Design of an Amphiphilic Poly(aspartamide)-Mediated Self-Assembled Nanoconstruct for Long-Term Tumor Targeting and Bioimaging
Abstract
:1. Introduction
2. Results and Discussion
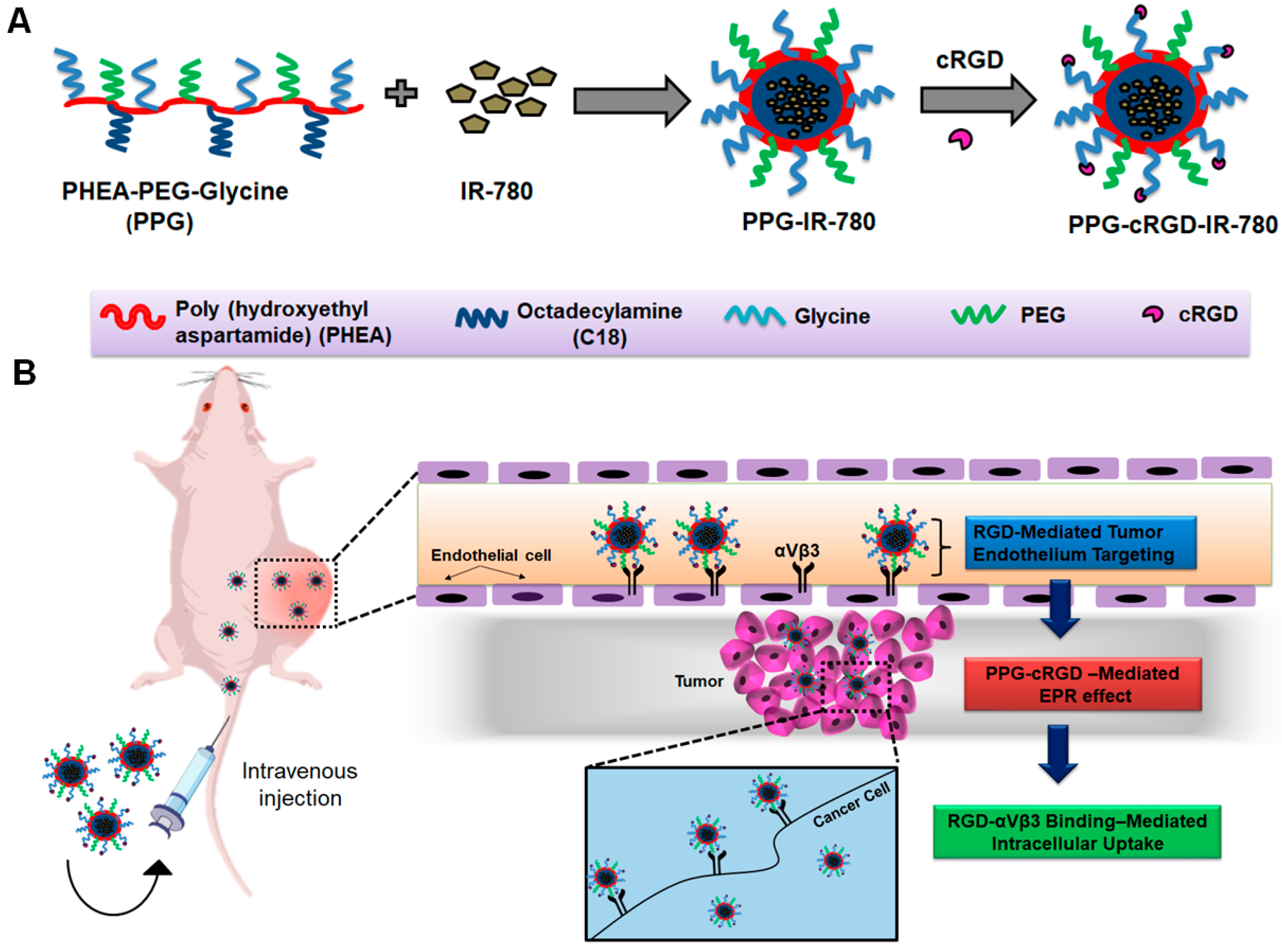
2.1. Synthesis of PPG and PPG-cRGD
2.2. Physicochemical Characterization
2.3. Critical Micelle Concentration and Stability of PPG NPs
2.4. In Vitro Cytocompatibility and Intracellular Uptake
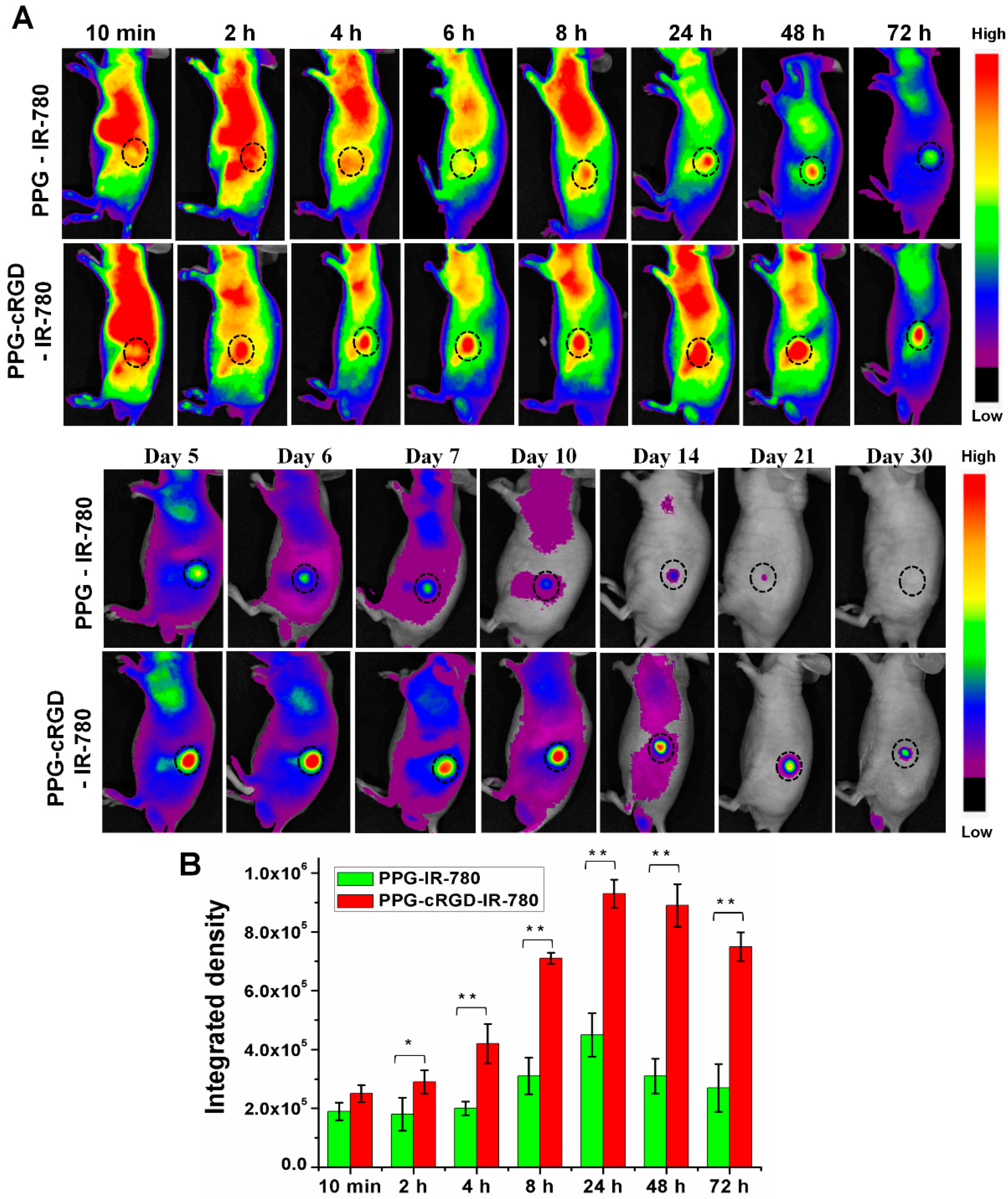
2.5. Long-Term Tumor Targeting and Bioimaging
3. Materials and Methods
3.1. Materials
3.2. Synthesis of PPG and PPG-cRGD NPs
3.3. Measurement of Particle Size Distribution, Zeta Potential, and Stability
3.4. Nuclear Magnetic Resonance Spectroscopy and Field-Emission Transmission Electron Microscopy
3.5. Critical Micelle Concentration Determination
3.6. IR 780 Loading
3.7. In Vitro Cytocompatibility and Intracellular Uptake
3.8. In Vivo Imaging and Biodistribution Analysis
3.9. Hematoxylin and Eosin Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. Handb. Exp. Pharmacol. 2010, 3–53. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-D.; Huang, L.; Nanoparticles, B. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zheng, J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Eliceiri, B.P.; Cheresh, D.A. Adhesion events in angiogenesis. Curr. Opin. Cell Biol. 2001, 13, 563–568. [Google Scholar] [CrossRef]
- Yancopoulos, G.D.; Klagsbrun, M.; Folkman, J. Vasculogenesis, angiogenesis, and growth factors: Ephrins enter the fray at the border. Cell 1998, 93, 661–664. [Google Scholar] [CrossRef]
- Reinmuth, N.; Liu, W.; Ahmad, S.A.; Fan, F.; Stoeltzing, O.; Parikh, A.A.; Bucana, C.D.; Gallick, G.E.; Nickols, M.A.; Westlin, W.F.; et al. αvβ3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003, 63, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Gamble, L.J.; Borovjagin, A.V.; Matthews, Q.L. Role of RGD-containing ligands in targeting cellular integrins: Applications for ovarian cancer virotherapy (Review). Exp. Ther. Med. 2010, 1, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Haubner, R.; Gratias, R.; Diefenbach, B.; Goodman, S.L.; Jonczyk, A.; Kessler, H. Structural and functional aspects of RGD-containing cyclic pentapeptides as highly potent and selective integrin α(v)β3 antagonists. J. Am. Chem. Soc. 1996, 118, 7461–7472. [Google Scholar] [CrossRef]
- Lee, H.J.; Yang, S.R.; An, E.J.; Kim, J.D. Biodegradable polymersomes from poly(2-hydroxyethyl aspartamide) grafted with lactic acid oligomers in aqueous solution. Macromolecules 2006, 39, 4938–4940. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Materials science: Soft surfaces: Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Shenton, J. Vitamin a in health foods. Lancet 1985, 325, 460. [Google Scholar] [CrossRef]
- Liu, D.E.; Han, H.; Lu, H.; Wu, G.; Wang, Y.; Ma, J.; Gao, H. Synthesis of amphiphilic polyaspartamide derivatives and construction of reverse micelles. RSC Adv. 2014, 4, 37130–37137. [Google Scholar] [CrossRef]
- Muthiah, M.; Lee, S.J.; Moon, M.; Lee, H.J.; Bae, W.K.; Chung, I.J.; Jeong, Y.Y.; Park, I.-K. Surface tunable polymersomes loaded with magnetic contrast agent and drug for image guided cancer therapy. J. Nanosci. Nanotechnol. 2013, 13, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, H.J.; Moon, M.-J.; Vu-Quang, H.; Lee, H.-J.; Muthiah, M.; Che, H.-L.; Heo, S.U.; Jeong, H.J.; Jeong, Y.Y.; et al. Superparamagnetic iron oxide nanoparticles-loaded polymersome-mediated gene delivery guided by enhanced magnetic resonance signal. J. Nanosci. Nanotechnol. 2011, 11, 7057–7060. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy, 3rd ed.; Saunders College Division: Philadelphia, PA, USA, 2001; ISBN 978-85-221-0708-7. [Google Scholar]
- Lai, Y.; Xie, C.; Zhang, Z.; Lu, W.; Ding, J. Design and synthesis of a potent peptide containing both specific and non-specific cell-adhesion motifs. Biomaterials 2010, 31, 4809–4817. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.H.; Jeong, J.H.; Devolder, R.J.; Brockman, C.; Schroeder, C.; Kong, H. Ellipsoidal polyaspartamide polymersomes with enhanced cell-targeting ability. Adv. Funct. Mater. 2012, 22, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Liu, P.; Zheng, M.; Zhao, P.; Wang, Y.; Ma, Y.; Cai, L. IR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapy. Biomaterials 2013, 34, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-B.; Cai, W.; Cao, Q.; Chen, K.; Wu, Z.; He, L.; Chen, X. 64Cu-labeled tetrameric and octameric RGD peptides for small-animal PET of tumor v3 integrin expression. J. Nucl. Med. 2007, 48, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, B.; Balalaie, S.; Shaygan, P. A new and efficient protocol for preparation of thiol esters from carboxylic acids and thiols in the presence of 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TBTU). Arkivoc 2007, 2007, 47–52. [Google Scholar] [CrossRef]
- Höfle, G.; Steglich, W.; Vorbrüggen, H. 4-Dialkylaminopyridines as highly active acylation catalysts. Angew. Chem. Int. Ed. 1978, 17, 569–583. [Google Scholar] [CrossRef]
- Yuan, W.; Guo, W.; Zou, H.; Ren, J. Tunable thermo-, pH- and light-responsive copolymer micelles. Polym. Chem. 2013, 4, 3934–3937. [Google Scholar] [CrossRef]
Sample Availability: Samples of the PPG-IR-780 and PPG-cRGD-IR-780 are available from the authors upon request for research collaborations. |







| Formulation | D/P a Ratio | Hydrodynamic Size (nm) | Zeta Potential (mV) | Polydispersity Index (PDI) b | EE | DL |
|---|---|---|---|---|---|---|
| PPG | - | 235 ± 5 | −24 ± 0.8 | 0.262 | - | - |
| PPG-cRGD | - | 238 ± 6 | −28 ± 1 | 0.258 | - | - |
| PPG-IR-780 | 0.01 | 219 ± 7 | −27 ± 2 | 0.201 | 90 | 1.06 |
| PPG-cRGD-IR-780 | 0.01 | 222 ± 3 | −31 ± 4 | 0.232 | 93 | 1.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherukula, K.; Uthaman, S.; Park, I.-K. Design of an Amphiphilic Poly(aspartamide)-Mediated Self-Assembled Nanoconstruct for Long-Term Tumor Targeting and Bioimaging. Molecules 2019, 24, 885. https://doi.org/10.3390/molecules24050885
Cherukula K, Uthaman S, Park I-K. Design of an Amphiphilic Poly(aspartamide)-Mediated Self-Assembled Nanoconstruct for Long-Term Tumor Targeting and Bioimaging. Molecules. 2019; 24(5):885. https://doi.org/10.3390/molecules24050885
Chicago/Turabian StyleCherukula, Kondareddy, Saji Uthaman, and In-Kyu Park. 2019. "Design of an Amphiphilic Poly(aspartamide)-Mediated Self-Assembled Nanoconstruct for Long-Term Tumor Targeting and Bioimaging" Molecules 24, no. 5: 885. https://doi.org/10.3390/molecules24050885
APA StyleCherukula, K., Uthaman, S., & Park, I.-K. (2019). Design of an Amphiphilic Poly(aspartamide)-Mediated Self-Assembled Nanoconstruct for Long-Term Tumor Targeting and Bioimaging. Molecules, 24(5), 885. https://doi.org/10.3390/molecules24050885







