Synthesis of New Indanyl Nucleoside Analogues and their Biological Evaluation on Hepatitis C Virus (HCV) Replicon
Abstract
:1. Introduction
2. Results and Discussion
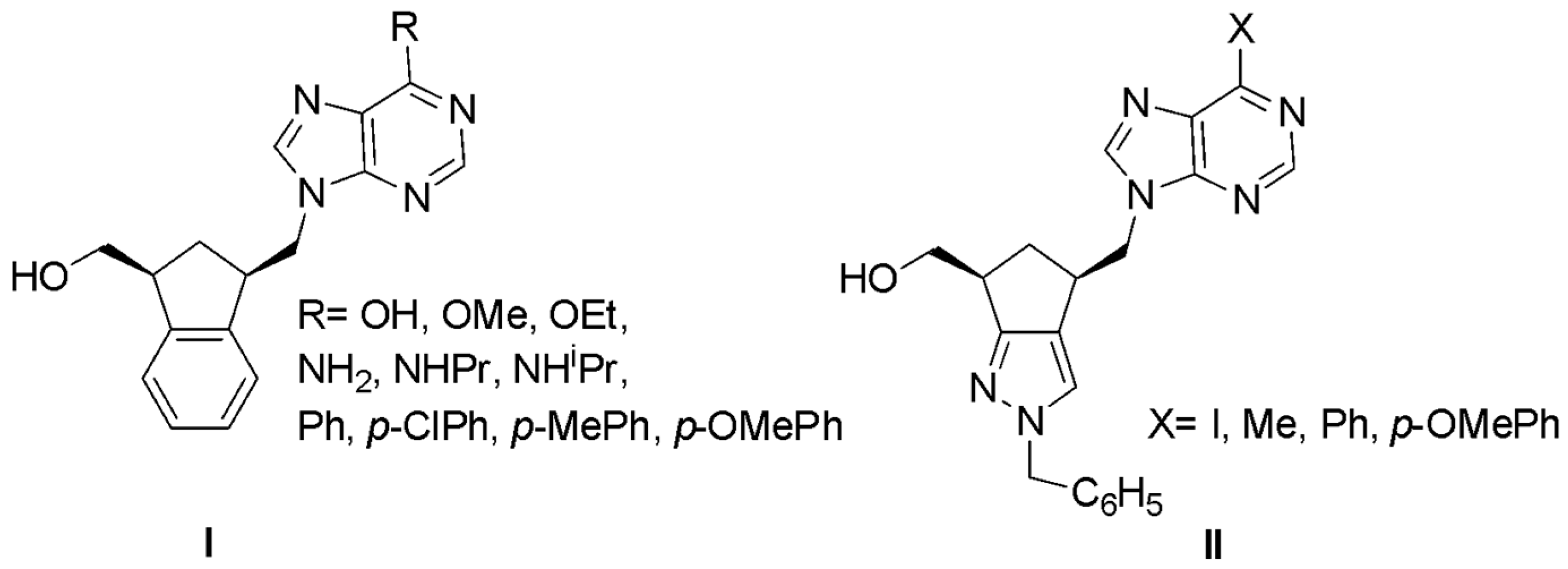
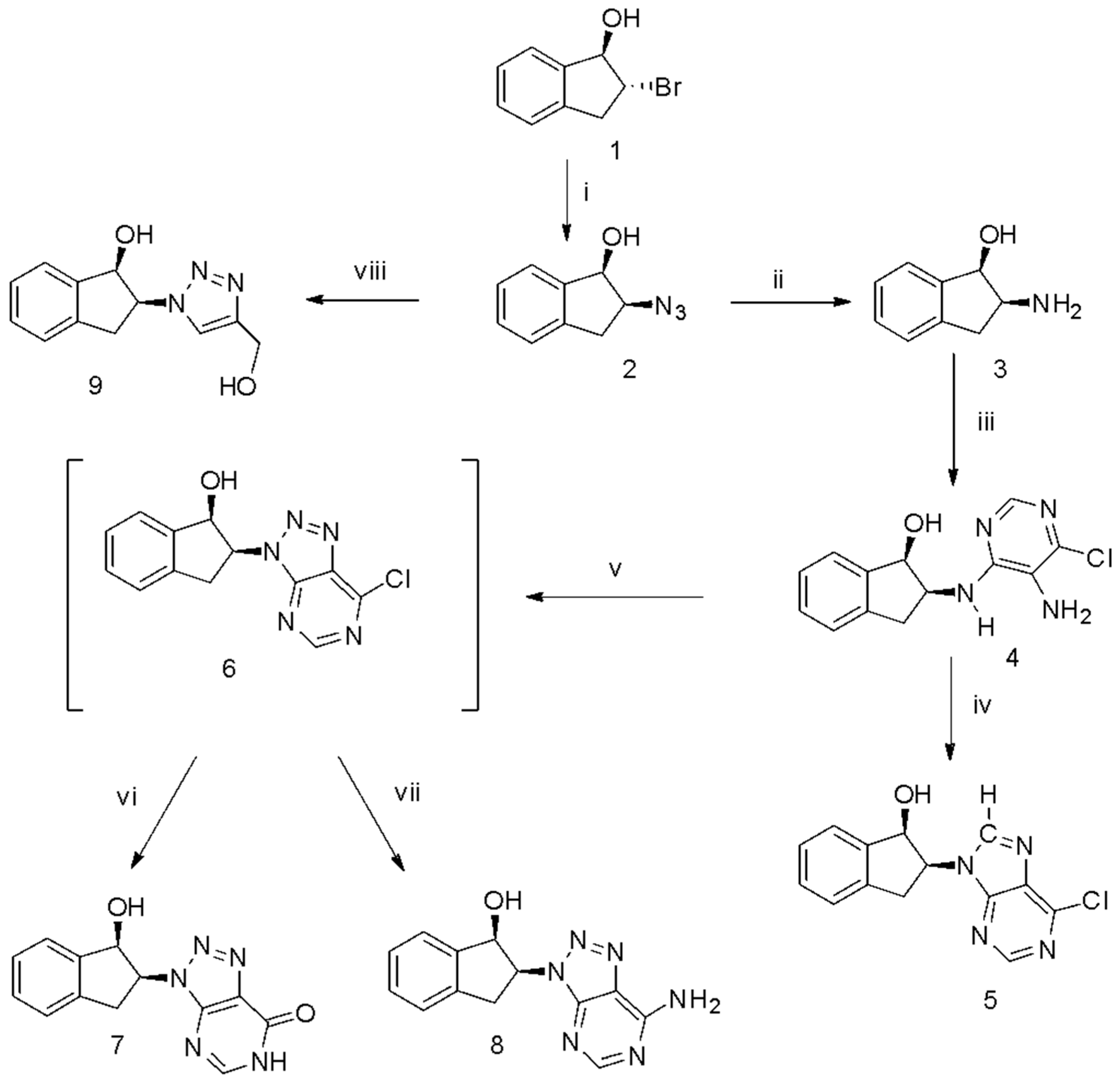
2.1. Chemistry
2.2. Biological Results
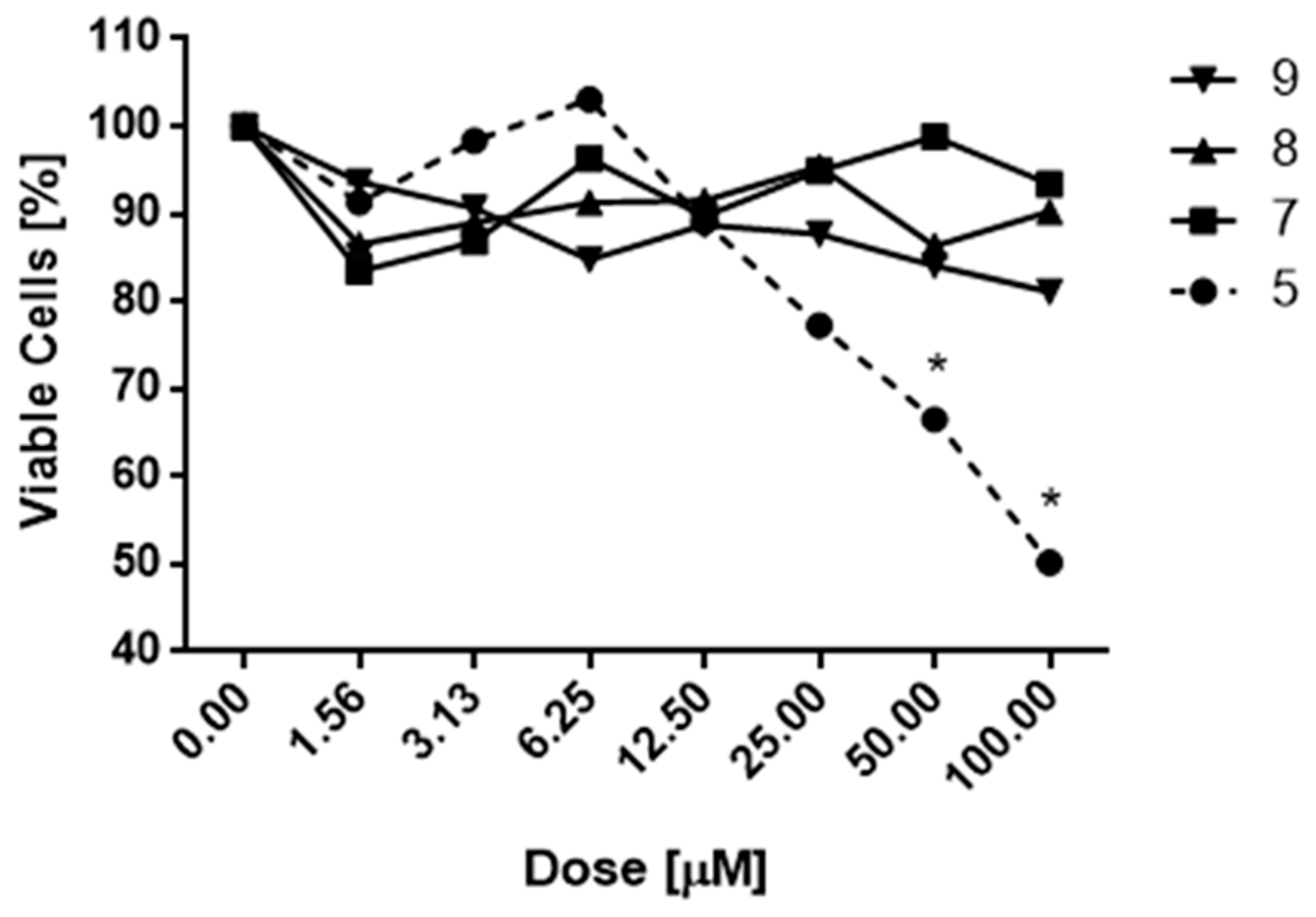
2.2.1. Cytotoxicity of Indanyl Derivatives
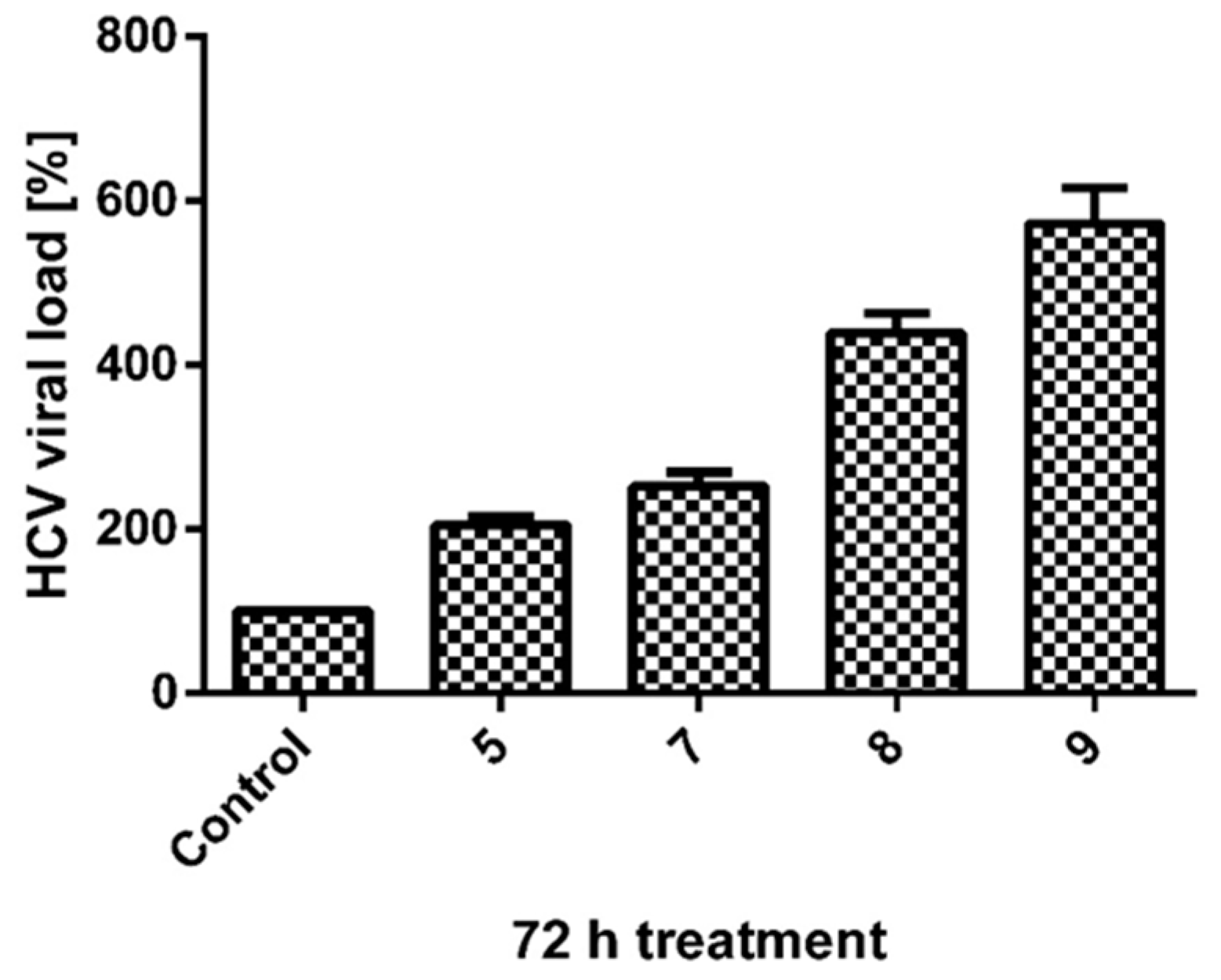
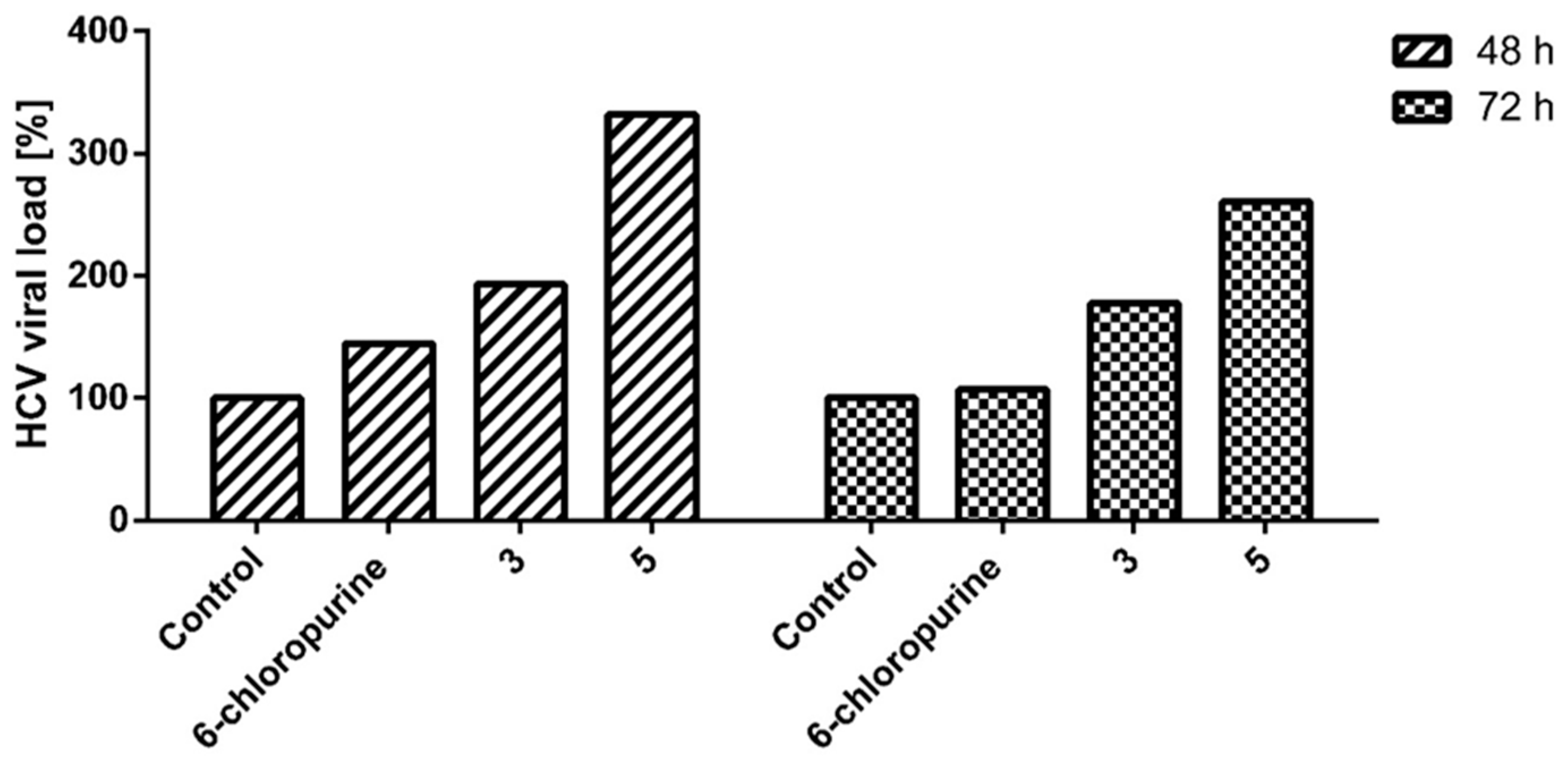
2.2.2. HCVg1b Replication Assay
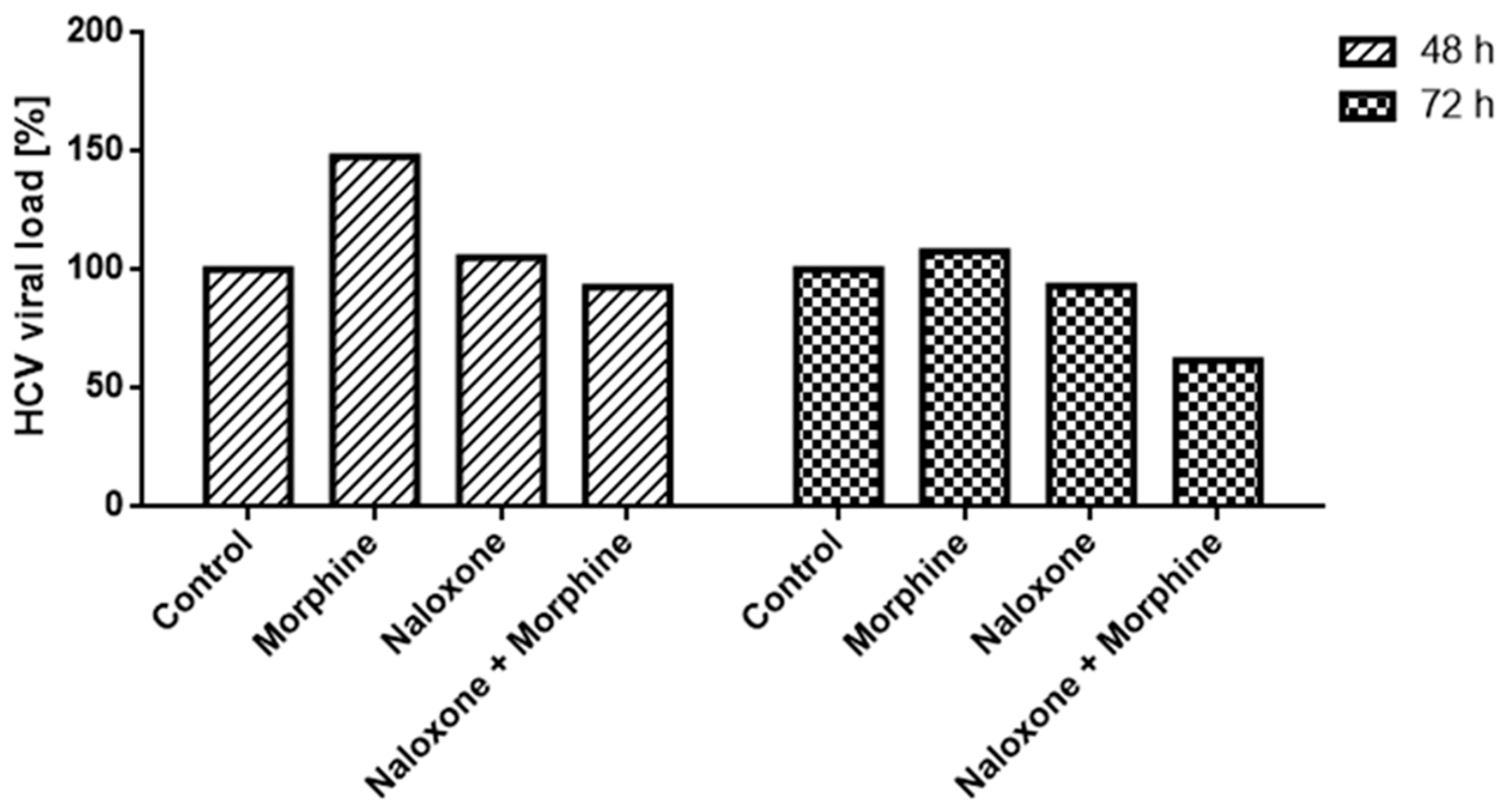
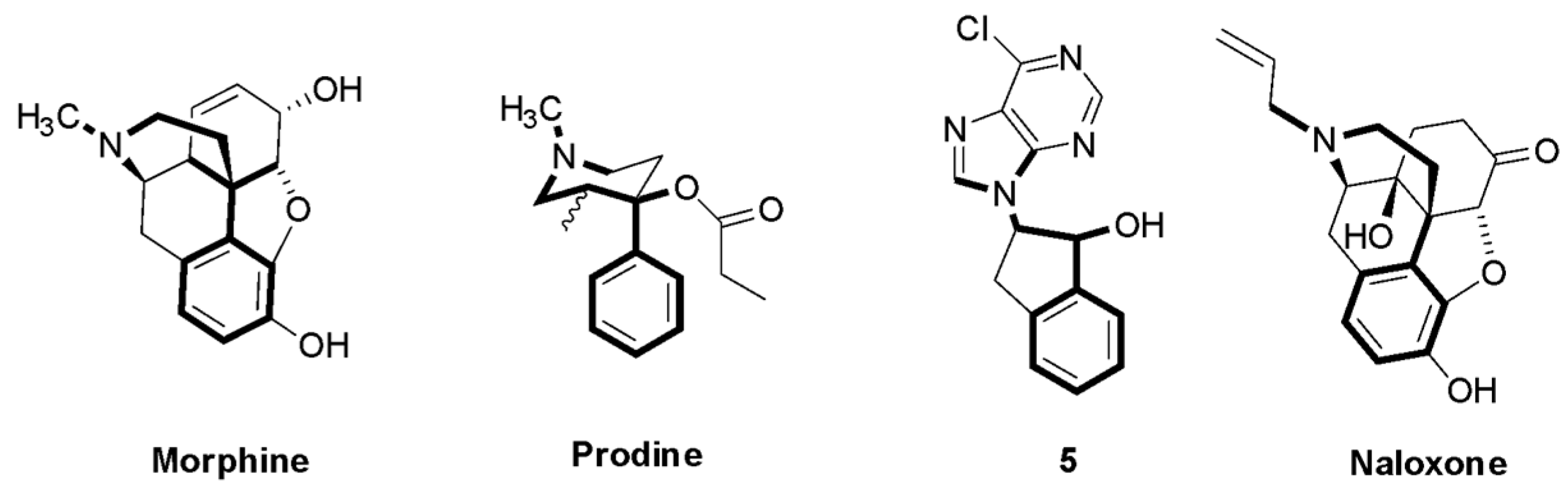
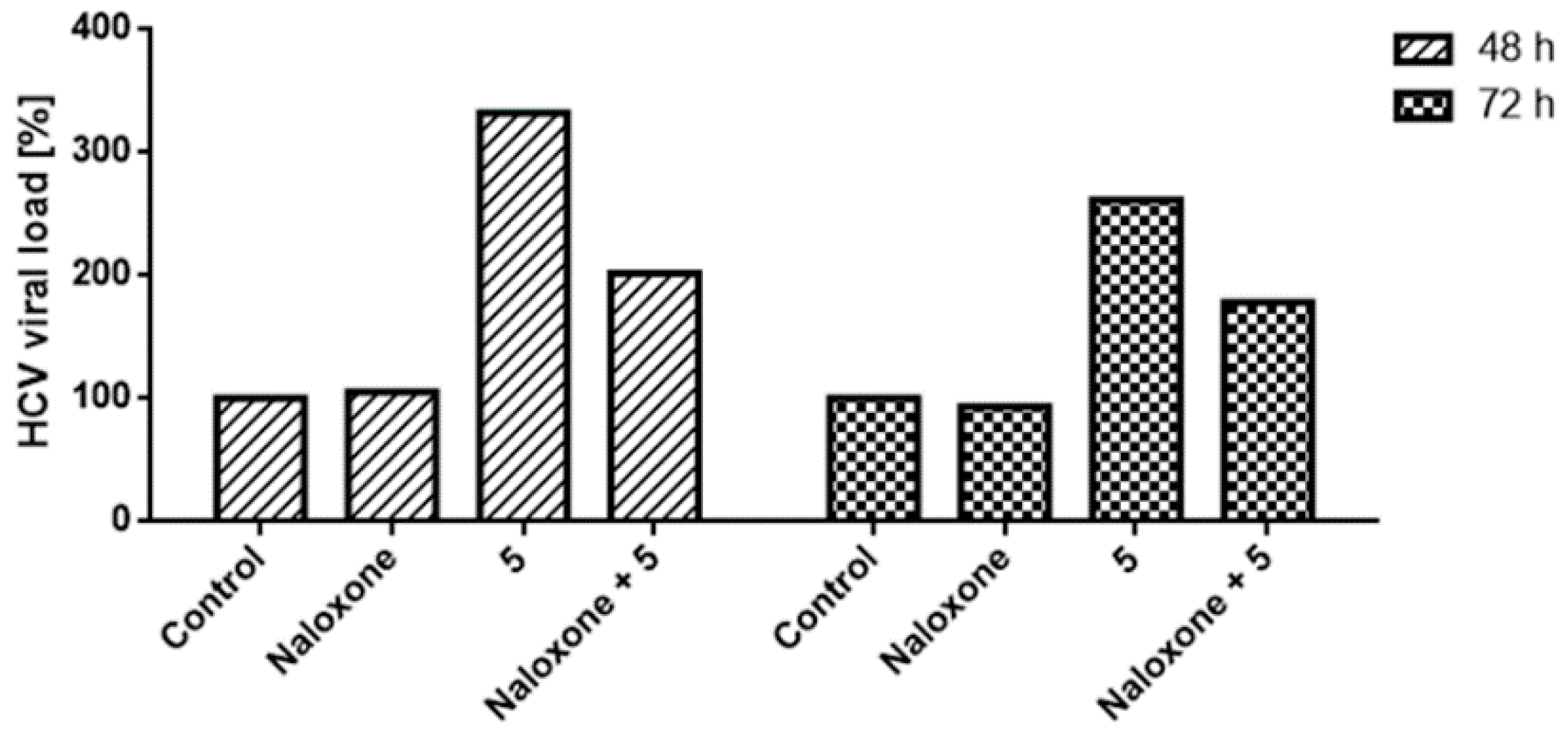
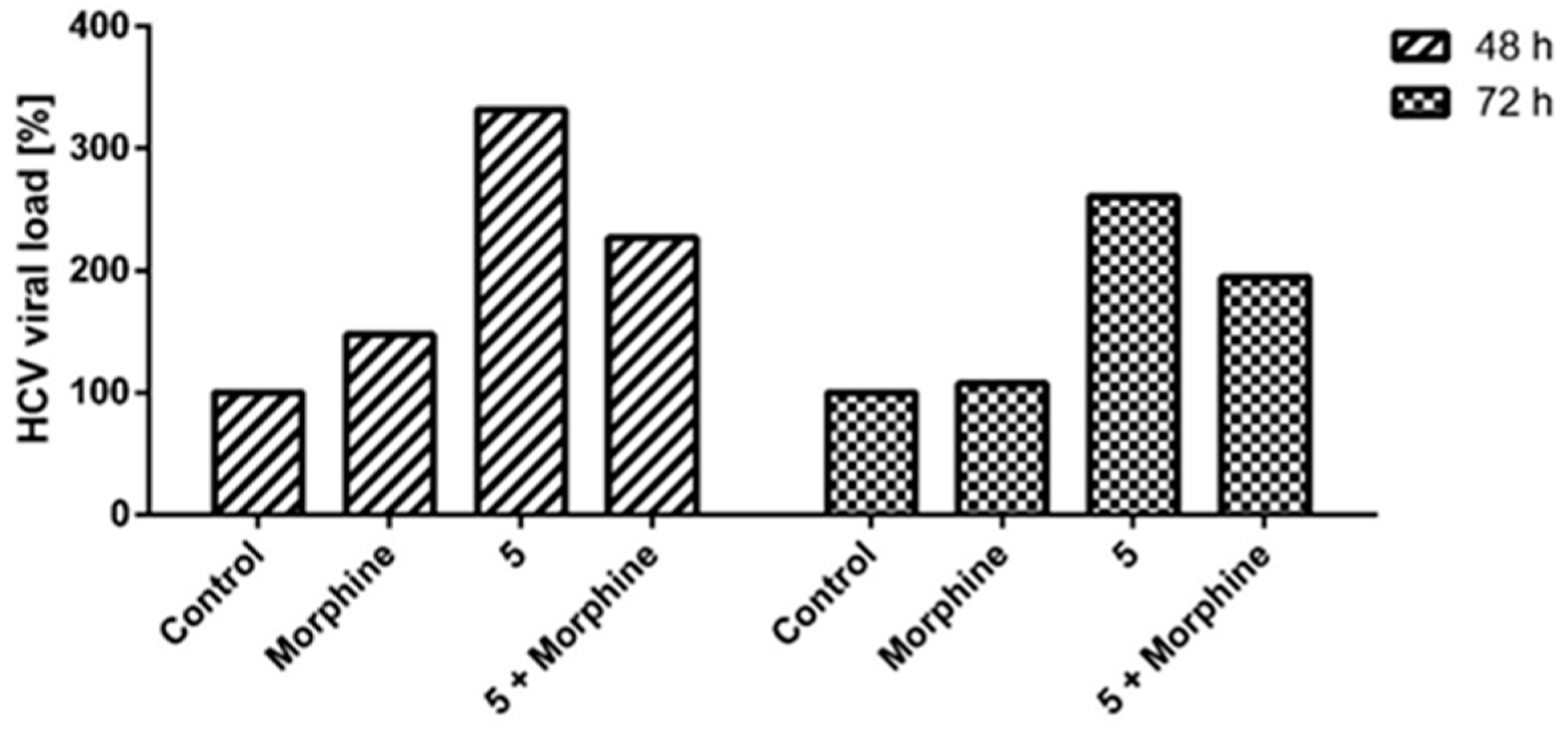
2.2.3. Indanyl Nucleoside Activity Related to the Opioid Receptor
3. Materials and Methods
3.1. Chemical Experimental Section
3.1.1. (+/−)-trans-2-bromo-1-indanol (1)
3.1.2. (+/−)-cis-2-azido-1-indanol (2)
3.1.3. (+/−)-cis-2-amino-1-indanol (3)
3.1.4. (+/−)-2-(5-amino-6-chloropyrimidin-4-yl-amino)-2,3-dihydro-1H-inden-1-ol (4)
3.1.5. (+/−)-2-(6-chloro-9H-purin-9-yl)-2,3-dihydro-1H-inden-1-ol (5)
3.1.6. 3-((+/−)1-hydroxy-2,3-dihydro-1H-inden-2-yl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-7-ol (7)
3.1.7. (+/−)2-(7-amino-3H-[1,2,3]triazolo[4,5–d]pyrimidin-3-yl)-2,3-dihydro-1H-inden-1-ol (8)
3.1.8. (+/−)-2-(4-hydroxymethyl-1H-1,2,3-triazol-1-yl)-2,3-dihydro-1H-inden-1-ol (9)
3.2. Evaluation of Biological Activity
3.2.1. Cells
3.2.2. Drug Preparation
3.2.3. Cytotoxic Assays
3.2.4. HCVg1b Replication Assay
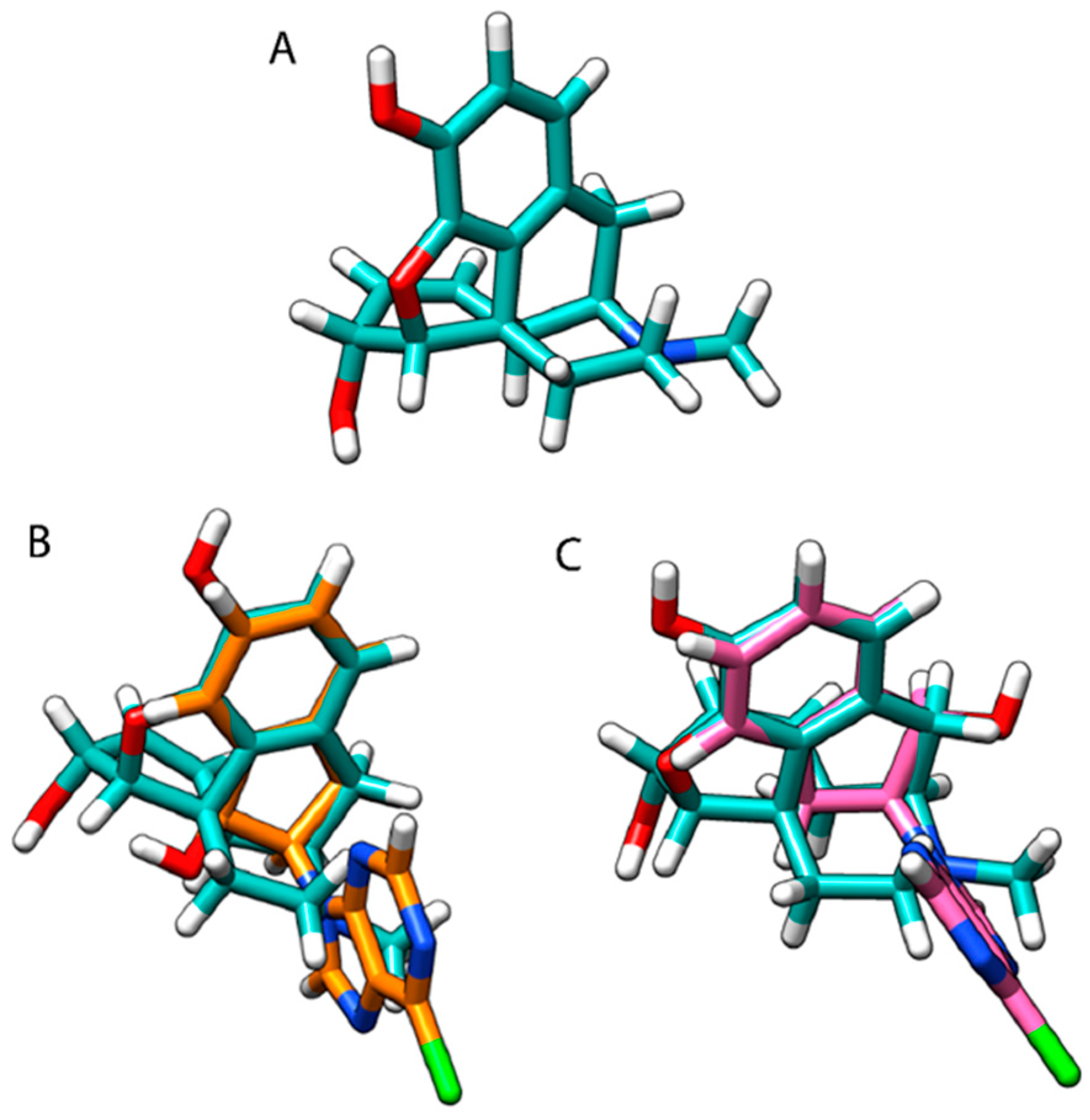
3.3. Molecular Modelling and Superimpose Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Clerc, E. Toward improved anti-HIV chemotherapy: Therapeutic strategies for intervention with HIV infections. J. Med. Chem. 1995, 38, 2491–2517. [Google Scholar] [CrossRef]
- Huang, P.; Farquhar, D.; Plunkett, W. Selective action of 3′-azido-3′-deoxythymidine 5′-triphosphate on viral reverse transcriptases and human DNA polymerases. J. Biol. Chem. 1990, 265, 11914–11918. [Google Scholar]
- Bressi, J.C.; Choe, J.; Hough, M.T.; Buckner, F.S.; van Voorhis, W.C.; Verlinde, C.L.; Hol, W.G.J.; Gelb, M.H. Adenosine analogues as Inhibitors of Trypanosoma brucei phosphoglycerate kinase: Elucidation of a novel binding mode for a 2-amino-N6-substituted adenosine. J. Med. Chem. 2000, 43, 4135–4150. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.I.; Hoth, D.F. Present status and future prospects for HIV therapies. Science 1993, 260, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Norbeck, D.W.; Kern, E.; Hayashi, S.; Rosenbrook, W.; Sham, H.; Herrin, T.; Plattner, J.J.; Erickson, J.; Clement, J. Cyclobut-A and cyclobut-G: Broad-spectrum antiviral agents with potential utility for the therapy of AIDS. J. Med. Chem. 1990, 33, 1281–1285. [Google Scholar] [CrossRef]
- Fernandez, F.; García-Mera, X.; Morales, M.; Vilariño, L.; Caamaño, O.; De Clercq, E. Synthesis of new 6-substituted purinyl-5′-nor-1′-homocarbanucleosides based on indanol. Tetrahedron 2004, 60, 9245–9253. [Google Scholar] [CrossRef]
- Ugliarolo, E.A.; Lantaño, B.; Moltrasio, G.Y.; Moglioni, A.G. An efficient approach to homochiralindane nucleosides. Tetrahedron Asymmetry 2009, 20, 1848–1853. [Google Scholar] [CrossRef]
- Shealy, Y.F.; Clayton, J.D. 9-[β-DL-2α,3α-Dihydroxy-4β-(hydroxymethyl)-cyclopentyl]adenine, the carbocyclic analog of adenosine. J. Am. Chem. Soc. 1966, 88, 3885–3887. [Google Scholar] [CrossRef]
- Ugliarolo, E.A.; Gagey, D.; Lantaño, B.; Moltrasio, G.Y.; Campos, R.H.; Cavallaro, L.V.; Moglioni, A.G. Synthesis and biological evaluation of novel homochiral carbocyclic nucleosides from 1-amino-2-indanols. Bioorg. Med. Chem. 2012, 20, 5986–5991. [Google Scholar] [CrossRef] [PubMed]
- Zampino, R.; Marrone, A.; Restivo, L.; Guerrera, B.; Sellitto, A.; Rinaldi, L.; Romano, C.; Adinolfi, L.E. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J. Hepatol. 2013, 5, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, I.M.; Lim, J.K.; Fried, M.W. American Gastroenterological Association Institute clinical practice update-expert review: Care of patients who have achieved a sustained virologic response after antiviral therapy for chronic hepatitis C infection. Gastroenterology 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; De Clercq, E. Current therapy for chronic hepatitis C: The role of direct-acting antivirals. Antivir. Res. 2017, 442, 83–122. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, P. Interferon-based combination treatment for chronic hepatitis C in the era of direct-acting antivirals. Ann. Gastroenterol. 2015, 28, 55–65. [Google Scholar]
- Sherman, K.E.; Flamm, S.L.; Afdhal, N.H.; Nelson, D.R.; Sulkowski, M.S.; Everson, G.T.; Fried, M.W.; Adler, M.; Reesink, H.W.; Martin, M.; et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N. Engl. J. Med. 2011, 365, 1014–1024. [Google Scholar] [CrossRef]
- Wilby, K.J.; Partovi, N.; Ford, J.A.; Greanya, E.; Yoshida, E.M. Review of boceprevir and telaprevir for the treatment of chronic hepatitis C. Can. J. Gastroenterol. 2012, 26, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Greig, S. Sofosbuvir/Velpatasvir: A review in chronic hepatitis C. Drugs 2016, 76, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Balo, C.; López, C.; Caamaño, O.; Fernández, F.; García-Mera, X.; Rodríguez-Borges, J.E. Synthesis of novel purinyl-1′-homocarbanucleosides based on a cyclopenta[b]pyrazine system. Chem. Pharm. Bull. 2008, 56, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.W.; Lopes, V.H.C.; Fernández, F.; García-Mera, X.; Morales, M.; Rodríguez-Borges, J.E.; Cordeiro, M.N.D.S. Synthesis and QSAR study of the anticancer activity of some novel indane carbocyclic nucleosides. Bioorg. Med. Chem. 2003, 11, 4999–5006. [Google Scholar] [CrossRef] [PubMed]
- Hergueta, A.R.; Fernandez, F.; López, C.; Balzarini, J.; De Clercq, E. Novel carbocyclic nucleosides containing a cyclobutyl ring: Adenosine analogues. Chem. Pharm. Bull. 2001, 49, 1174–1177. [Google Scholar] [CrossRef]
- Borges, J.E.; Fernández, F.; García, X.; Hergueta, A.R.; López, C.; Andrei, G.; Snoeck, R.; Witvrounw, M.; Balzarini, J.; De Clercq, E. Novel carbocyclic nucleosides containing a cyclobutyl ring. Guanosine and adenosine analogues. Nucleosides Nucleotides 1998, 17, 1237–1253. [Google Scholar] [CrossRef] [PubMed]
- Imuta, M.; Ziffer, H. Synthesis and absolute stereochemistry of cis- and trans-1,2-indandiols: Metabolites of indene and 2-indanone. J. Org. Chem. 1978, 43, 4540–4552. [Google Scholar] [CrossRef]
- Kozhushkov, S.I.; Yufit, D.S.; de Meijere, A. Convenient and Inexpensive Synthesis of (1R,2R)-trans-1-Amino-6-nitroindan-2-ol. Adv. Synth. Catal. 2005, 347, 255–265. [Google Scholar] [CrossRef]
- Iacazio, G.; Réglier, M. Chemo-enzymatic synthesis of all four diastereoisomers of 1-fluoro-2-amino-indane. Tetrahedron Asymmetry 2005, 16, 3633–3639. [Google Scholar] [CrossRef]
- Mitrochkine, A.; Gil, G.; Règlier, M. Synthesis of enantiomerically pure cis and trans-2-amino-1-indanol. Tetrahedron Asymmetry 1995, 6, 1535–1538. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G. and Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Douglas, S.D.; Lai, J.P.; Xiao, W.D.; Pleasure, D.E.; Ho, W.Z. Morphine enhances hepatitis C virus (HCV) Replicon Expression. Am. J. Pathol. 2003, 163, 1167–1175. [Google Scholar] [CrossRef]
- Wang, C.Q.; Li, Y.; Douglas, S.D.; Wang, X.; Metzger, D.S.; Zhang, T.; Ho, W.Z. Morphine withdrawal enhances hepatitis C virus replicon expression. Am. J. Pathol. 2005, 167, 1333–1340. [Google Scholar] [CrossRef]
- Delgado Cirilo, A.; Minguillón Llombart, C.; Joglar Tamargo, J. Introducción a la Química Terapéutica, 2nd ed.; Ediciones Díaz de Santos: Madrid, Spain, 2003; p. 307. [Google Scholar]
Sample Availability: Samples of the compounds 7, 8 and 9 are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, M.E.; Gentile, E.A.; Martini, M.F.; Cuestas, M.L.; Mathet, V.L.; Moltrasio, G.Y.; Moglioni, A.G. Synthesis of New Indanyl Nucleoside Analogues and their Biological Evaluation on Hepatitis C Virus (HCV) Replicon. Molecules 2019, 24, 990. https://doi.org/10.3390/molecules24050990
Gómez ME, Gentile EA, Martini MF, Cuestas ML, Mathet VL, Moltrasio GY, Moglioni AG. Synthesis of New Indanyl Nucleoside Analogues and their Biological Evaluation on Hepatitis C Virus (HCV) Replicon. Molecules. 2019; 24(5):990. https://doi.org/10.3390/molecules24050990
Chicago/Turabian StyleGómez, Matías E., Emiliano A. Gentile, M. Florencia Martini, María L. Cuestas, Verónica L. Mathet, Graciela Y. Moltrasio, and Albertina G. Moglioni. 2019. "Synthesis of New Indanyl Nucleoside Analogues and their Biological Evaluation on Hepatitis C Virus (HCV) Replicon" Molecules 24, no. 5: 990. https://doi.org/10.3390/molecules24050990
APA StyleGómez, M. E., Gentile, E. A., Martini, M. F., Cuestas, M. L., Mathet, V. L., Moltrasio, G. Y., & Moglioni, A. G. (2019). Synthesis of New Indanyl Nucleoside Analogues and their Biological Evaluation on Hepatitis C Virus (HCV) Replicon. Molecules, 24(5), 990. https://doi.org/10.3390/molecules24050990





