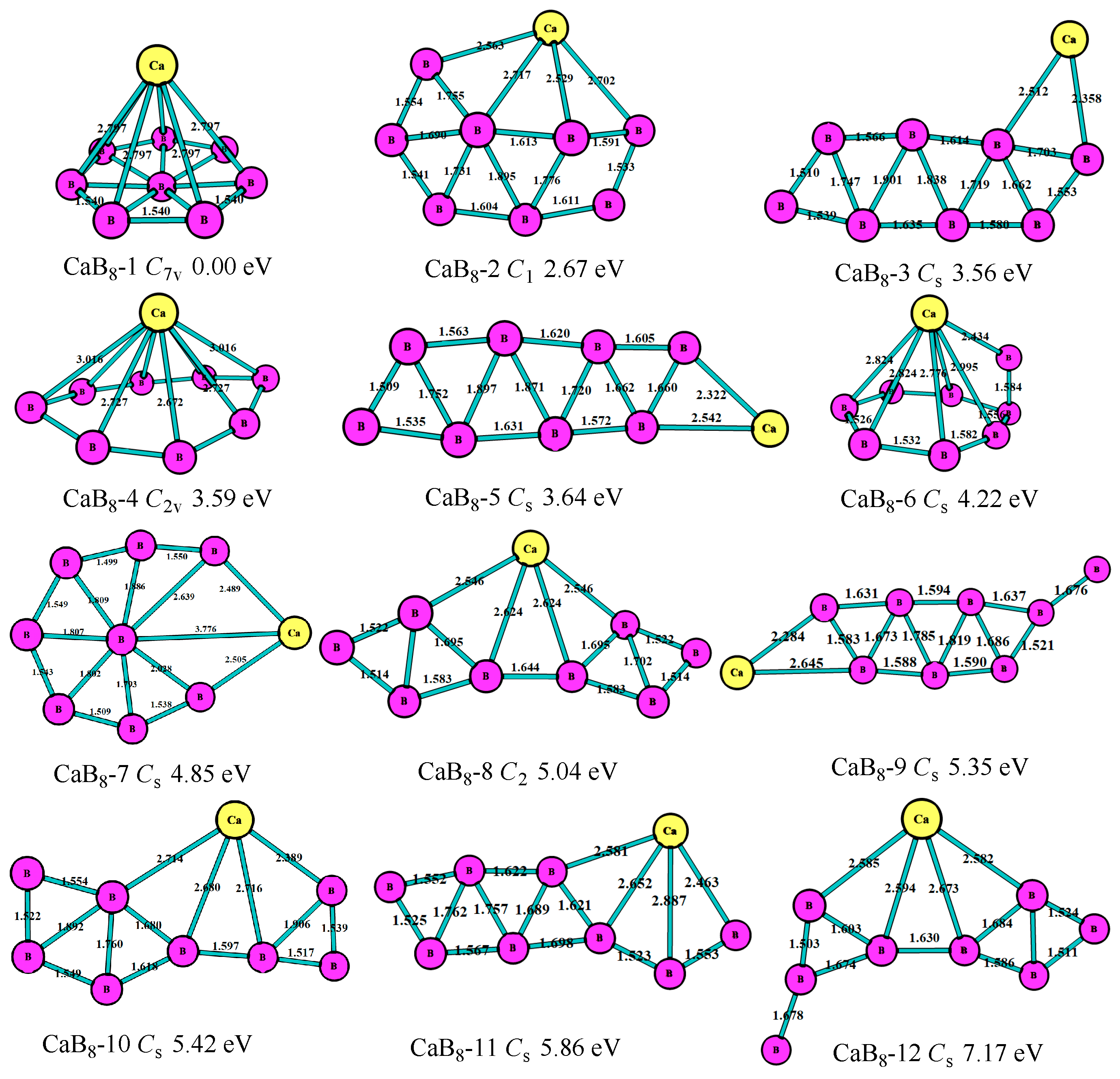
3.1. Stable Geometric Structures
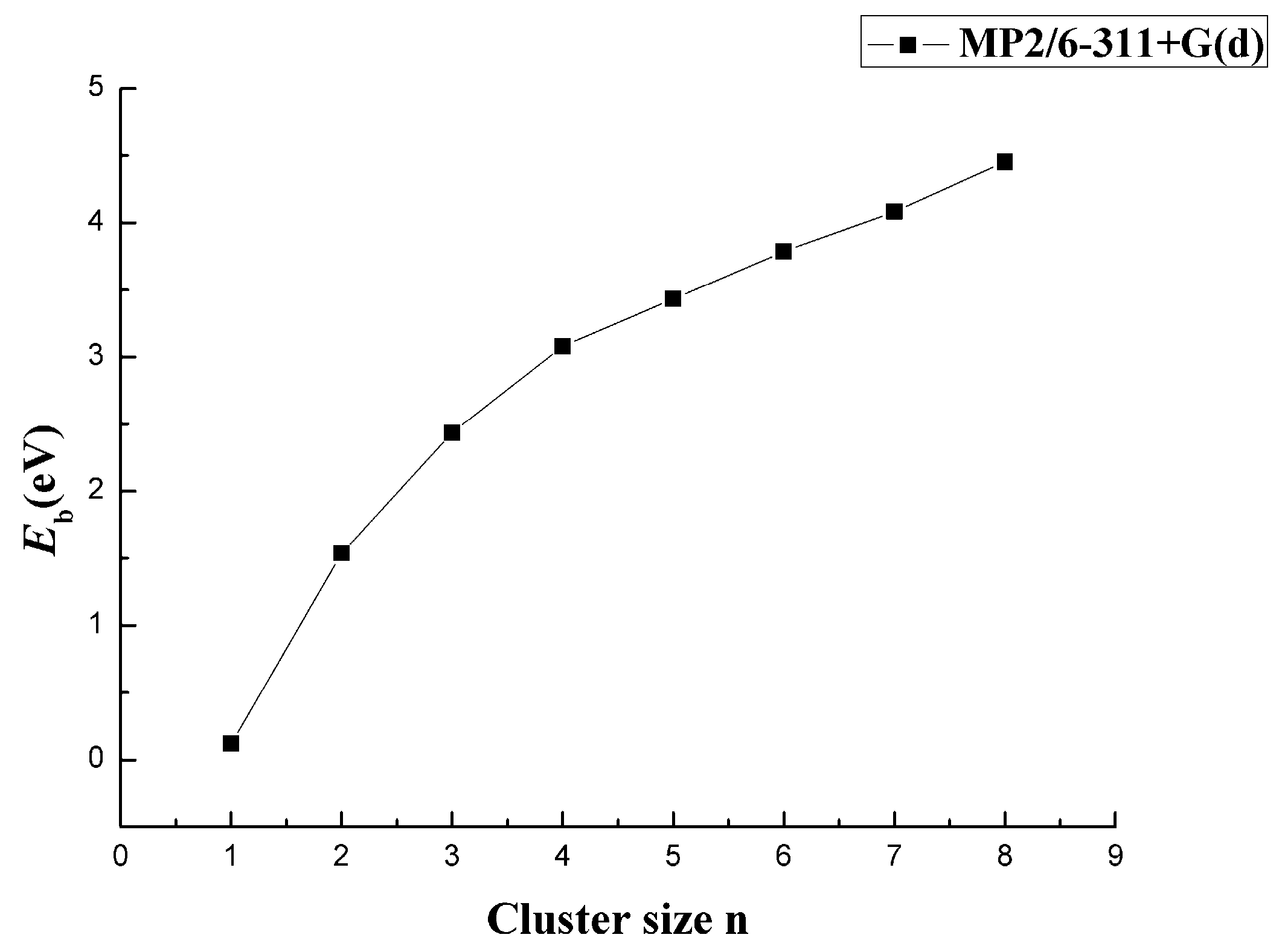
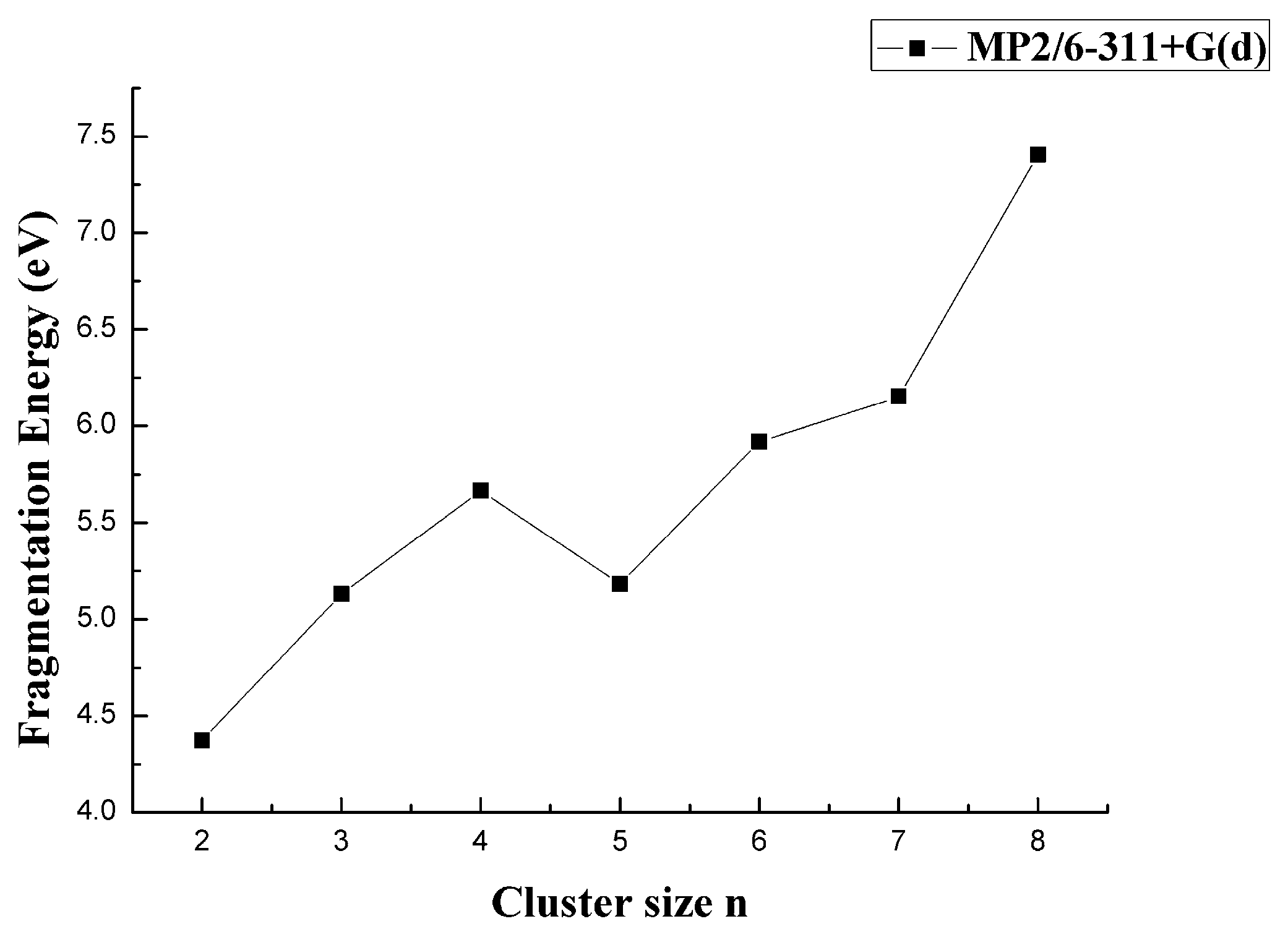
3.2. Relative Stability
3.3. The Ability of Obtaining or Removing an Electron
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buzea, C.; Yamashita, T. Review of the superconducting properties of MgB2. Supercond. Sci. Technol. 2001, 14, R115–R146. [Google Scholar] [CrossRef]
- Liu, Z.; Han, X.; Yu, D.; Sun, Y.; Xu, B.; Zhou, X.; He, J.; Wang, H.; Tian, Y. Formation, structure, and electric property of CaB4 single crystal synthesized under high pressure. Appl. Phys. Lett. 2010, 96, 031903. [Google Scholar] [CrossRef]
- Xin, S.; Han, X.; Liu, S.; Liu, Z.; Bo, X.; Tian, Y.; Yu, D. CaB6 single crystals grown under high pressure and hightemperature. J. Cryst. Growth. 2010, 313, 47–50. [Google Scholar] [CrossRef]
- Zhang, L.; Min, G.; Yu, H. Sintering process and high temperature stability investigation for nano-scale CaB6 materials. Ceram. Int. 2010, 36, 2253–2257. [Google Scholar] [CrossRef]
- Zhang, L.; Min, G.; Yu, H. Reaction mechanism and size control of CaB6 micron powder synthesized by the boroncarbide method. Ceram. Int. 2009, 35, 3533–3536. [Google Scholar]
- Chen, F.; Ju, M.; Kuang, X.Y.; Yeung, Y. Insights into the Microstructure and Transition Mechanism for Nd3+ Doped Bi4Si3O12: A Promising Near-Infrared Laser Material. Inorg. Chem. 2018, 57, 4563–4570. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Jin, Q. Aromaticity of Planar B5− Anion in the MB5 (M = Li, Na, K, Rb, and Cs) and MB5+ (M = Be, Mg, Ca, and Sr) Clusters. J. Phys. Chem. A 2004, 108, 855–860. [Google Scholar] [CrossRef]
- Li, Q.S.; Jin, Q. Theoretical Study on the Aromaticity of the Pyramidal MB6 (M = Be, Mg, Ca, and Sr) Clusters. J. Phys. Chem. A 2003, 107, 7869–7873. [Google Scholar] [CrossRef]
- Gong, L.F.; Guo, W.L.; Wu, X.M.; Li, Q.S. B7− as a novel ligand: Theoretical investigations on structures and chemical bonding of LiB7 and BeB7+. Chem. Phys. Lett. 2006, 429, 326–334. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Zhao, F.Q.; Ju, X.H. DFT study on structure and stability of MgBn±m clusters. Comput. Theor. Chem. 2014, 1027, 151–159. [Google Scholar] [CrossRef]
- Liu, C.; Si, H.; Han, P.; Tang, M. Density functional theory study on structure and stability of BeBn+ clusters. Rapid Commun. Mass Spectrom. 2017, 31, 1437–1444. [Google Scholar] [CrossRef]
- Lei, X.L.; Zhu, H.J.; Ge, G.X.; Wang, X.M.; Luo, Y.H. Structures and magnetism of BnNi (n = 6–12) clusters from density-functional theory. Acta Phys.Sin. 2008, 57, 5491–5499. [Google Scholar]
- Yang, Z.; Yan, Y.L.; Zhao, W.J.; Lei, X.L.; Ge, G.X.; Luo, Y.H. Structures and magnetism of FeBN (N ≤ 6) clusters. Acta. Phys. Sin. 2007, 56, 2590–2595. [Google Scholar]
- Liu, X.; Zhao, G.F.; Guo, L.J.; Jing, Q.; Luo, Y.H. Structural, electronic, and magnetic properties of MBn (M = Cr, Mn, Fe, Co, Ni, n ≤ 7) clusters. Phys. Rev. A 2007, 75, 063201. [Google Scholar] [CrossRef]
- Böyükata, M.; Güvenc, Z.B. Density functional study of AlBn clusters for n = 1–14. J. Alloys Compd. 2011, 509, 4214–4234. [Google Scholar] [CrossRef]
- Truong, B.T.; Nguyen, M.T. Thermochemical properties, electronic structure and bonding of mixed lithium boron clusters (BnLi, n = 1–8) and their anions. Chem. Phys. 2010, 375, 35–45. [Google Scholar]
- Yao, J.G.; Wang, X.W.; Wang, Y.X. A theoretical study on structural and electronic properties of Zr-doped B clusters: ZrBn (n = 1–12). Chem. Phys. 2008, 351, 1–6. [Google Scholar] [CrossRef]
- Feng, X.J.; Luo, Y.H. Structure and Stability of Al-Doped Boron Clusters by the Density-Functional Theory. J. Phys. Chem. A 2007, 111, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.F.; Ma, L.J.; Wang, J.F. Structures and stabilities of ScBn (n = 1–12) clusters: An ab initio investigation. J. Mol. Model. 2013, 19, 3255–3261. [Google Scholar] [CrossRef]
- Jia, J.F.; Li, X.R.; Li, Y.N.; Ma, L.J.; Wu, H.S. Density functional theory investigation on the structure and stability of Sc2Bn (n = 1–10) clusters. Comput. Theor. Chem. 2014, 1027, 128–134. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Pople, J.A.; Frisch, M.J. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 1988, 153, 503–506. [Google Scholar] [CrossRef]
- Aihara, J.; Ishida, T. Aromaticity of planar boron clusters confirmed. J. Am. Chem. Soc. 2005, 127, 13324–13330. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, G.H.; Zhao, J. Structure and electronic properties of Gen (n = 2–25) clusters from density-functional theory. Phys. Rev. B. 2001, 64, 205411. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
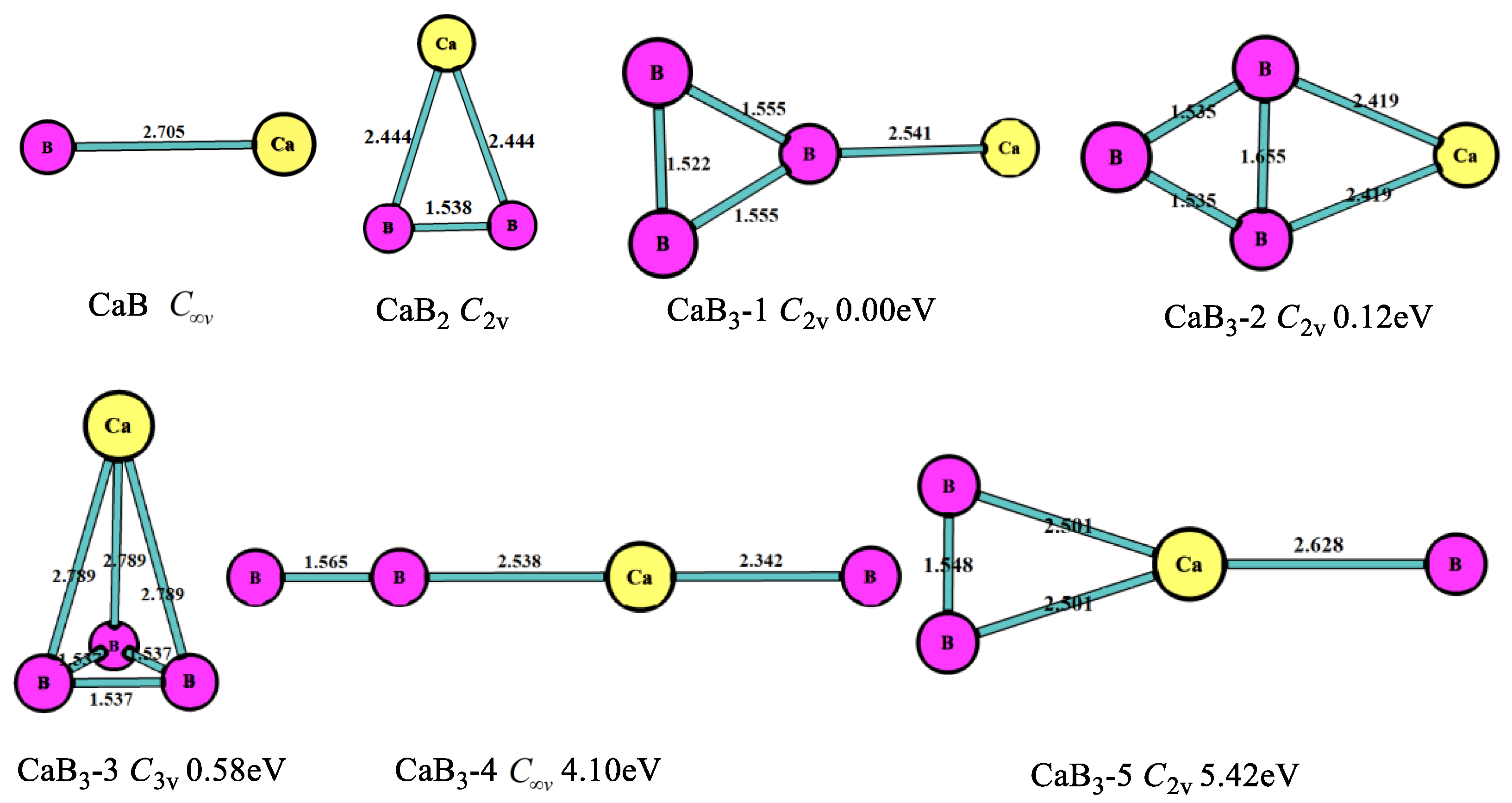
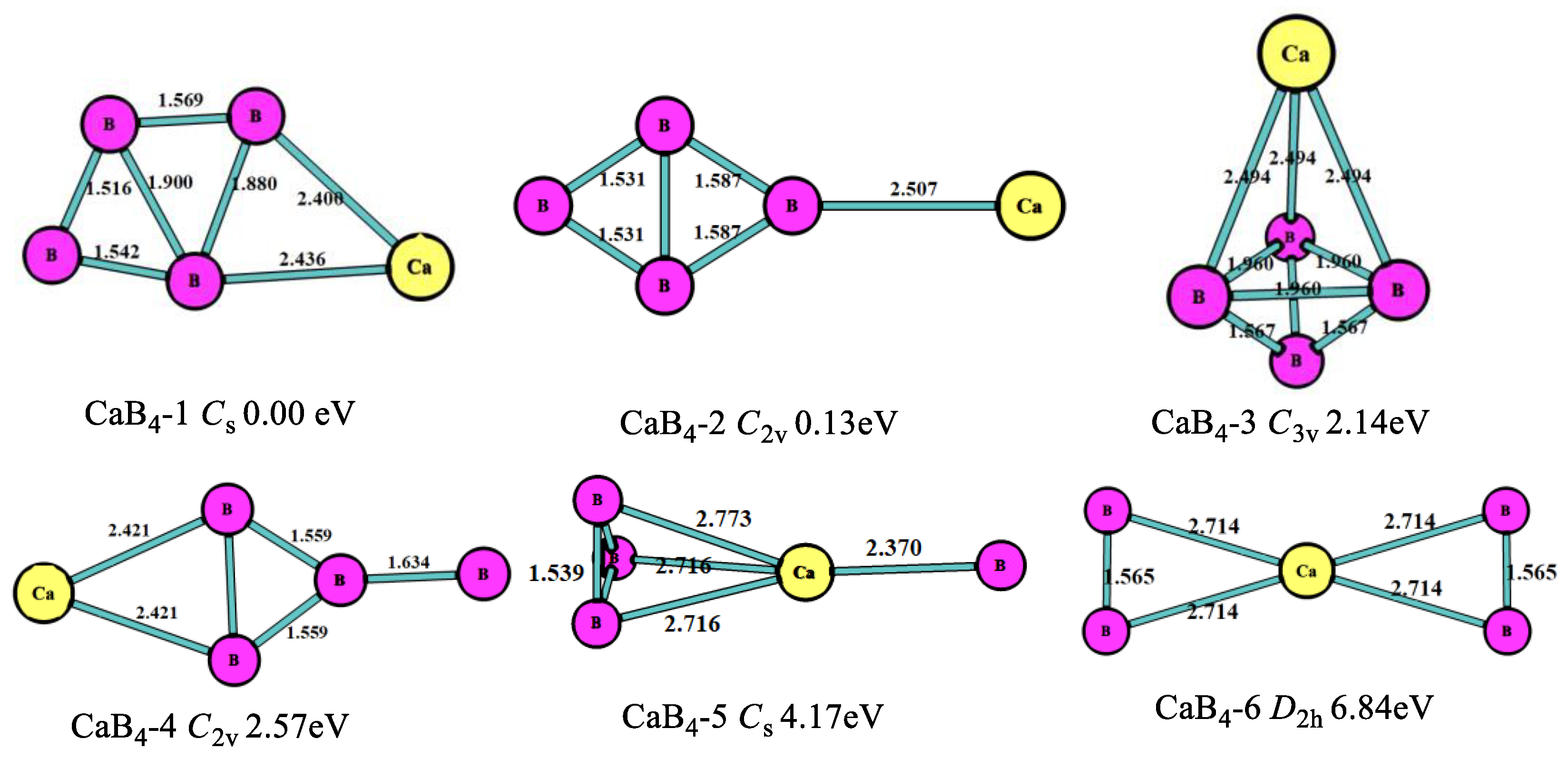
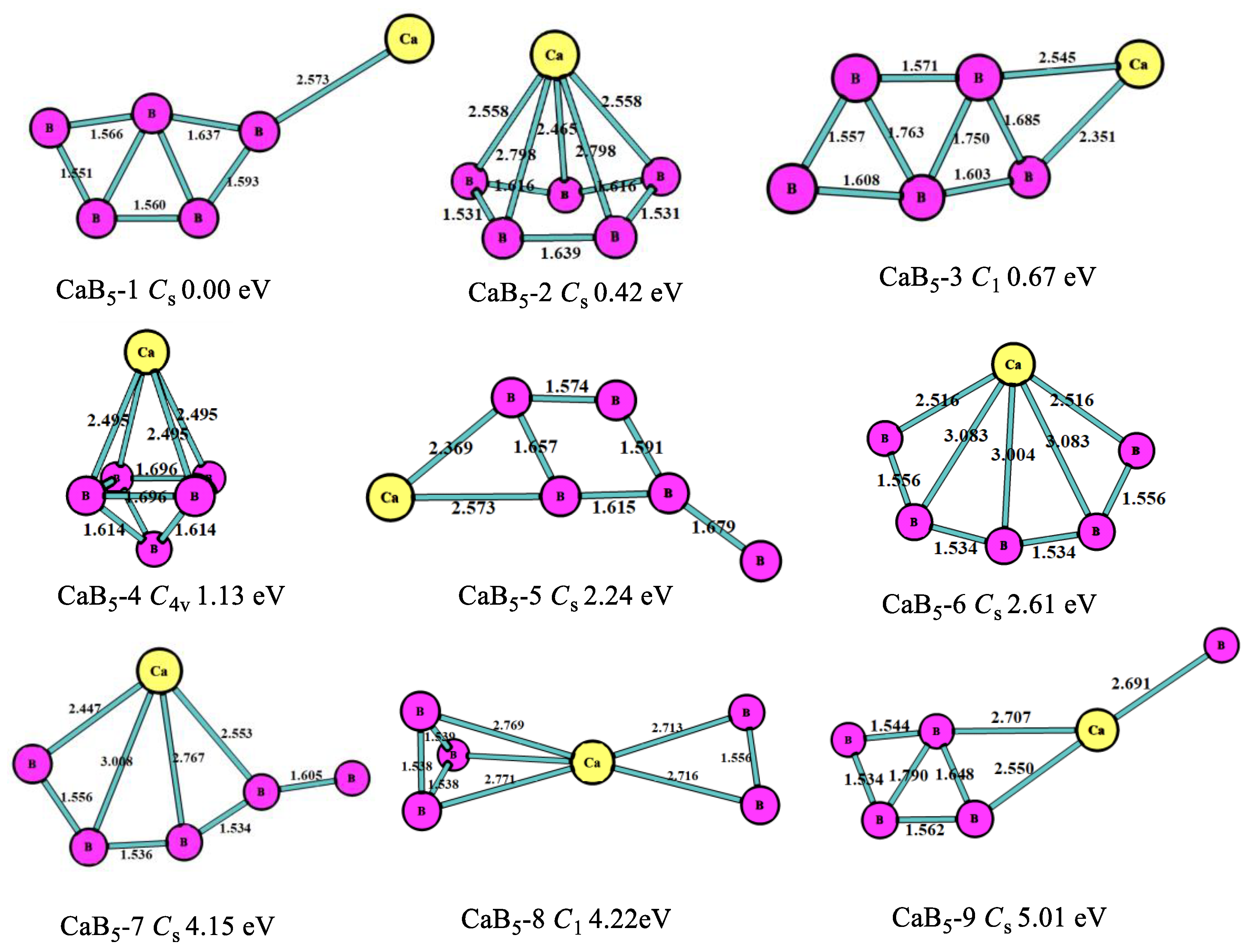
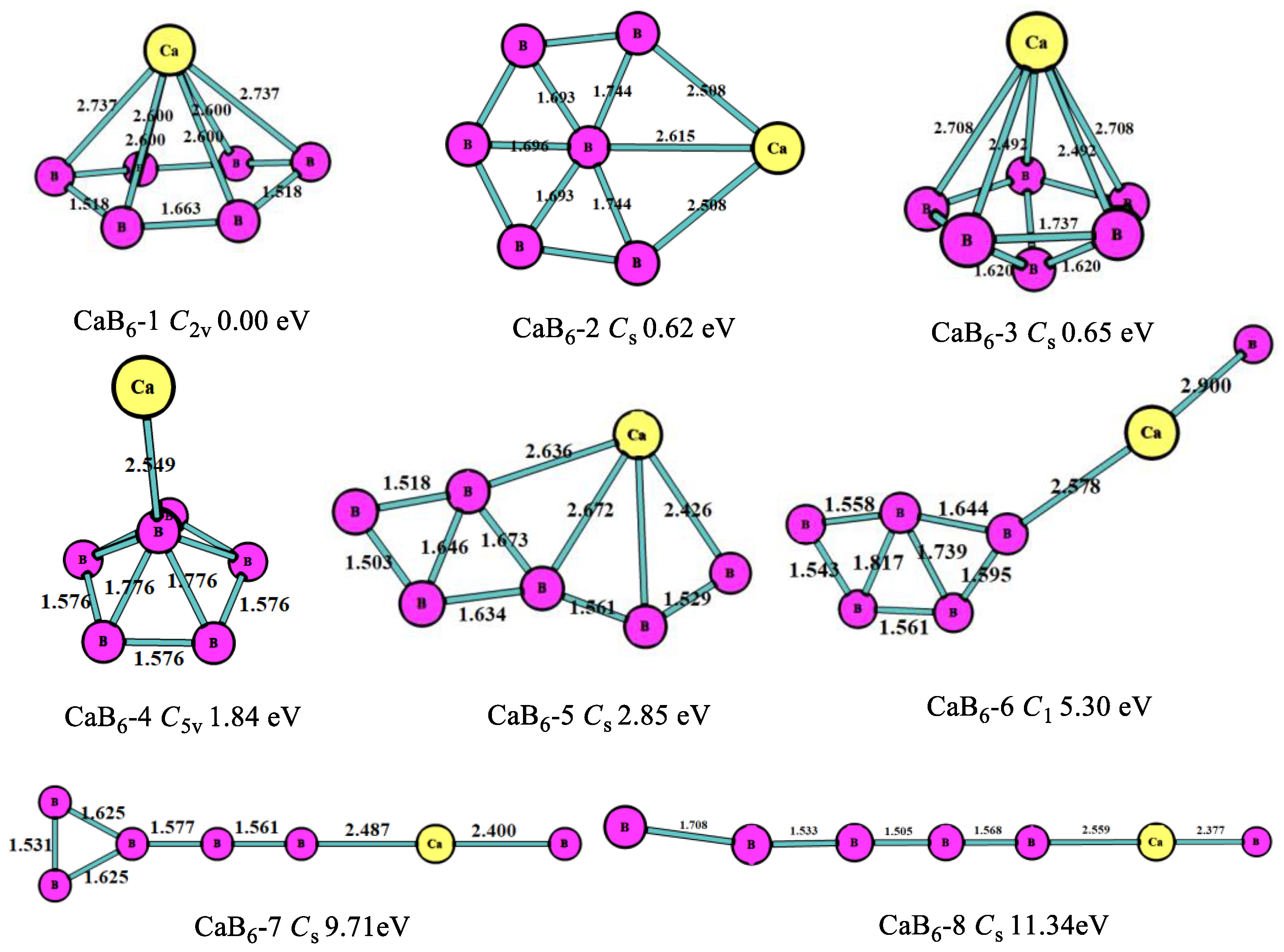
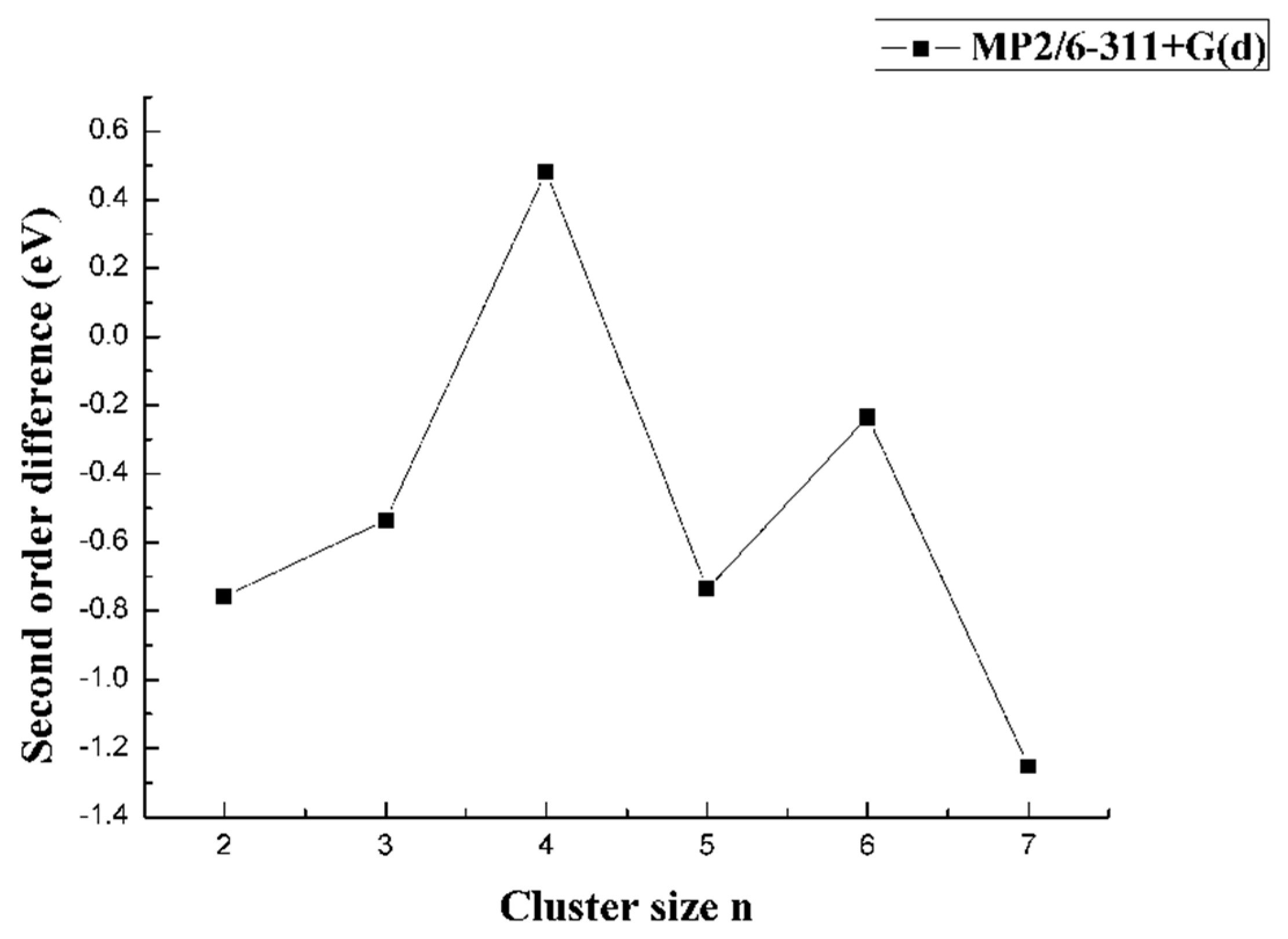
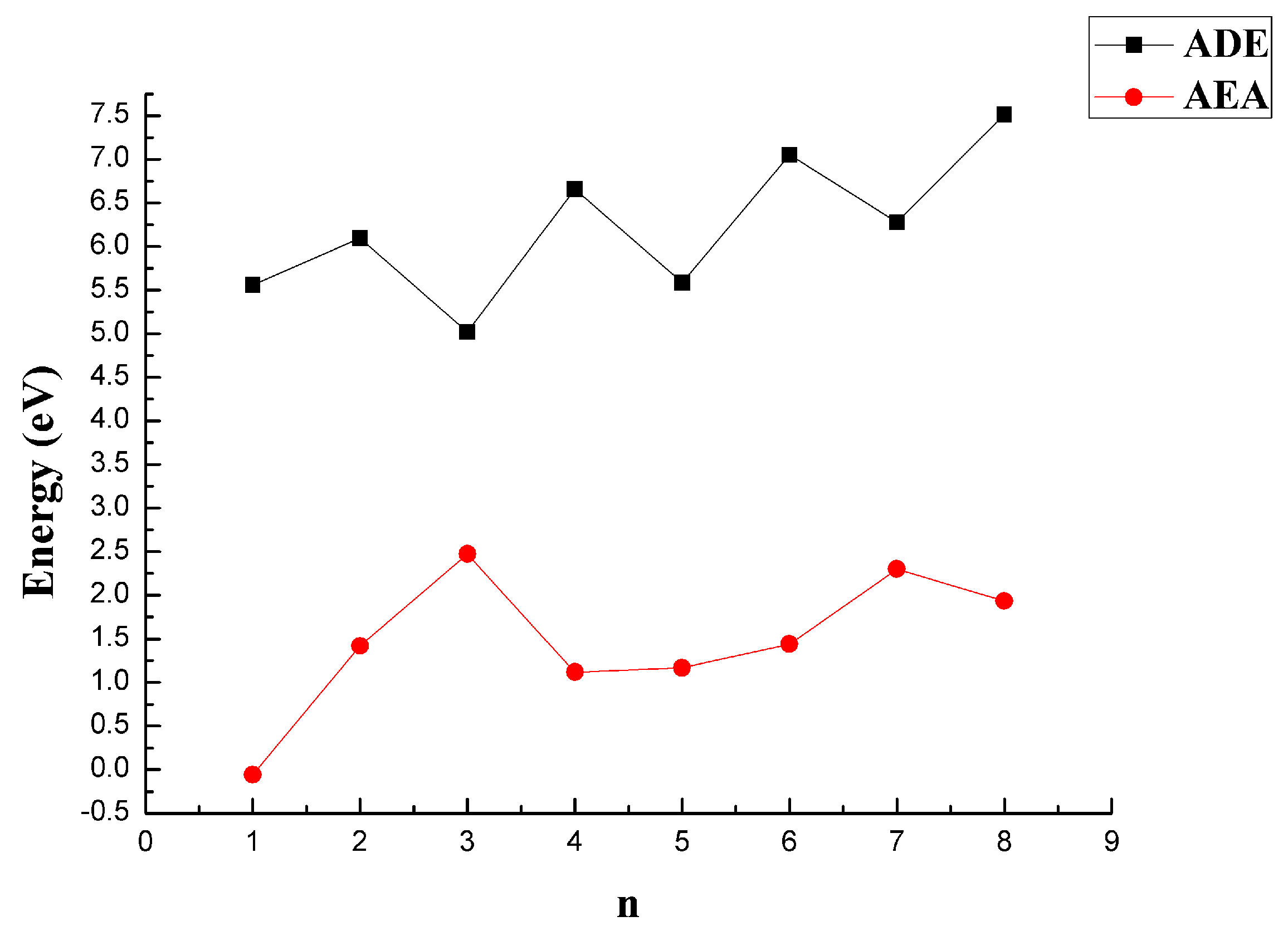
| CaBn | Energies/a.u. | Eb/eV | EF/eV | Δ2E/eV | ADE/eV | AEA/eV |
|---|---|---|---|---|---|---|
| CaB | −701.5209 | 0.1196 | — | — | 5.5593 | −0.0595 |
| CaB2 | −726.2515 | 1.5378 | 4.3741 | −0.7567 | 6.0984 | 1.4198 |
| CaB3 | −751.0098 | 2.4361 | 5.1308 | −0.5352 | 5.0175 | 2.4738 |
| CaB4 | −775.7879 | 3.0820 | 5.6660 | 0.4833 | 6.6577 | 1.1190 |
| CaB5 | −800.5481 | 3.4321 | 5.1826 | −0.7357 | 5.5863 | 1.1684 |
| CaB6 | −825.3354 | 3.7873 | 5.9184 | −0.2340 | 7.0507 | 1.4423 |
| CaB7 | −850.1314 | 4.0829 | 6.1523 | −1.2530 | 6.2788 | 2.3017 |
| CaB8 | −874.9733 | 4.4521 | 7.4053 | — | 7.5131 | 1.9359 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, P.; Chai, F.; Qiao, B.; Liu, C. The Studies on Structure and Stability of CaBn Clusters. Molecules 2019, 24, 1011. https://doi.org/10.3390/molecules24061011
Han P, Chai F, Qiao B, Liu C. The Studies on Structure and Stability of CaBn Clusters. Molecules. 2019; 24(6):1011. https://doi.org/10.3390/molecules24061011
Chicago/Turabian StyleHan, Peilin, Fengli Chai, Bolin Qiao, and Chunhui Liu. 2019. "The Studies on Structure and Stability of CaBn Clusters" Molecules 24, no. 6: 1011. https://doi.org/10.3390/molecules24061011
APA StyleHan, P., Chai, F., Qiao, B., & Liu, C. (2019). The Studies on Structure and Stability of CaBn Clusters. Molecules, 24(6), 1011. https://doi.org/10.3390/molecules24061011





