1. Introduction
Spicatoside A (SpiA) belongs to the class of steroidal saponins, which are known complex mixtures having highly branched oligosaccharide moieties and are found to be distributed in various plants [
1,
2]. They are known to exert a wide range of pharmacological effects including anti-inflammatory, anti-asthma, anti-osteoclastogenesis, neurite outgrowth, memory consolidation and anti-cancer [
3]. The anti-inflammatory, anti-asthma and neurite outgrowth activities of SpiA have received great attention since these properties are correlated with chronic human diseases [
4,
5,
6]. Prosapogenin III of SpiA significantly decreases the nitric oxide (NO) production and suppresses the nuclear translocation of nuclear factor (NF)-κB in RAW264.7 cells [
4]. SpiA is known to enhance the basal production of mucin 5AC (MUC5AC) mucin in the airway surface epithelium and NCI-H292 cells [
5,
7,
8]. Moreover, it has been shown to stimulate the differentiation and survival of neuronal cells through regulation of neurotrophic factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) [
6,
9,
10]. Although the above studies provide some information regarding the possible correlation between improvement of intestinal bowel diseases and steroidal saponin, no studies have directly investigated the laxative effects of SpiA in the constipated animal model.
Several reports on the effects of extracts and compounds containing saponins have provided evidence for the possibility that steroidal saponin contributes to the decrease of constipation symptoms. Traditional medicines are known to administer saponin containing extracts of
Aloe ferox Mill. and
Morinda morindoes for the treatment of constipation [
11,
12]. Moreover, saponin containing extracts from
Fumaria parviflora Linn. and
Phyllanthus emblica fruit showed enhanced movement of charcoal meal and the recovery in the total number of feces, depending on the concentration administered to mice [
13,
14]. Furthermore, the crude extract of
Viola betonicifolia containing alkaloid and saponins, and the aqueous extracts of
Liriope platyphylla containing isoflavone and saponin, also effectively decreased the symptoms of constipation in the loperamide (Lop)-induced model [
14,
15]. Similar laxative effects have recently been observed in Lop-induced constipation rats treated with the red
L. platyphylla extract (EtRLP) containing SpiA [
16]. However, no study has showed direct evidence of the laxative effects and molecular mechanism of SpiA when administered as a single compound.
Therefore, the present study investigated the possibility that, of the various steroidal saponins, SpiA is a key component for inducing laxative effects in the Lop-induced constipation Institute of Cancer Research (ICR) mice model. We believe that our results provide the first scientific evidence that SpiA successfully improves functional regulation of the neural, Cajal, smooth muscle and epithelial cells in the transverse colon of the Lop-induced constipation ICR mice model.
3. Discussion
Laxative effects to decrease constipation symptoms were investigated in phytochemicals-containing products of several herbal plants without any significant toxicity [
13,
14]. Among these, saponin-containing products such as
L. platyphylla, Red
L. platyphylla,
Aloe ferox, F. parviflora and
P. emblica can be considered as potential candidate because they significantly recovered the stool excretion, intestinal motility, mucin secretion and histological structure in Lop-induced constipation model [
11,
13,
14,
15,
18]. In this study, we investigated the laxative effects and mechanism of action of SpiA, based on previous studies reporting that the SpiA containing red
L. platyphylla alleviated symptoms in the Lop-induced constipation ICR mice model [
19]. We believe that our results of the current study are the first to provide direct evidence that SpiA stimulates stool excretion, recovery of histological and cytological structures, and mucin secretion in Lop-induced constipation ICR mice model. These results especially indicate that the laxative effects of SpiA are tightly correlated with the cholinergic regulation of enteric nerve in the colon of Lop-induced constipation ICR mice model.
Determining the therapeutic dose of drug candidates is considered an important parameter, since these data can be provided as basic information to predict the clinical therapeutic index (TI) of a drug candidate at an early stage [
20]. Various concentrations of saponin-containing laxatives have actually been used to evaluate their efficacy in animal model. Among these, 100–300 mg/kg is determined as the optimal concentration of saponin containing extracts of
Aloe ferox, F. parviflora and
P. emblica for laxative effects [
13,
14,
18]. Other natural products such as
L. platyphylla and red
L. platyphylla extracts which prepared with nine repetitions of a two-step process (steaming 200 g of dry root samples at 99 °C for 3 h after air-drying at 70 °C for 24 h) show an optimal laxative effect at 1000 mg/kg [
15,
19]. Meanwhile, quercetin as a single compound has been administered at relative low concentrations (10, 20 and 40 mg/kg) [
17]. In this study, 20 mg/kg of SpiA was used as the optimal concentration, based on the concentration of quercetin used in a previous study. This dose was the equivalent to 1.62 mg/kg daily dose in humans, according to the Food and Drug Administration in USA (Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers).
The laxative effects of constipation model could be induced by natural products containing various phytochemicals such as saponins, flavonoids, tannins, sterols, terpenoids, alkaloids, phenolic compounds [
13,
14]. Of these, some flavonoids such as naringenin and quercetin induce the enhancement of stool excretion and mucin secretion in the Lop-induced constipation model [
17,
21]. Saponins have been investigated to relate with the regulation of smooth muscle contraction in the intestine. Rhizoma Parisdis total saponins (RPS) are known to remarkably inhibit the gastric antral smooth muscle contractility through regulation of muscarinic receptors, while SpiA-containing EtRLP improves the symptoms of Lop-induced constipation in SD rats via the regulation of the mAChR downstream signaling pathway and the ER stress response [
22]. The current study is the first to administer SpiA as a single compound into Lop-induced ICR constipation model to verify the laxative effect and mechanism of action. Various effects representing the improvement of constipation were observed after treatment of SpiA, with most outcomes being similar to previous studies. Therefore, we believe that our results are the first direct evidences regarding the molecular mechanism of SpiA exerting its laxative effects in Lop-induced constipation ICR mice model.
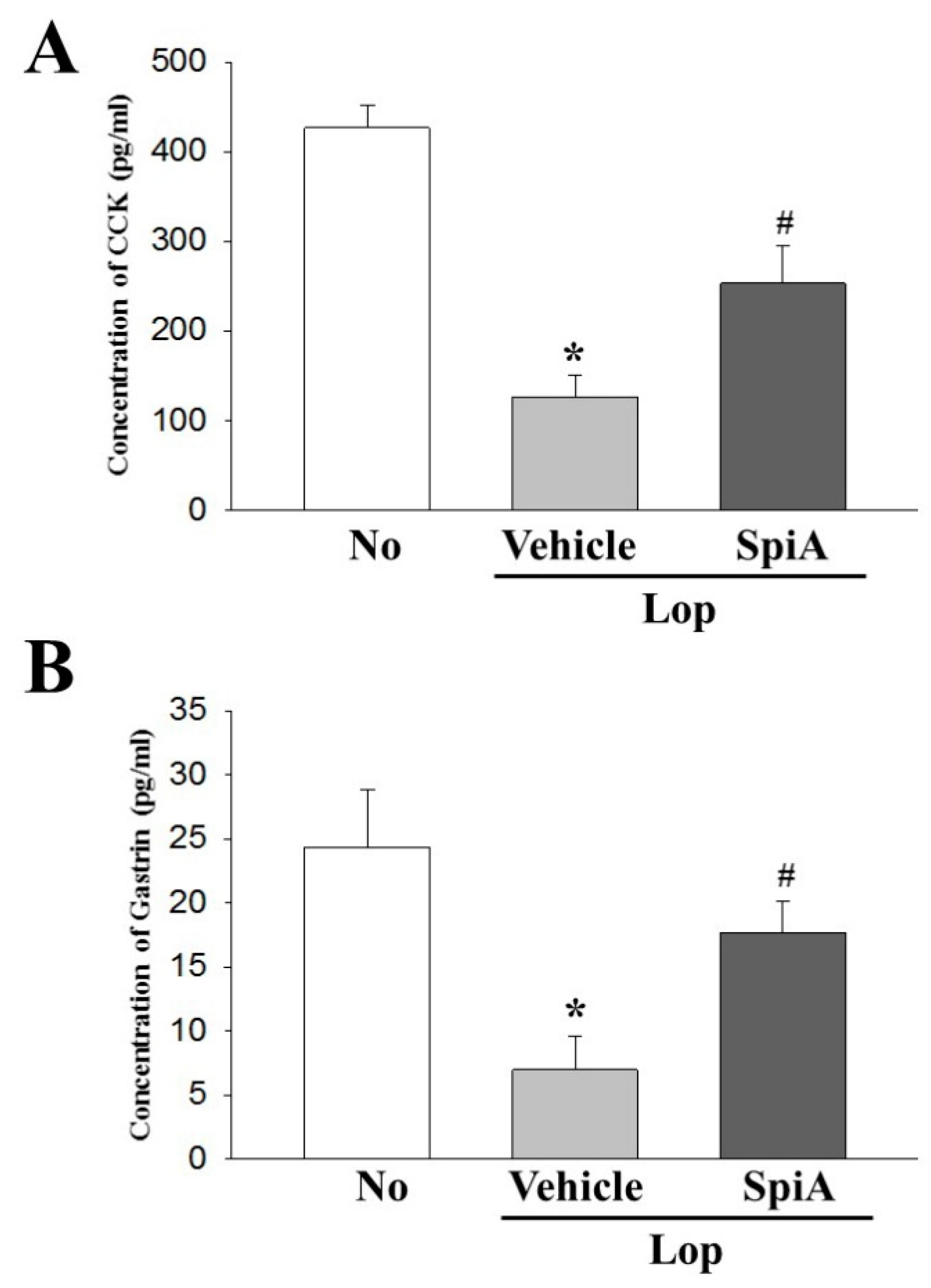
The digestive system is controlled by complex mechanisms regulating motility and secretion. During these processes, CNS, ENS and GI peptides participate as key members to control the function of the enteric nerve, Cajal cells, smooth muscle cells and epithelial cells [
23]. Some natural products with laxative effects induce the recovery of GI metabolic components and enteric nerve-related factors.
Lactobacillus fermentum Suo (LF-Suo) significantly increase the serum levels of MTL, gastrin (GAS), endothelin (ET), AChE, substance P (SP) and vasoactive intestinal peptide (VIP) as well as the expression levels of c-Kit, SCF and GDNF during the preventive effects for constipation in mice model [
24]. Also, a similar effect was observed in the Lop-induced constipation model after administering aqueous extracts of
Herba Cistanche and naringenin [
25,
26]. Furthermore, gallotannin-enriched extracts isolated from Galla Rhois (GEGR) successfully improves the concentration of four GI hormones, including CCK, GAS, somatostatin (SS) and MTL, and their receptor signaling pathways in the constipated animal model [
27]. In the current study, we measured the levels of GI metabolic components and enteric nerve-related factors in the Lop + SpiA treated group to investigate the molecular mechanism of SpiA on laxative activity. Our results are consistent with above previous findings, although there are some differences in the analyzed factors. These results therefore provide novel evidences that SpiA contributes to improving the level of GI metabolic components and enteric nerve-related factors during the laxative effects. However, the present study provides limited information since only five factors were analyzed in the serum and colon. Furthermore, multifactor analyses and mechanism studies are necessary to clarify the laxative role and mechanism of SpiA.
4. Materials and Methods
4.1. Preparation of SpiA
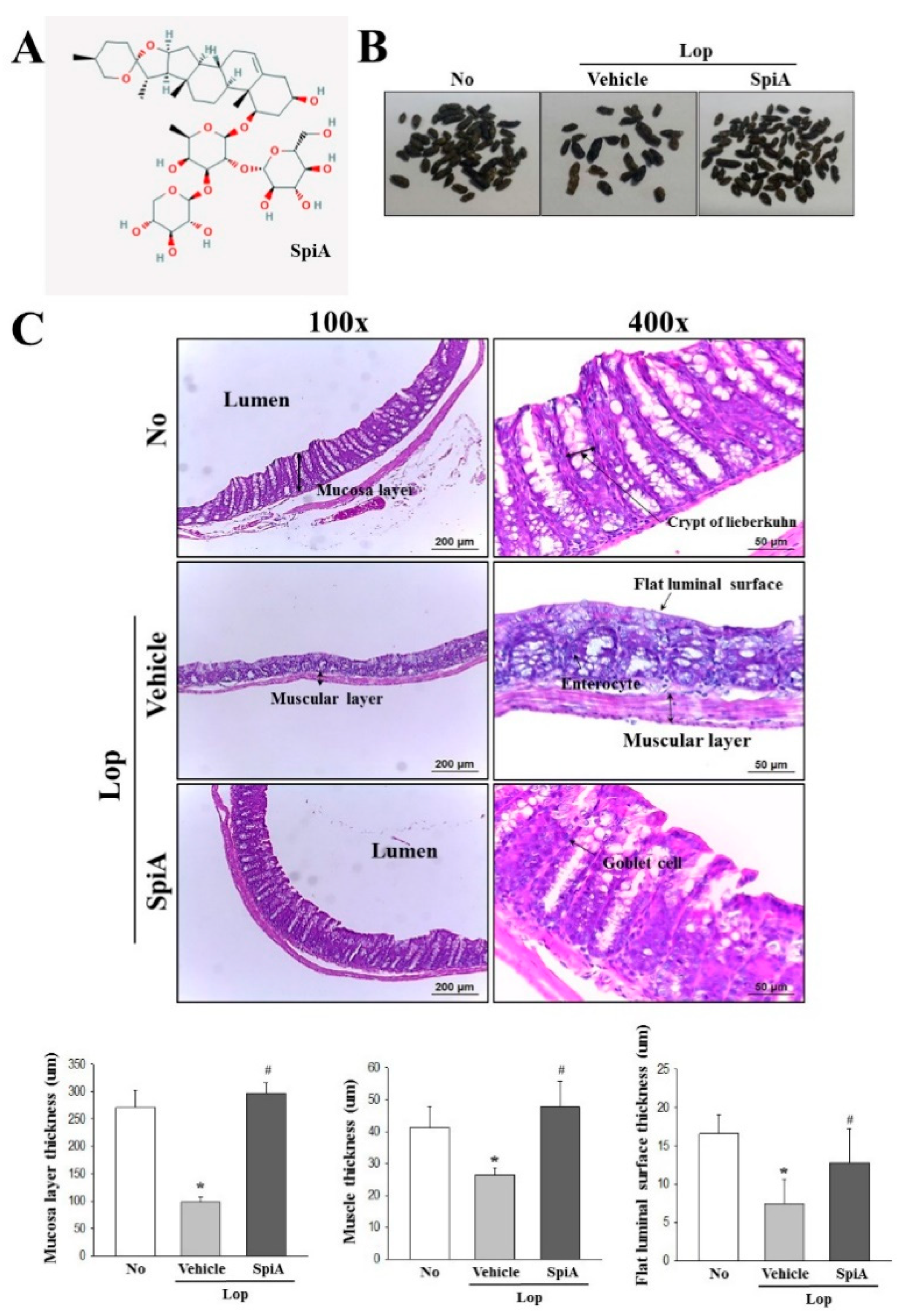
SpiA used in this study (
Figure 1A) was kindly provided by the National Development Institute of Korean Medicine (NIKOM). It was isolated from dried roots of
Liriope platyphylla F.T. Wang & Tang (Liliaceae) through a series of extraction steps, purification steps and nuclear magnetic resonance (NMR) analyses, as described in a previous study [
6].
4.2. Experimental Design for Animal Study
To study the laxative effects of SpiA, the animal protocol was reviewed and approved based on the ethical procedures for scientific care guidelines by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2017-1713). Adult ICR mice purchased from Samtako BioKorea Inc. (Osan, Korea) were handled at the Pusan National University-Laboratory Animal Resources Center, which is accredited by the Korea Food and Drug Administration (KFDA) (Accredited Unit Number-000231) and The Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International (Accredited Unit Number; 001525). Animals were provided with ad libitum access to a standard irradiated chow diet (Samtako BioKorea Inc.) and water. During the experiment, all mice were maintained in a specific pathogen-free (SPF) state under a strict light cycle (lights on at 08:00 h and off at 20:00 h) at 23 ± 2 °C and 50 ± 10% relative humidity.
Constipation of ICR mice were induced based on novel methods, as described in a previous study [
28]. Briefly, eight-week-old ICR mice in each group (
n = 27) were assigned to either a nonconstipation group (untreated group,
n = 9) or a constipation group (
n = 18). ICR mice of the Lop-induced constipation group were further subdivided into Vehicle treated group (Lop + Vehicle treated group,
n = 9) and SpiA treated group (Lop + SpiA treated group,
n = 9). ICR mice were subcutaneously injected with Lop (Sigma-Aldrich, MO, USA) (4 mg/kg weight) in 0.9% sodium chloride, twice a day for 4 days. After a 3-day stationary phase, 8 mg/kg of Lop was subcutaneously administered for 4 days. Following the induction of constipation, the SpiA treated group was orally administered 20 mg/kg of SpiA, whereas the Vehicle treated group was injected with 0.9% sodium chloride alone. At 24 h after the final treatment, constipation parameters were measured in all the mice subset groups, after which all animals were euthanized using CO
2 gas. Tissue samples were acquired and stored in Eppendorf tubes at −70 °C until further assay.
4.3. Analysis of Food Intake, Water Consumption and Body Weight
The food weight, water volume in bottle, and body weight of ICR mice treated with Vehicle or SpiA were measured daily at 10:00 a.m. throughout the experimental period, using an electrical balance (for food and body weight) and a measuring cylinder (for water volume). The average food intake and water consumption of each mice was then calculated using the above data. All measurements were performed in triplicate to ensure accuracy.
4.4. Measurement of Stool Parameters and Urine Volume
During all experimental periods, ICR mice of subset groups were bred in metabolic cages to avoid any contamination and harvest pure stools and urine (Daejong Instrument Industry Co., LTD, Seoul, Korea). The stool number and weight were measured as previously described [
15,
29]. Briefly, stools excreted from each ICR mice were collected daily at 10:00 am; the number of stools were counted three times, and each sample was weighed thrice using an electric balance. The water content of stool was also analyzed using the following formula:
where, A is the weight of fresh stools collected from ICR mice of subset groups, and B is the weight of stools after drying at 60 °C for 12 h. Furthermore, urine volume was measured three times per sample using a cylinder.
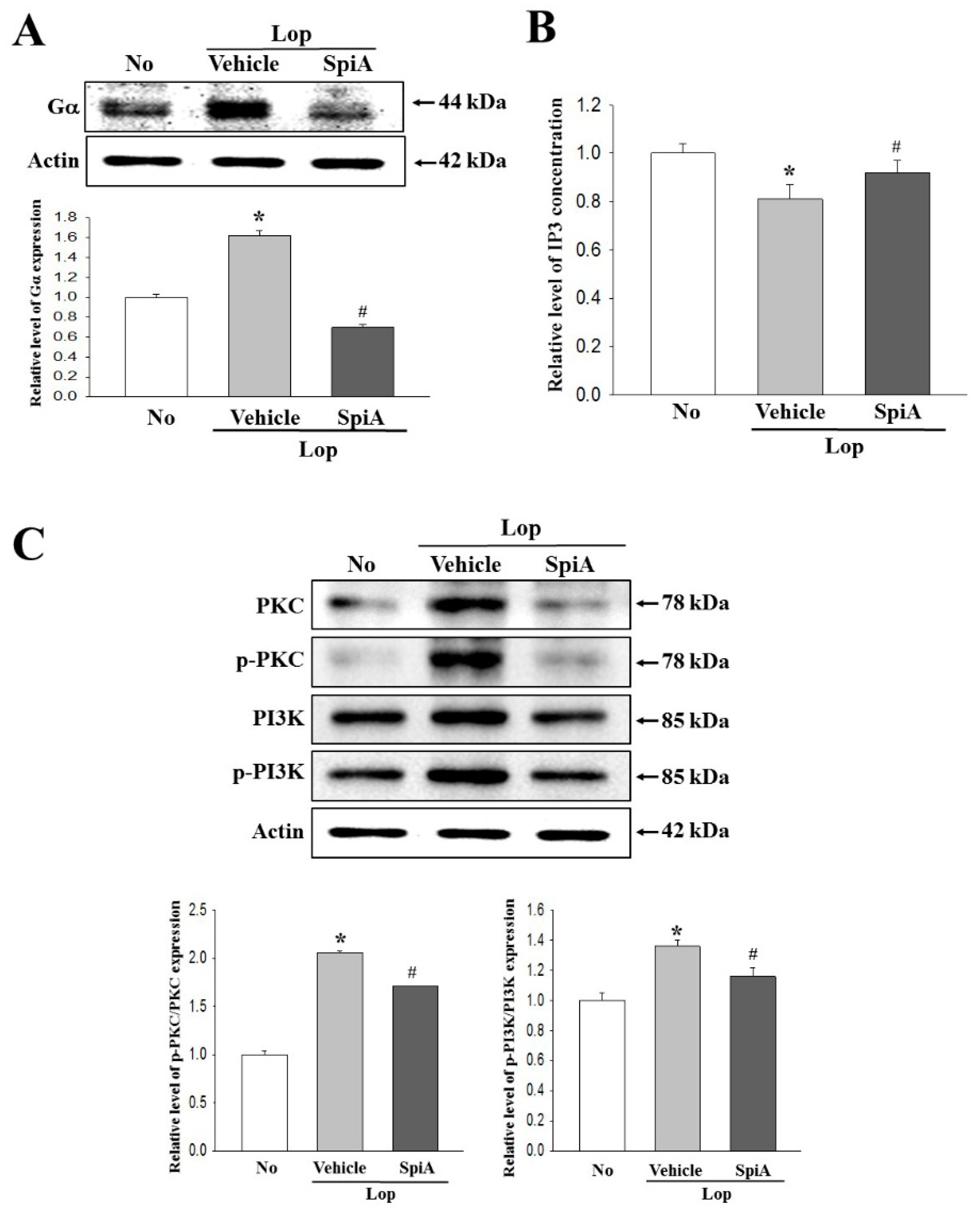
4.5. Western Blotting Analysis
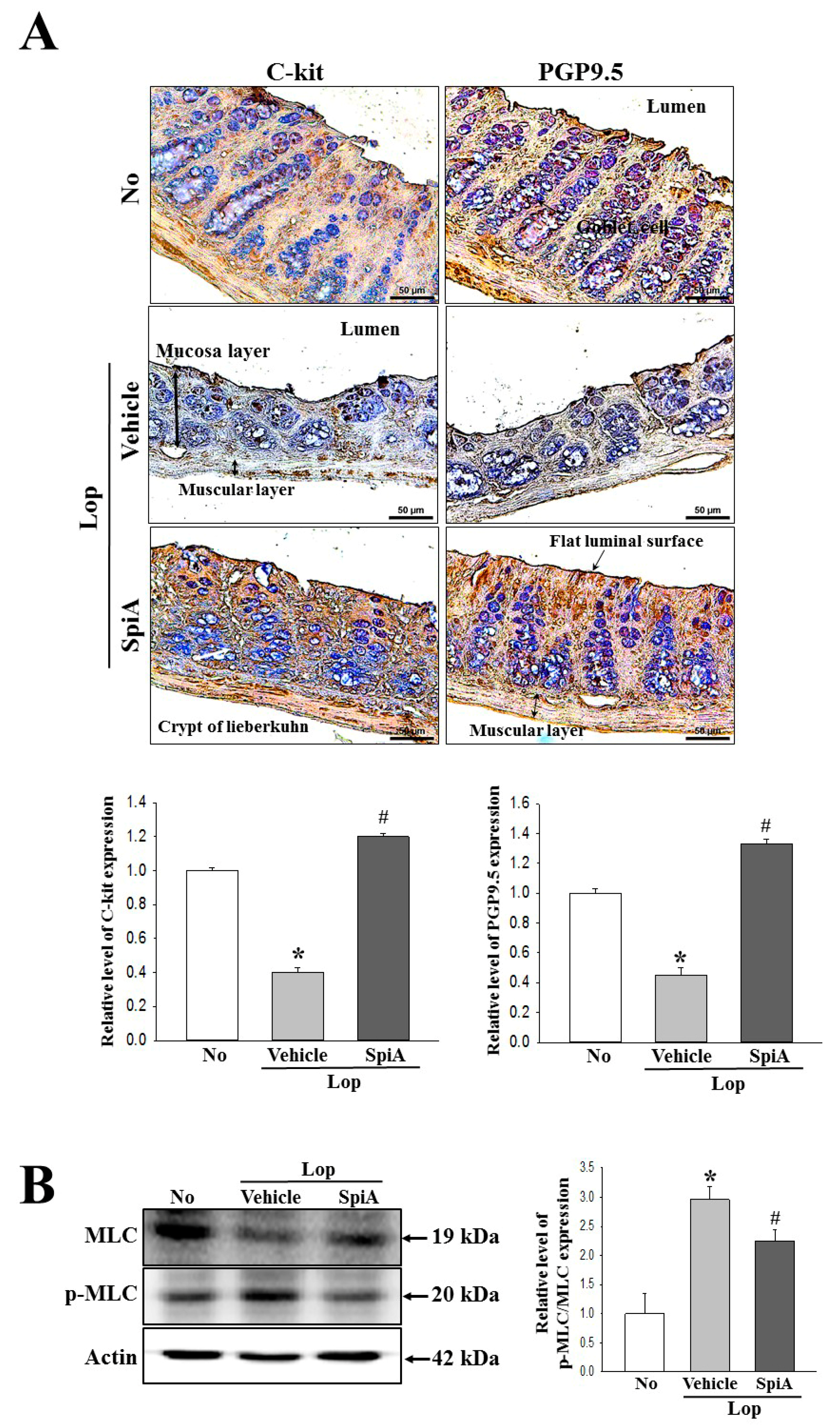
Total homogenate proteins were extracted from the colons of all subset groups (No, Lop + Vehicle and Lop + SpiA treated ICR mice) using the Pro-Prep Protein Extraction Solution (Intron Biotechnology Inc., Seongnam, Korea). Following centrifugation at 13,000 rpm for 5 min, protein concentrations were determined using a SMARTTM Bicinchoninic Acid Protein assay kit (Thermo Fisher Scientific Inc.). Proteins (30 μg) were then separated by 4%–20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for 3 h, following which the resolved proteins were transferred to nitrocellulose membranes for 2 h at 40 V. Each membrane was then incubated separately with primary antibody, overnight at 4 °C: anti-Gα (Abcam, Cambridge, UK), anti-PI-3K (Cell Signaling Technology Inc., Cambridge, MA, USA), anti-p-PI3K (Cell Signaling Technology Inc.), anti-PKC (Cell Signaling Technology Inc.), anti-p-PKC (Cell Signaling Technology Inc.), anti-MLC (Cell Signaling Technology Inc.), anti-p-MLC (Cell Signaling Technology Inc.), or anti-actin (Sigma-Aldrich Co.). The membranes were washed with washing buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, and 0.05% Tween 20), and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Zymed Laboratories, South San Francisco, CA, USA) at a dilution of 1:1000 and room temperature for 2 h. Finally, the blots were developed using a Chemiluminescence Reagent Plus kit (Pfizer Inc., Gladstone, NJ, USA). The signal band image for each protein was acquired using the digital camera (1.92 MP resolution) of the FluorChem® FC2 Imaging system (Alpha Innotech Corporation, San Leandro, CA, USA). Protein densities were semiquantified using the AlphaView Program version 3.2.2 (Cell Biosciences Inc., Santa Clara, CA, USA).
4.6. Semi-Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
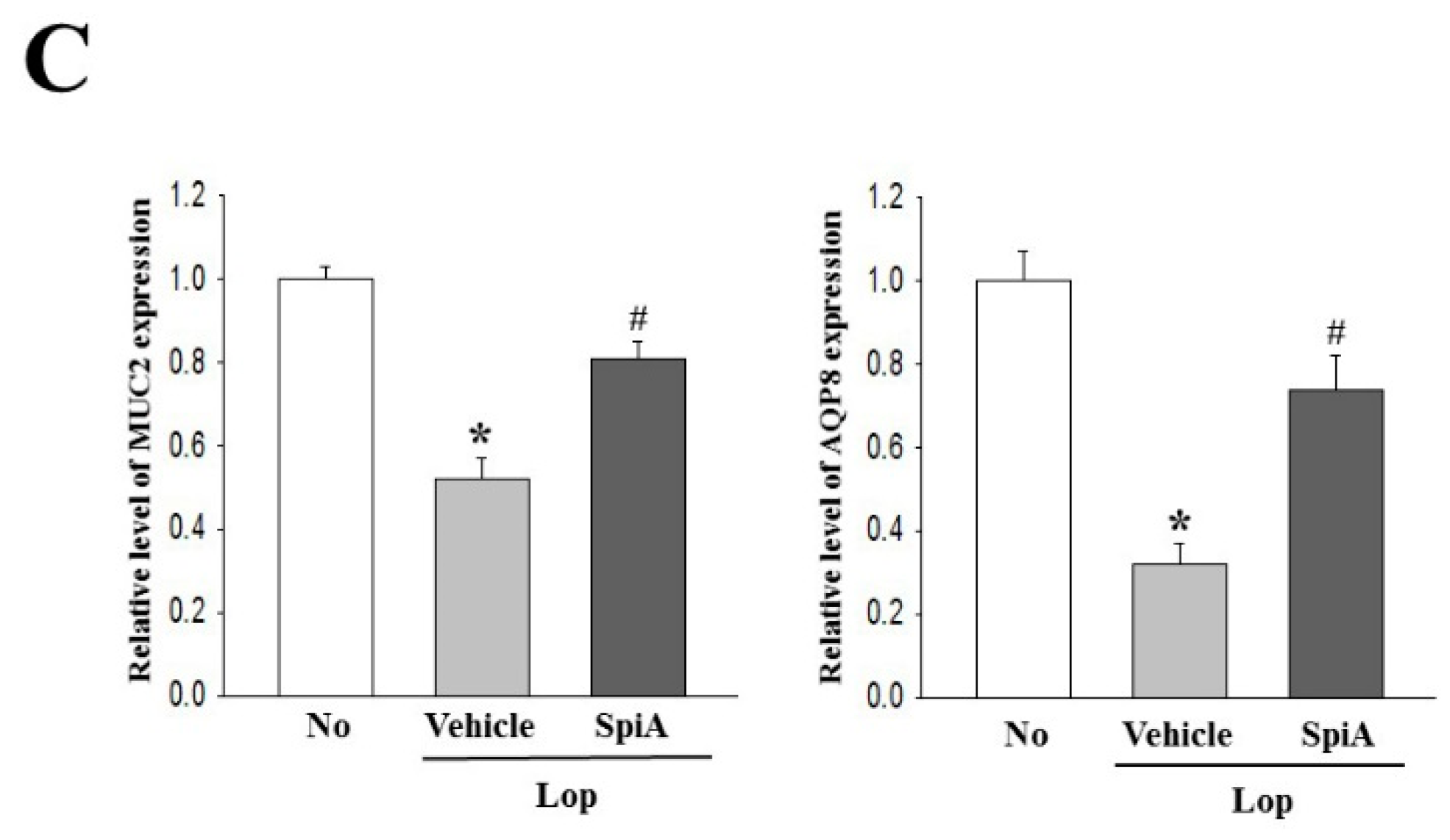
Total RNA was isolated from the frozen colons using RNAzol B solution (Tel-Test Inc.), according to the manufacturer’s protocols. Following synthesis of cDNA, genes were amplified by subjecting the samples to 28 cycles of 30 s at 94 °C, 30 s at 62 °C and 45 s at 72 °C, in a Perkin-Elmer Thermal Cycler. The primer sequences used to evaluate the level of mRNA were as follows: MUC2, sense primer, 5′-GCTGC TCATT GAGAA GAACG ATGC-3′, antisense primer, 5′-CTCTC CAGGT ACACC ATGTT ACCAG G-3′; AQP8, sense primer, 5′-GTAGT ATGGA CCTAC GTGAG ATCAA GG-3′, antisense primer, 5′-AGAAC CTTTC CTCTG GACTC ACCAC C-3′; β-actin, sense and antisense primers were 5′-TGGAA TCCTG TGGCA TCCAT GAAAC-3′ and 5′-TAAAA CGCAG CTCAG TAACA GTCCG-3′, respectively. The PCR products were quantified using 1% agarose gels and a Kodak Electrophoresis Documentation and Analysis System 120.
4.7. Quantitative Real-Time PCR Analysis
Quantitative real-time PCR assessed the relative quantities of mRNA for MUC2 and AQ8. Total RNA molecules were isolated from frozen colon tissues using RNA Bee solution (Tet-Test Inc., Friendswood, TX, USA). After quantification of the RNA concentration, the complement DNA (cDNA) was synthesized using a mixture of oligo-dT primer (Invitrogen, Carlsbad, CA, USA), dNTP and reverse transcriptase (Superscript II, 18064-014, Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Q-PCR was then conducted using a cDNA template and 2×Power SYBR Green (TOYOBO Co., Osaka, Japan) as described in previous studies [
16]. The primer sequences used in quantitative real-time PCR was the same as the one in semi-quantitative RT-PCR analysis. The reaction cycle at which PCR products exceeded this fluorescence intensity threshold during the exponential phase of PCR amplification was considered as the threshold cycle (CT).
4.8. Histopathological Analysis
Colons collected from No, Lop + Vehicle and Lop + SpiA treated ICR mice were fixed with 10% formalin for 48 h, embedded in paraffin wax, and then sectioned into 4-μm thick slices which were subsequently stained with hematoxylin and eosin (H&E, Sigma-Aldrich Co.). Morphological features of the stained sections were observed by light microscopy, after which the mucosa thickness, flat luminal surface thickness and muscle thickness were measured using the Leica Application Suite (Leica Microsystems, Heerbrugg, Switzerland).
For mucin staining, colons collected from ICR mice of subset groups were fixed with 10% formalin for 48 h, embedded in paraffin wax, and then sectioned into 4-μm thick slices that were subsequently deparaffinized with xylene and rehydrated. Next, the tissue sections on the slides were rinsed with distilled water and stained with an Alcian Blue Stain kit (IHC WORLD, Woodstock, MD, USA). Finally, the morphological features in the stained colon sections were observed by light microscopy.
Immunohistochemical analysis was performed as previously described [
30]. Briefly, the histological distribution of C-kit and PGP9.5 protein was observed using optical microscopy after fixing the tissue samples in 10% formalin for 48 h, embedding the tissues in paraffin, and acquiring sections 4-μm in thickness. Each section was de-paraffinized with xylene, rehydrated, and pretreated for 30 min at room temperature with a phosphate buffered saline (PBS)-based blocking buffer containing 10% goat serum. The samples were then incubated with mouse anti-C-kit (DAKO, Kyoto, Japan) and anti-PGP9.5 antibody (Abcam, Cambridge, UK), diluted 1:1000 in PBS-blocking buffer. Antigen-antibody complexes were visualized with goat anti-rabbit HRP-conjugated streptavidin secondary antibody (Histostain-Plus Kit, Zymed Laboratories) diluted 1:1000 in PBS-blocking buffer. A 3,3’-Diaminodbenzidine (DAB) substrate (Invitrogen, Carlsbad, CA, USA) and a model GS-690 imaging densitometer (Bio-Rad Laboratories, Hercules, CA, USA) were used to detect the C-kit and PGP9.5 proteins.
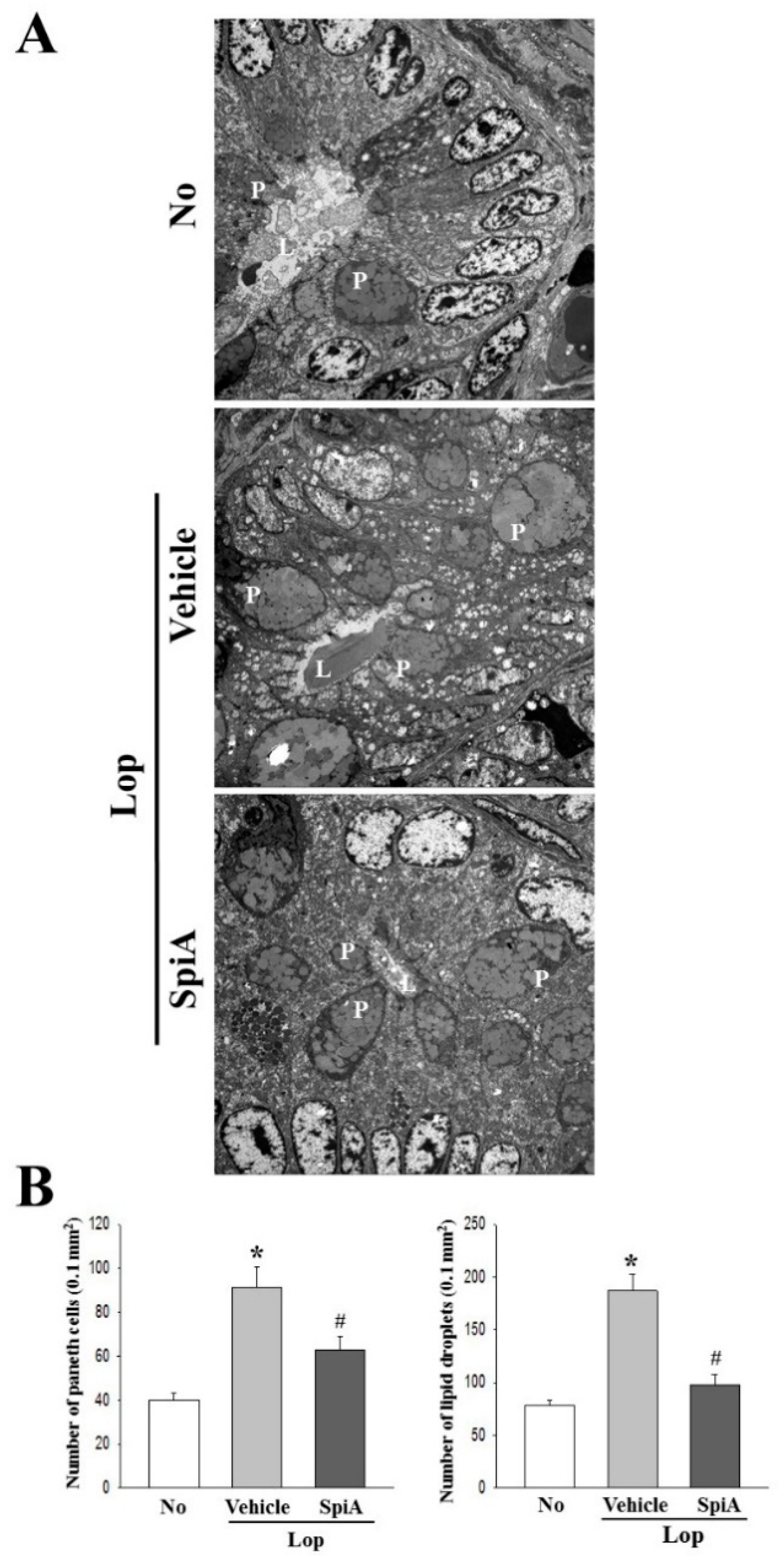
4.9. Transmission Electron Microscopy (TEM) Analysis
Colon tissues collected from 5–6 mice from each of the five treatment groups were fixed in 2.5% glutaraldehyde solution, rinsed with 1× PBS solution, dehydrated with ascending concentrations of EtOH solution, post-fixed in 1% osmium tetroxide (OsO4) for 1–2 h at room temperature, and embedded in Epon 812 media (Polysciences, Hirschberg an der Bergstrasse, Germany). Subsequently, ultra-thin sections of colon tissue (70-nm thick) were placed on a holey formvar-carbon coated grid, after which the grids were negatively stained using uranyl acetate and lead citrate. Morphological features of tissues were examined by TEM (Hitachi, Tokyo, Japan).
4.10. Measurement of Inositol Trisphosphate (IP3) Concentration
Levels of IP3 were determined using an IP3 ELISA kit (Cusabio Biotech Co., Ltd., Wuhan, China), according to the manufacturer’s instructions. Briefly, the frozen colon tissues were washed and homogenized in ice-cold PBS (pH 7.2–7.4) using a glass homogenizer (Sigma-Aldrich Co.). Tissue lysates were centrifuged at 1000 rpm for 5 min at room temperature, after which the supernatant was collected for analysis. An anti-IP3 detection antibody was added and incubated at 37 °C for 60 min, after which the substrate solution was added and the samples were further incubated for 15 min at 37 °C. The reaction was terminated following the addition of stop solution, and the plates were read at an absorbance of 450 nm using a Molecular Devices VersaMax Plate Reader (Molecular Devices, Sunnyvale, CA, USA).
4.11. Measurement of Gastrointestinal (GI) Hormone Concentrations
The concentration of CCK and gastrin were quantified using ELISA kits (Cusabio Biotech Co., Ltd., Wuhan, China), according to the manufacturer’s instructions. Briefly, the tissue of colons (100 mg) were homogenized in ice-cold 1× PBS (pH 7.2–7.4) using a glass homogenizer (Sigma-Aldrich Co.). The tissue lysates were then centrifuged at 1000 rpm for 5 min at 4 °C, after which the supernatant was collected for analysis. After addition of the two specific hormone antibodies (separately in each well), the supernatant was incubated for 60 min at 37 °C, to which HRP-Streptavidin solution was subsequently added and incubated for 60 min at 37 °C. This was followed by addition of the TMP One-Step Substrate Reagent, and the mixture was further incubated for 30 min at 37 °C. The reaction was terminated following addition of the stop solution. Finally, the absorbance of the reaction mixture was read at 450 nm using the Molecular Devices VersaMax Plate Reader (Sunnyvale, CA, USA).
4.12. AChE Activity Analysis
The AChE activity was determined using an Acetylcholinesterase Assay Kit (Abcam, Cambridge, UK), according to the manufacturer’s protocols. Briefly, the colon of each mouse was homogenized in PRO-PREP protein extraction solution (1.0 mM PMSF, 1.0 mM EDTA, 1.0 µM pepstatin, 1.0 µM leupeptin, and 1.0 µM aprotinin) (iNtRON Biotechnology Inc., Seoul, Korea), after which the homogenates were stored at −70 °C until analysis. The sample or standards and ACh reaction mixture were then incubated in a 96-well plate for 10 min at room temperature, protected from the light. Color alterations were read using a Vmax plate reader (Molecular Devices, Sunnyvale, CA, USA) at 410 nm.
4.13. Statistical Analysis
Statistical significance was evaluated using one-way analysis of variance (ANOVA) (SPSS for Windows, Release 10.10, Standard Version, Chicago, IL, USA) followed by Tukey’s post hoc t-test for multiple comparisons. Data are presented as means ± SD (standard deviation). p < 0.05 is considered to indicate a statistically significant difference.