A Validated HPLC-MS/MS Assay for 14-O-[(4,6-Diaminopyrimidine-2-yl)thioacetyl] Mutilin in Biological Samples and Its Pharmacokinetic, Distribution and Excretion via Urine and Feces in Rats
Abstract
1. Introduction
2. Results and Discussion
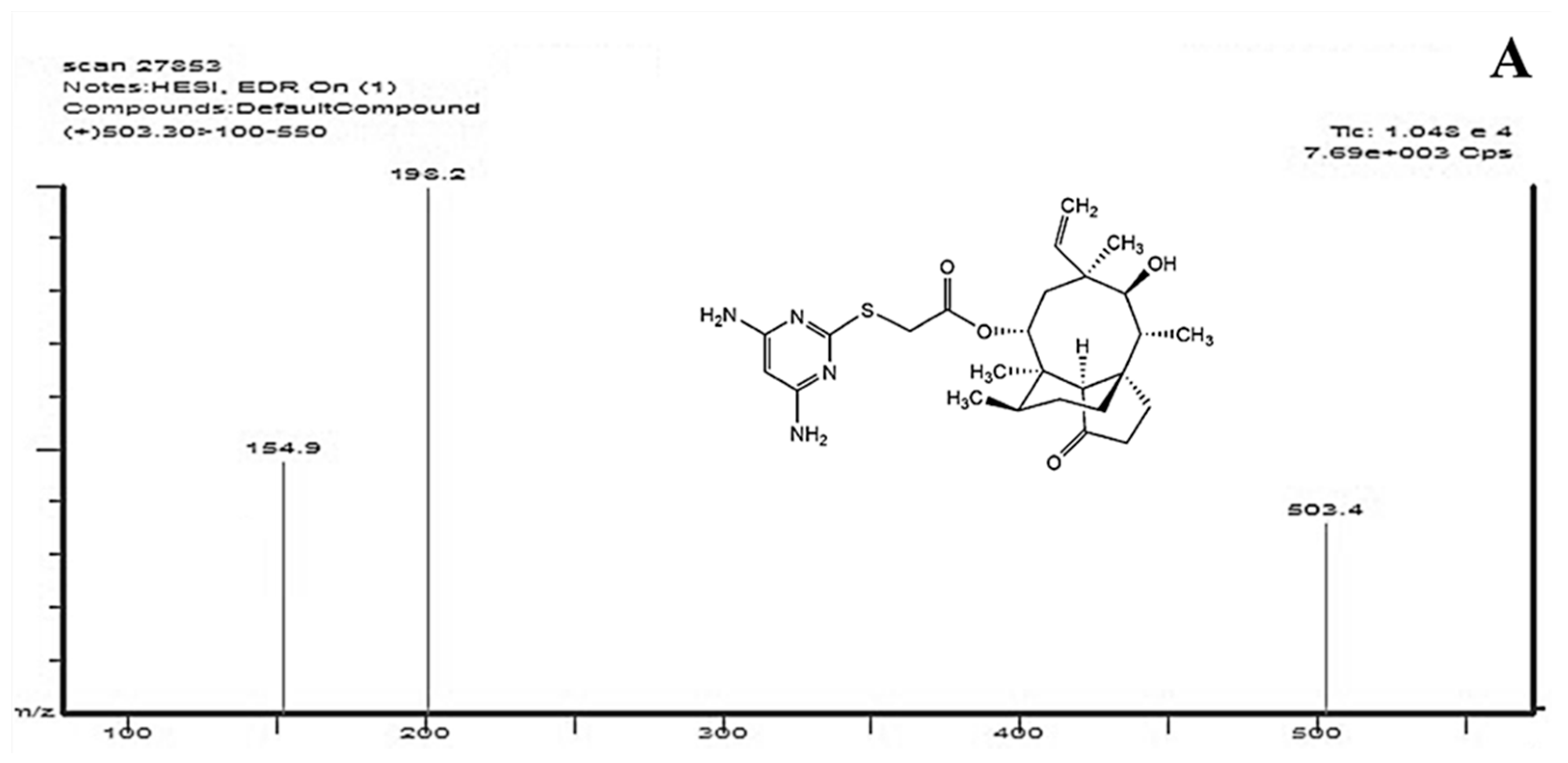
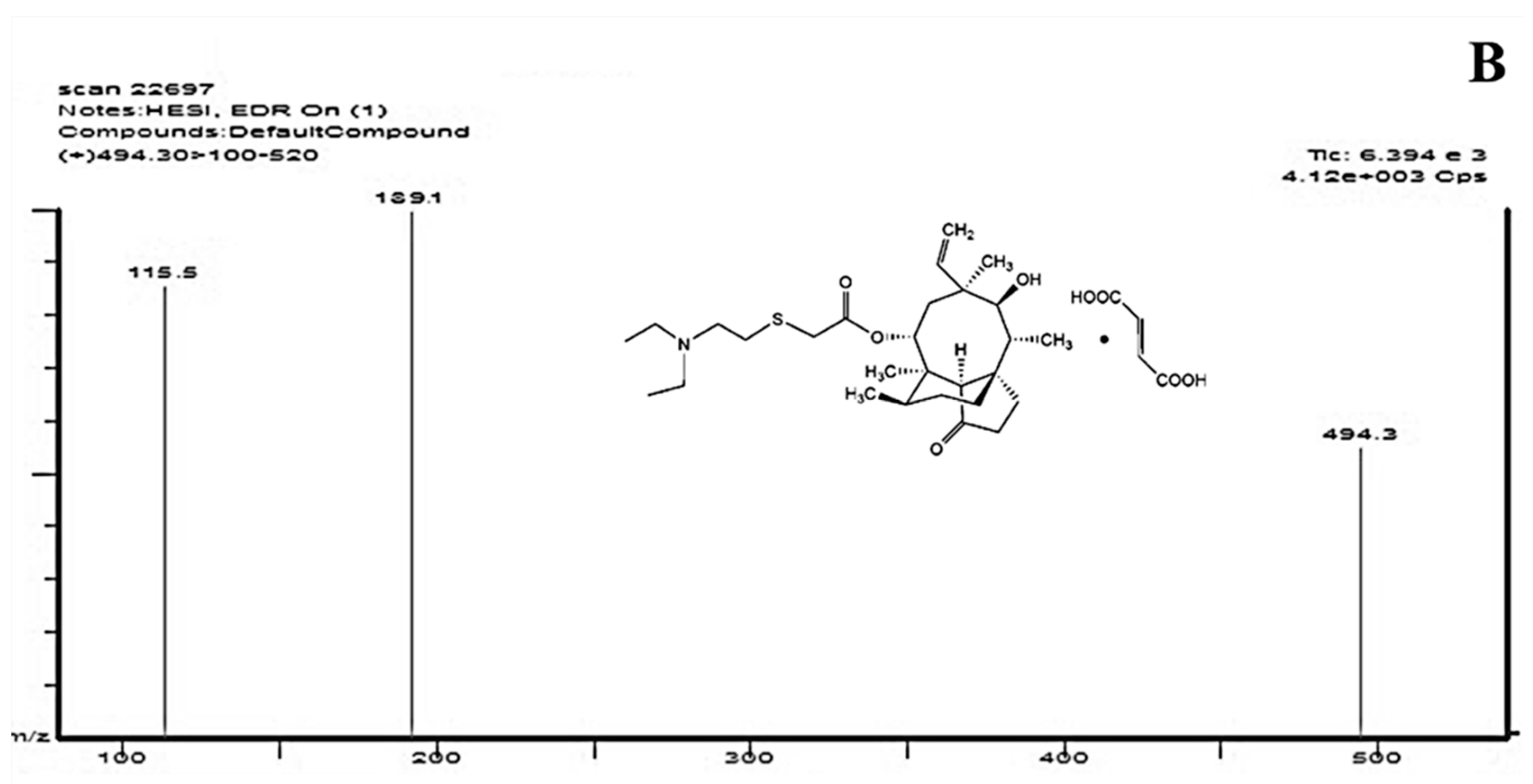
2.1. Optimization of HPLC-MS/MS Conditions
2.2. Method Validation
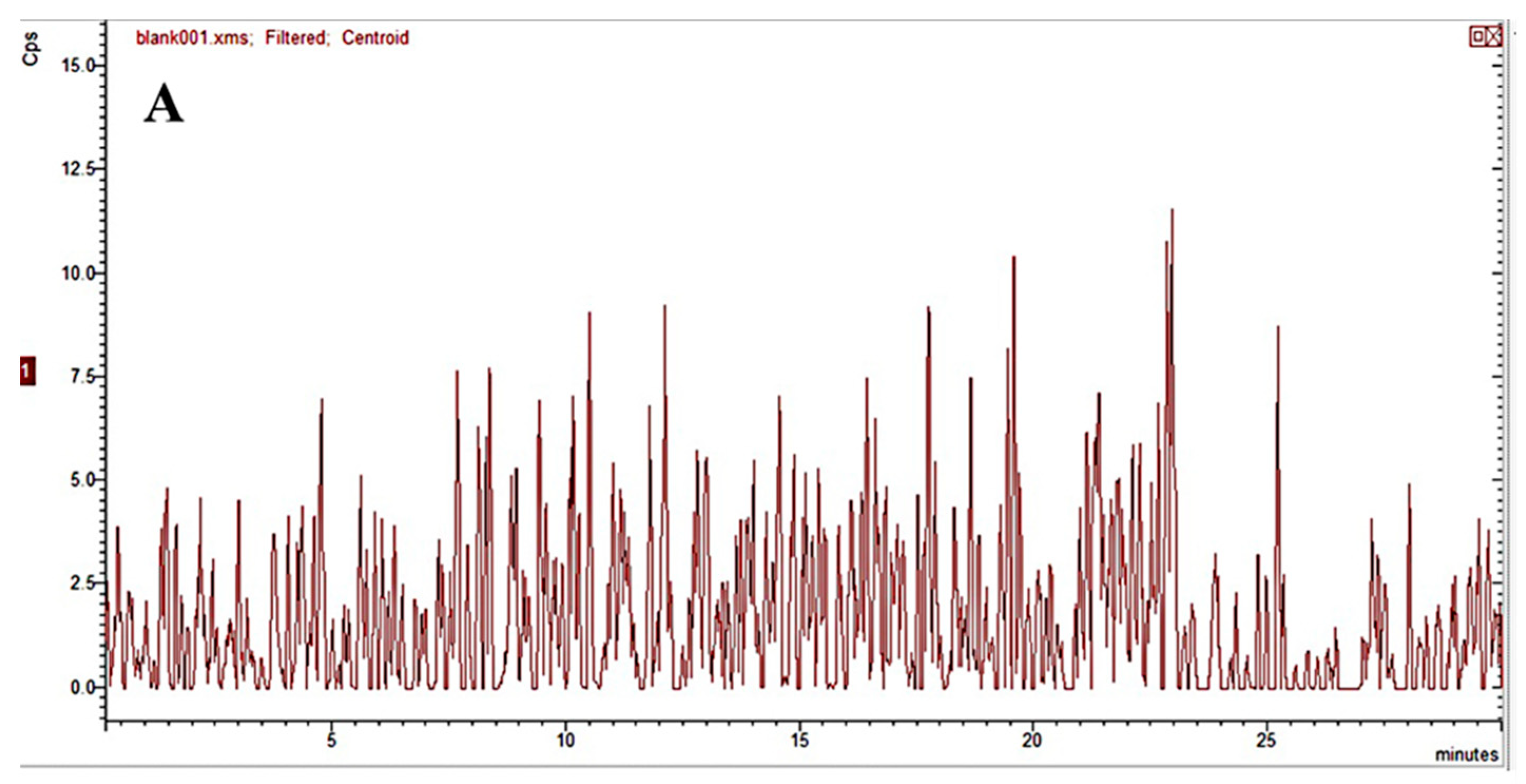
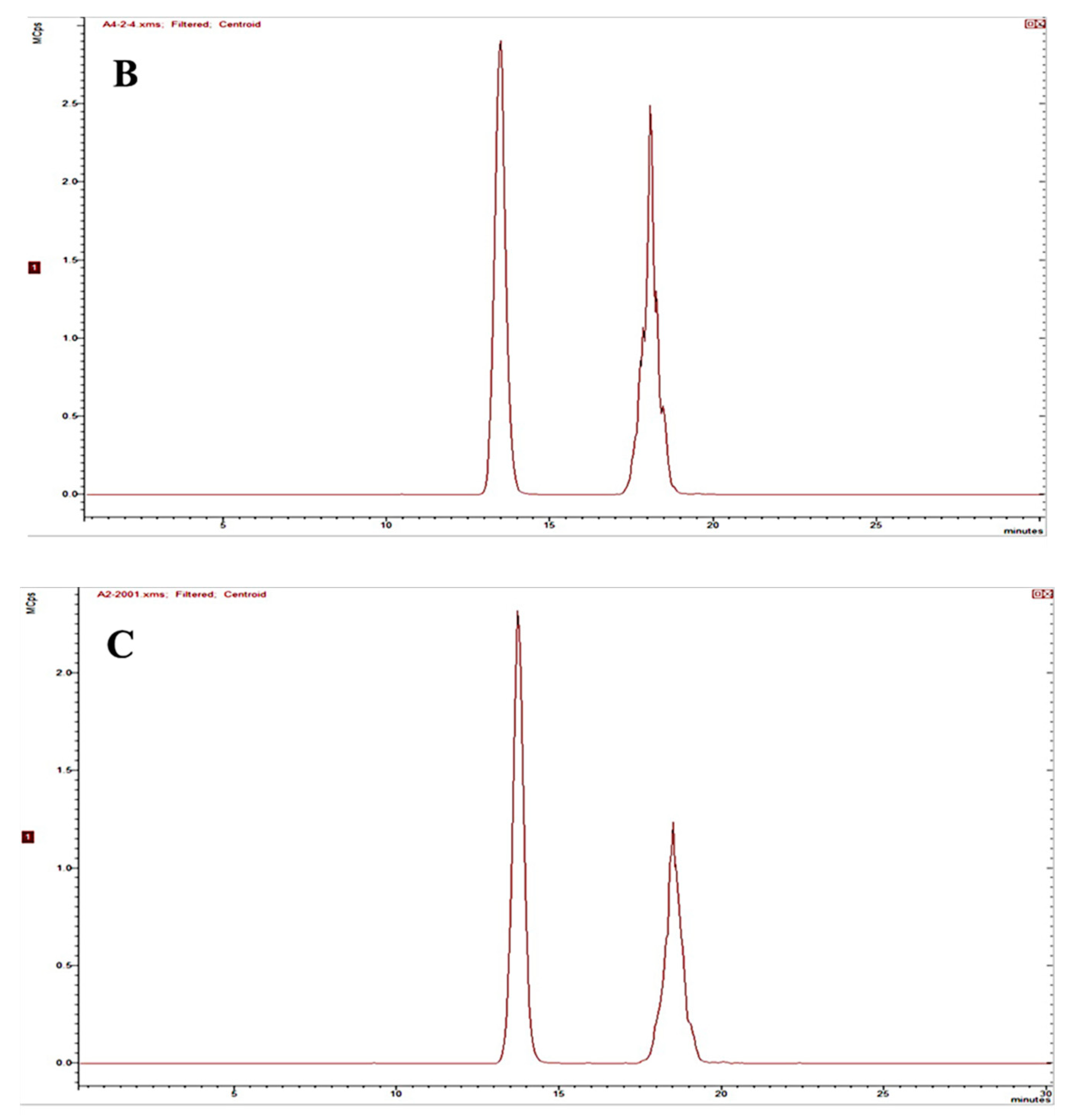
2.2.1. Specificity
2.2.2. Linearity and Sensitivity
2.2.3. Accuracy and Precision
2.2.4. Extraction Recovery and Matrix Effects
2.2.5. Dilution Integrity
2.2.6. Stability
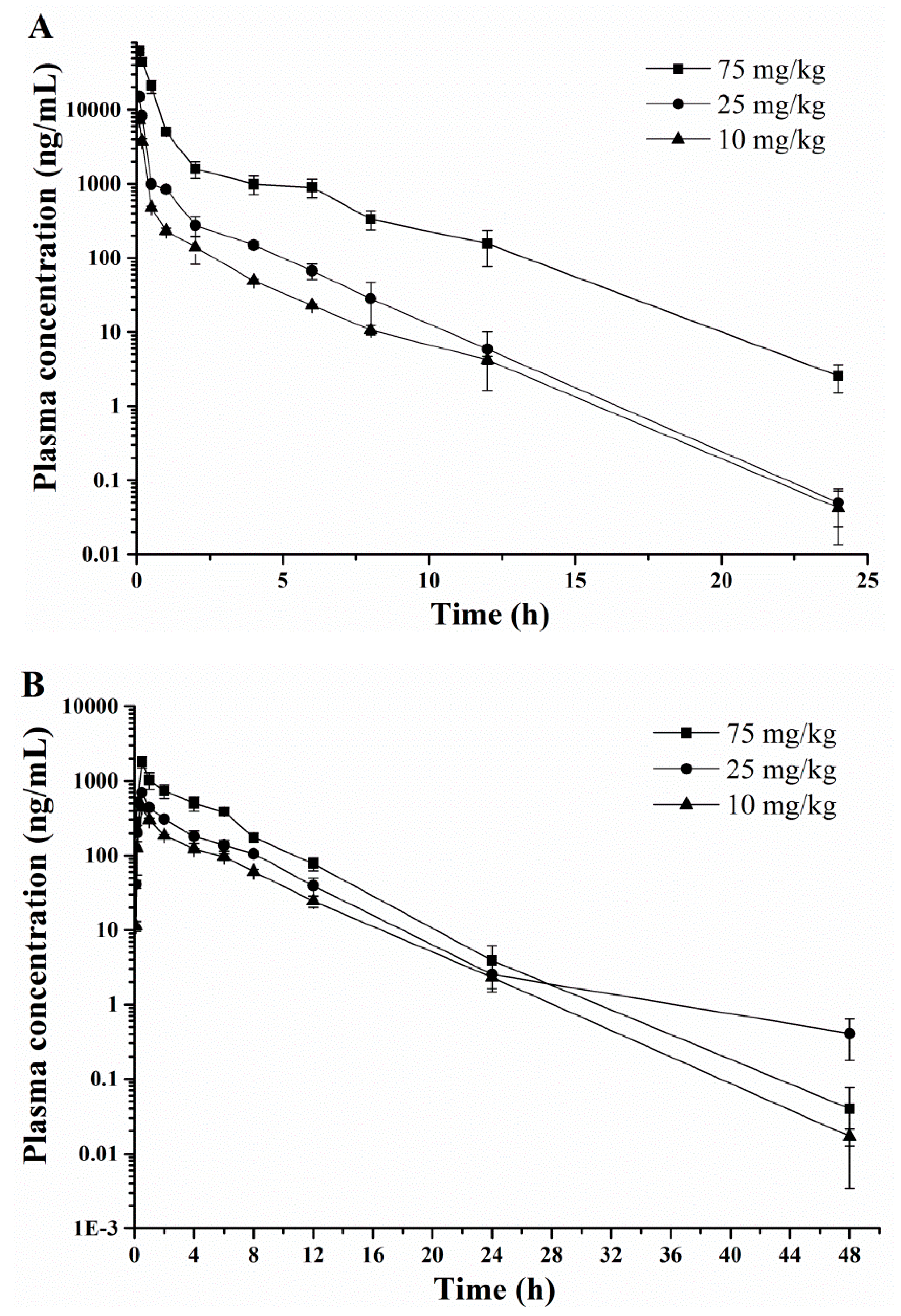
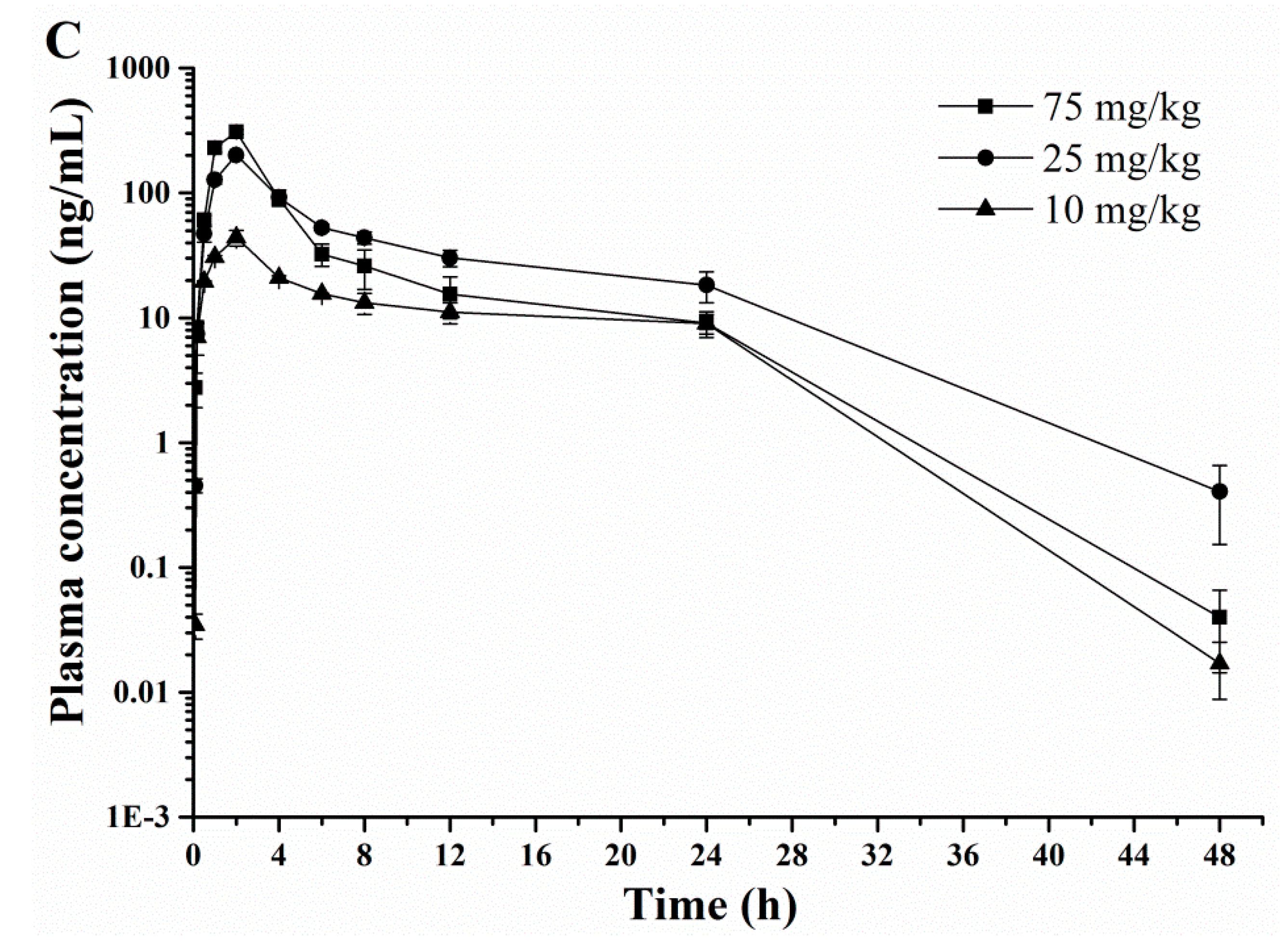
2.3. Pharmacokinetic Profiles
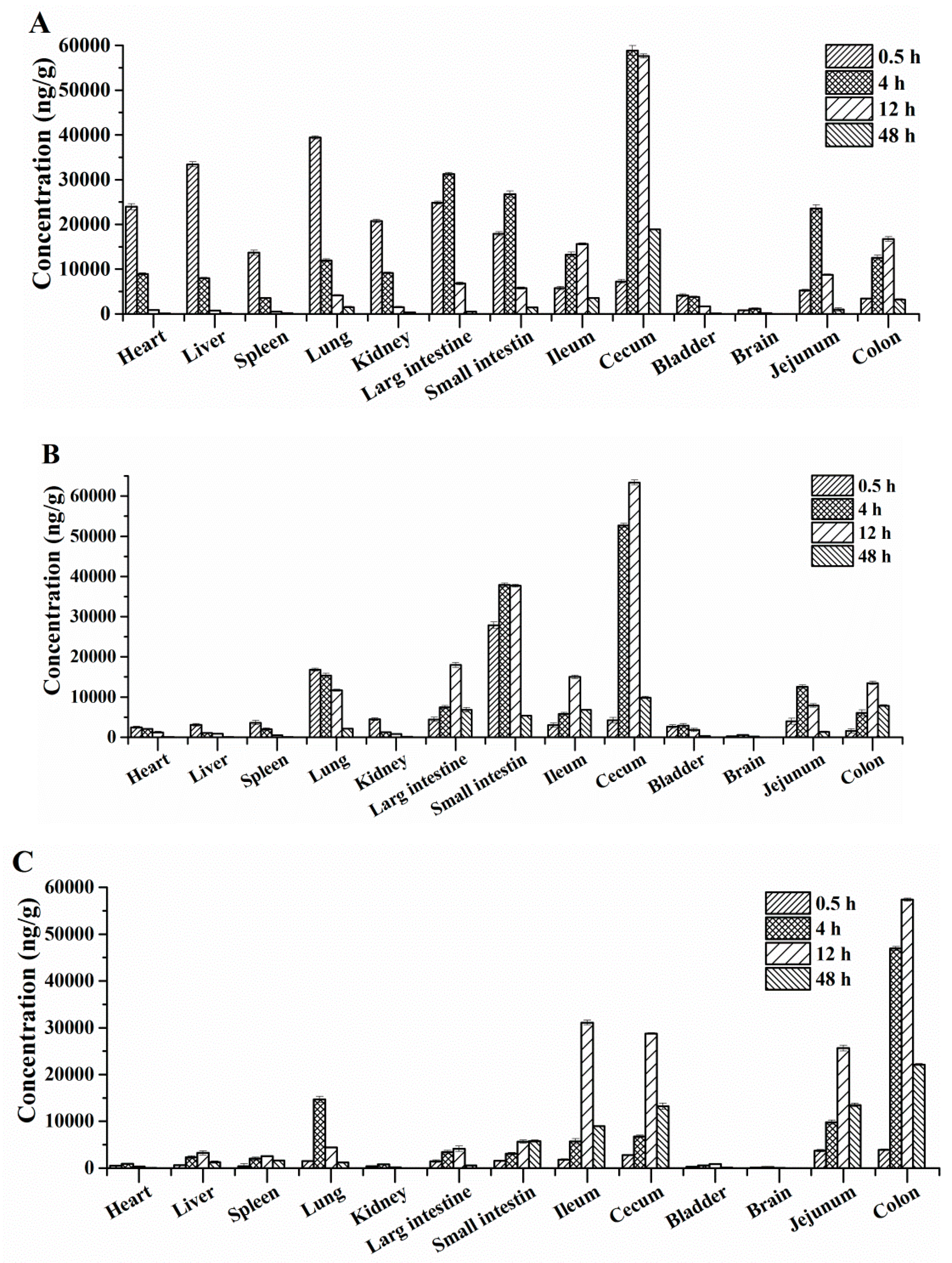
2.4. Tissue Distribution
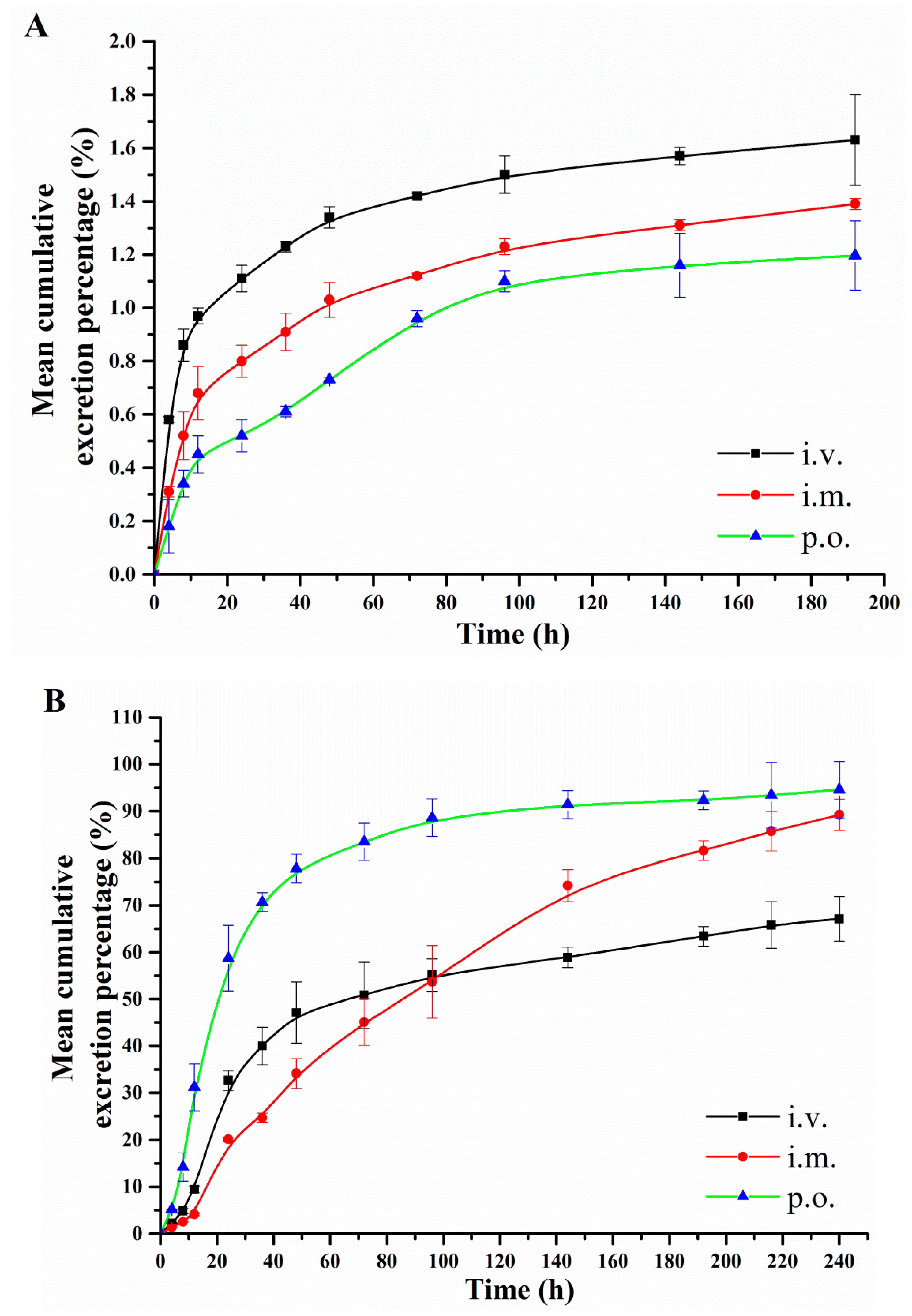
2.5. Excretion via Urine and Feces
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Animals
3.3. HPLC and Mass Spectrometry
3.4. Preparation of Stock and Working Solutions
3.5. Preparation of Calibration Standards and Quality Control (QC) Samples
3.6. Sample Treatment
3.7. Method Validation
3.8. Pharmacokinetic Study
3.9. Tissue Distribution Study in Rats
3.10. Excretion via Urine and Feces Study in Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kavanagh, F.; Hervey, A.; Robbins, W.J. Antibiotic substances from basidiomycetes: VIII. Pleurotus multilus (Fr.) sacc. and pleurotus passeckerianus pilat. Proc. Natl. Acad. Sci. USA 1951, 37, 570–574. [Google Scholar]
- Farney, E.P.; Feng, S.S.; Schafers, F.; Reisman, S.E. Total synthesis of (+)-pleuromutilin. J. Am. Chem. Soc. 2018, 140, 1267–1270. [Google Scholar] [CrossRef]
- Sun, S.J.; Ai, L.Y.; Zhang, H.Y.; Weng, C.H.; Lai, C.F.; Liu, L. Enhanced production of pleuromutilin by Pleurotus mutilus and study on its molecular structure. Food Chem. 2017, 230, 350–353. [Google Scholar] [CrossRef]
- Siricilla, S.; Mitachi, K.; Yang, J.S.; Eslamimehr, S.; Lemieux, M.R.; Meibohm, B.; Ji, Y.; Kurosu, M. A New Combination of a Pleuromutilin Derivative and Doxycycline for Treatment of Multidrug-Resistant Acinetobacter baumannii. J. Med. Chem. 2017, 60, 2869–2878. [Google Scholar] [CrossRef]
- Kaku, N.; Yanagihara, K.; Morinaga, Y.; Yamada, K.; Harada, Y.; Migiyama, Y.; Kohno, S. Influence of antimicrobial regimen on decreased in-hospital mortality of patients with MRSA bacteremia. J. Infect. Chemother. 2014, 20, 350–355. [Google Scholar] [CrossRef]
- Poulsen, S.M.; Karlsson, M.; Johansson, L.B.; Vester, B. The pleuromutilin drugs tiamulin and valnemulin bind to the RNA at the peptidyl transferase center on the ribosome. Mol. Microbiol. 2001, 41, 1091–1099. [Google Scholar] [CrossRef]
- Shang, R.F.; Wang, J.T.; Guo, W.Z.; Liang, J.P. Efficient antibacterial agents: A review of the synthesis, biological evaluation and mechanism of pleuromutilin derivatives. Curr. Top. Med. Chem. 2013, 13, 3013–3025. [Google Scholar] [CrossRef]
- Novak, R.; Shlaes, D.M. The pleuromutilin antibiotics: A new class for human use. Curr. Opin. Invest. Drugs. 2010, 11, 182–191. [Google Scholar]
- Schlunzen, F.; Pyetan, E.; Fucini, P.; Yonath, A.; Harms, J.M. Inhibition of peptide bond formation by pleuromutilins: The structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol. Microbiol. 2004, 54, 1287–1294. [Google Scholar] [CrossRef]
- Davidovich, C.; Bashan, A.; Auerbach-Nevo, T.; Yaggie, R.D.; Gontarek, R.R.; Yonath, A. Induced-fit tightens pleuromutilins, binding to ribosomes and remote interactions enable their selectivity. Proc. Natl. Acad. Sci. USA 2007, 104, 4291–4296. [Google Scholar] [CrossRef]
- Goethe, O.; Heuer, A.; Ma, X.S.; Wang, Z.X.; Herzon, S.B. Antibacterial properties and clinical potential of pleuromutilins. Nat. Prod. Rep. 2018, 7, 1–28. [Google Scholar] [CrossRef]
- Burch, D.G. Tiamulin activity against Brachyspira hyodysenteriae. Vet. Rec. 2008, 163, 760. [Google Scholar]
- Stipkovits, L.; Ripley, P.H.; Tenk, M.; Glavits, R.; Molnar, T.; Fodor, L. The efficacy of valnemulin (Econor) in the control of disease caused by experimental infection of calves with Mycoplasma bovis. Res. Vet. Sci. 2005, 78, 207–215. [Google Scholar] [CrossRef]
- Loreto, E.S.; Tondolo, J.S.M.; Oliveira, D.C.; Santurio, J.M.; Alvesa, S.H. In vitro activities of miltefosine and antibacterial agents from the macrolide, oxazolidinone, and pleuromutilin classes against pythium insidiosum and pythium aphanidermatum. Antimicrob. Agents Chemother. 2018, 62, 1678–1684. [Google Scholar] [CrossRef]
- Scangarella-Oman, N.E.; Shawar, R.M.; Bouchillon, S.; Hoban, D. Microbiological profile of a new topical antibacterial: Retapamulin ointment 1%. Expert. Rev. Anti Infect. Ther. 2009, 7, 269–279. [Google Scholar] [CrossRef]
- Jones, R.N.; Fritsche, T.R.; Sader, H.S.; Ross, J.E. Activity of retapamulin (SB-275833), a novel pleuromutilin, against selected resistant gram-positive cocci. Antimicrob. Agents Chemother. 2006, 50, 2583–2586. [Google Scholar] [CrossRef]
- Rubino, C.M.; Xue, B.; Bhavnani, S.M.; Prince, W.T.; Ivezic-Schoenfeld, Z.; Wicha, W.W.; Ambrose, P.G. Population Pharmacokinetic Analyses for BC-3781 Using Phase 2 Data from Patients with Acute Bacterial Skin and Skin Structure Infections. Antimicrob. Agents Chemother. 2015, 59, 282–288. [Google Scholar] [CrossRef]
- Ling, C.Y.; Tao, Y.L.; Chu, W.J.; Wang, H.; Wang, H.D.; Yang, Y.S. Design, synthesis and antibacterial activity of novel pleuromutilin derivatives with 4H-pyran-4-one and pyridin-4-one substitution in the C-14 side chain. Chin. Chem. Lett. 2016, 27, 235–240. [Google Scholar] [CrossRef]
- Waites, K.B.; Crabb, D.M.; Duffy, L.B.; Jensen, J.S.; Liu, Y.; Paukner, S. In Vitro Activities of Lefamulin and Other Antimicrobial Agents against Macrolide-Susceptible and Macrolide-Resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob. Agents Chemother. 2017, 61, 2008–2016. [Google Scholar] [CrossRef]
- Zeitlinger, M.; Schwameis, R.; Burian, A.; Burian, B.; Matzneller, P.; Muller, M.; Wicha, W.W.; Strickmann, D.B.; Prince, W. Simultaneous assessment of the pharmacokinetics of a pleuromutilin, lefamulin, in plasma, soft tissues and pulmonary epithelial lining fluid. J. Antimicrob. Chemother. 2016, 71, 1022–1026. [Google Scholar] [CrossRef]
- Yi, Y.P.; Xu, X.M.; Liu, Y.; Xu, S.J.; Huang, X.; Liang, J.P.; Shang, R.F. Synthesis and antibacterial activities of novel pleuromutilin derivatives with a substituted pyrimidine moiety. Eur. J. Med. Chem. 2017, 126, 687–695. [Google Scholar] [CrossRef]
- Zhao, S.M.; Zhang, Y.Y.; Ju, P.; Gu, L.Q.; Zhuang, R.; Zhao, L.S.; Tang, X.; Bi, K.S.; Chen, X.H. Determination of 6258-70, a new semi-synthetic taxane, in rat plasma and tissues: Application to the pharmacokinetics and tissue distribution study. J. Pharmaceut. Anal. 2016, 6, 219–225. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, L.; Zhu, L.; He, L.; Luo, X.; Wang, R.; Liu, Y. Pharmacokinetics and bioavailability of valnemulin in muscovy ducks (cairina moschata). Brit. Poultry Sci. 2012, 53, 374–378. [Google Scholar] [CrossRef]
- Xiao, X.; Sun, J.; Yang, T.; Fang, X.; Cheng, J.; Xiong, Y.Q.; Liu, Y.H. Pharmacokinetic/pharmacodynamic profiles of tiamulin in an experimental intratracheal infection model of Mycoplasma gallisepticum. Front. Vet. Sci. 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Shang, R.F.; Zhang, C.; Yi, Y.P.; Liu, Y.; Pu, W.X. Determination of a new pleuromutilin derivative in broiler chicken plasma by RP-HPLC-UV and its application to a pharmacokinetic study. J. Chromatogr. Sci. 2018, 56, 604–610. [Google Scholar] [CrossRef]
- Shang, R.F.; Pu, X.Y.; Xu, X.M.; Xin, Z.J.; Zhang, C.; Guo, W.Z.; Liu, Y.; Liang, J.P. Synthesis and biological activities of novel pleuromutilin derivatives with a substituted thiadiazole moiety as potent drug-resistant bacteria inhibitors. J. Med. Chem. 2014, 57, 5664–5678. [Google Scholar] [CrossRef]
- Shang, R.F.; Yi, Y.P.; Zhang, C.; Fu, Y.X.; Liang, J.P.; Pu, W.X. Antibacterial activity and pharmacokinetic profile of a promising antibacterial agent: 14-O-[(4-Amino-6-hydroxy-pyrimidine-2-yl)thioacetyl] mutilin. Pharmacolog. Res. 2018, 129, 424–431. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Bioanalytical Method Validation; European Medicines Agency: London, UK, 2015.
- Marques, L.M.; Callejon, D.R.; Pinto, L.G.; Campos, M.L.D.; Oliveira, A.R.M.; Vessecchi, R.; Lopes, N.P. Pharmacokinetic properties, in vitro metabolism and plasma protein binding of govaniadine an alkaloid isolated from corydalis govaniana wall. J. Pharm. Biomed. Anal. 2016, 131, 464–472. [Google Scholar] [CrossRef]
- Bostrom, E.; Simonsson, U.S.; Hammarlund-Udenaes, M. Oxycodone pharmacokinetics and pharmacodynamics in the rat in the presence of the P-glycoprotein inhibitor PSC833. J. Pharm. Sci. 2005, 94, 1060–1066. [Google Scholar] [CrossRef]
- Yan, F.; Sun, M.M.; Hang, T.J.; Sun, J.; Zhou, X.; Deng, X.; Ge, L.; Qian, H.; Ya, D.; Huang, W.L. A rapid and sensitive UPLC–MS/MS method for determination of HZ08 in rat plasma and tissues: Application to a pharmacokinetic study of liposome injections. J. Pharm. Biomed. Anal. 2015, 102, 246–252. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 14-O-[(4,6-Diaminopyrimidine-2-yl)thioacetyl] Mutilin (DPTM) and tiamulin fumarate are available from the authors. |
| Biosamples | Y = a X + b | 95% CI for Slope | 95% CI for Ordinate | R2 | Linear Range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|---|---|
| Plasma | Y= 467.7 X + 46.8 | 397.5–537.9 | 42.6–51.0 | 0.9999 | 5–4000 | 5 |
| Heart | Y = 525.3 X + 131.4 | 439.2–609.4 | 120.9–141.9 | 0.9968 | 5–4000 | 5 |
| Liver | Y = 533.3 X + 50.4 | 465.0–601.6 | 43.2–57.6 | 0.9988 | 5–4000 | 5 |
| Spleen | Y = 682.8 X + 95.9 | 589.3–776.3 | 274.9–342.1 | 0.9963 | 5–4000 | 5 |
| Lung | Y = 715.6X + 308.5 | 609.4–821.8 | 42.3–68.7 | 0.9987 | 5–4000 | 5 |
| Kidney | Y = 664.5 X + 55.5 | 574.7–754.6 | 47.2–63.8 | 0.9951 | 5–4000 | 5 |
| Large intestine | Y = 893.0 X + 341.4 | 803.7–982.3 | 308.9–373.8 | 0.9923 | 5–4000 | 5 |
| Small intestine | Y = 733.3 X +82.5 | 674.6–791.9 | 43.5–121.5 | 0.9975 | 5–4000 | 5 |
| Ileum | Y = 620.8X + 86.0 | 574.2–667.4 | 66.7–105.3 | 0.9978 | 5–4000 | 5 |
| Cecum | Y = 689.9 X + 249.2 | 638.2–741.6 | 207.5–290.9 | 0.9993 | 5–4000 | 5 |
| Bladder | Y = 664.5 X + 55.5 | 604.7–724.3 | 41.9–69.1 | 0.9988 | 5–4000 | 5 |
| Brain | Y = 599.2 X + 25.3 | 563.2–635.2 | 17.4–33.2 | 0.9965 | 5–4000 | 5 |
| Jejunum | Y = 624.7X + 95.3 | 574.7–674.7 | 70.0–120.6 | 0.9985 | 5–4000 | 5 |
| Colon | Y = 664.8 X + 182.5 | 614.9–714.7 | 150.2–214.8 | 0.9996 | 5–4000 | 5 |
| Urinary | Y= 514.4 X + 35.6 | 468.1–560.7 | 28.1–43.1 | 0.9995 | 5–4000 | 5 |
| Fecal | Y=430.4 X + 24.1 | 387.4–473.4 | 19.1–29.1 | 0.9995 | 5–4000 | 5 |
| Biosamples | Spiked Concentration (ng/mL) | Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|
| Precision (% RSD) | Accuracy (% RE) | Precision (% RSD) | Accuracy (% RE) | ||
| Plasma | 10 | 6.1 | 7.9 | 5.9 | 8.1 |
| 200 | 3.2 | 4.6 | 3.1 | 4.3 | |
| 3000 | 2.1 | 3.8 | 1.9 | 3.5 | |
| Heart | 10 | 7.9 | 8.4 | 7.7 | 8.1 |
| 200 | 0.5 | 0.3 | 0.4 | 0.4 | |
| 3000 | 3.6 | 4.5 | 3.2 | 4.1 | |
| Liver | 10 | 7.9 | 6.8 | 7.7 | 6.5 |
| 200 | 3.5 | 5.7 | 3.3 | 6.1 | |
| 3000 | 2.5 | −0.8 | 5.3 | −0.9 | |
| Spleen | 10 | 3.5 | 4.6 | 2.3 | 4.3 |
| 200 | 3.6 | 3.8 | 3.7 | 3.5 | |
| 3000 | 2.6 | 5.6 | 3.5 | 5.8 | |
| Lung | 10 | 5.4 | 3.8 | 5.1 | 3.6 |
| 200 | 2.9 | −1.6 | 3.7 | −1.3 | |
| 3000 | 3.3 | 5.5 | 3.3 | 5.3 | |
| Kidney | 10 | 7.5 | 9.8 | 7.7 | 8.8 |
| 200 | 3.6 | 4.3 | 3.2 | 4.8 | |
| 3000 | 2.1 | 8.3 | 2.3 | 8.9 | |
| Large intestine | 10 | 4.6 | 4.3 | 3.9 | 4.1 |
| 200 | 9.8 | 7.9 | 9.5 | 7.5 | |
| 3000 | 6.5 | 9.6 | 6.4 | 9.7 | |
| Small intestine | 10 | 2.3 | −1.1 | 2.7 | −1.5 |
| 200 | 3.2 | 4.3 | 3.8 | 4.9 | |
| 3000 | 5.1 | 2.9 | 5.5 | 2.3 | |
| Bladder | 10 | 3.5 | 7.6 | 3.5 | 7.1 |
| 200 | 4.2 | 1.1 | 4.7 | 1.7 | |
| 3000 | 3.5 | −0.3 | 3.4 | −0.5 | |
| Brain | 10 | 5.4 | 2.1 | 5.1 | 1.8 |
| 200 | 3.9 | 7.5 | 3.7 | 6.9 | |
| 3000 | 7.5 | 2.4 | 7.6 | 2.3 | |
| Ileum | 10 | 3.3 | 5.5 | 3.8 | 5.2 |
| 200 | 4.3 | 6.7 | 4.5 | 7.1 | |
| 3000 | 3.3 | 4.4 | 3.1 | 4.3 | |
| Cecum | 10 | 5.5 | 7.6 | 5.4 | 7.9 |
| 200 | 3.2 | 5.3 | 3.1 | 5.1 | |
| 3000 | 4.9 | 6.7 | 5.3 | 7.1 | |
| Jejunum | 10 | 3.3 | 4.9 | 3.1 | 5.1 |
| 200 | 5.5 | 1.8 | 5.1 | 2.1 | |
| 3000 | 5.7 | 3.5 | 4.9 | 3.2 | |
| Colon | 10 | 1.8 | 2.5 | 2.1 | 2.9 |
| 200 | 1.4 | −1.5 | 1.2 | −1.8 | |
| 3000 | 2.1 | 1.7 | 1.9 | 1.9 | |
| Urinary | 10 | 3.8 | 2.1 | 3.6 | 1.9 |
| 200 | 5.4 | 3.9 | 5.7 | 3.5 | |
| 3000 | 3.6 | 5.4 | 3.3 | 5.1 | |
| Fecal | 10 | 4.1 | 6.3 | 3.2 | 4.6 |
| 200 | 2.9 | 4.6 | 2.5 | 3.8 | |
| 3000 | 3.3 | 5.1 | 3.5 | 4.3 | |
| Biosamples | Spiked Concentration (ng/mL) | Extraction Recovery | Matrix Effect | ||
|---|---|---|---|---|---|
| Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | ||
| Plasma | 10 | 101 ± 6 | 7.6 | 103 ± 3 | 6.9 |
| 200 | 103 ± 4 | 3.5 | 103 ± 6 | 7.3 | |
| 3000 | 100 ± 3 | 8.9 | 101 ± 4 | 6.5 | |
| IS | 2000 | 101 ± 5 | 3.4 | 98 ± 6 | 7.3 |
| Heart | 10 | 99 ± 3 | 9.8 | 97 ± 6 | 8.6 |
| 200 | 103 ± 5 | 7.6 | 99 ± 7 | 5.4 | |
| 3000 | 100 ± 7 | 9.3 | 99 ± 7 | 3.6 | |
| IS | 2000 | 97 ± 4 | 8.6 | 94 ± 7 | 5.3 |
| Liver | 10 | 99 ± 6 | 5.6 | 97 ± 6 | 7.8 |
| 200 | 103 ± 7 | 5.8 | 99 ± 5 | 2.1 | |
| 3000 | 101 ± 5 | 6.3 | 99 ± 3 | 2.5 | |
| IS | 2000 | 99 ± 2 | 7.9 | 100 ± 7 | 1.2 |
| Spleen | 10 | 95 ± 4 | 8.6 | 95 ± 3 | 6.5 |
| 200 | 97 ± 4 | 7.9 | 99 ± 6 | 7.3 | |
| 3000 | 103 ± 8 | 9.8 | 99 ± 7 | 6.5 | |
| IS | 2000 | 101 ± 7 | 7.8 | 98 ± 4 | 7.3 |
| Lung | 10 | 100 ± 7 | 6.2 | 99 ± 7 | 6.5 |
| 200 | 98 ± 4 | 7.5 | 97 ± 6 | 3.8 | |
| 3000 | 109 ± 6 | 9.8 | 98 ± 9 | 4.2 | |
| IS | 2000 | 103 ± 4 | 7.3 | 99 ± 9 | 5.1 |
| Kidney | 10 | 105 ± 6 | 3.2 | 99 ± 7 | 2.3 |
| 200 | 100 ± 5 | 2.6 | 102 ± 4 | 3.2 | |
| 3000 | 99 ± 6 | 3.8 | 102 ± 8 | 5.4 | |
| IS | 2000 | 99 ± 2 | 6.1 | 104 ± 7 | 6.9 |
| Large intestine | 10 | 107 ± 6 | 9.3 | 104 ± 7 | 7.2 |
| 200 | 103 ± 4 | 7.9 | 104 ± 6 | 6.7 | |
| 3000 | 107 ± 9 | 7.9 | 105 ± 6 | 3.2 | |
| IS | 2000 | 103 ± 4 | 7.6 | 106 ± 9 | 2.3 |
| Small intestine | 10 | 100 ± 6 | 6.5 | 99 ± 8 | 5.1 |
| 200 | 109 ± 4 | 7.3 | 100 ± 4 | 3.2 | |
| 3000 | 99 ± 6 | 3.2 | 98 ± 8 | 7.6 | |
| IS | 2000 | 99 ± 8 | 2.1 | 99 ± 4 | 8.5 |
| Bladder | 10 | 97 ± 4 | 3.5 | 98 ± 7 | 6.8 |
| 200 | 100 ± 8 | 6.3 | 99 ± 7 | 5.4 | |
| 3000 | 99 ± 7 | 5.9 | 97 ± 10 | 8.3 | |
| IS | 2000 | 109 ± 8 | 9.5 | 97 ± 4 | 5.8 |
| Brain | 10 | 110 ± 8 | 8.1 | 107 ± 5 | 3.8 |
| 200 | 107 ± 4 | 4.5 | 101 ± 5 | 6.2 | |
| 3000 | 103 ± 4 | 6.8 | 105 ± 7 | 5.8 | |
| IS | 2000 | 103 ± 5 | 7.8 | 110 ± 5 | 5.5 |
| Ileum | 10 | 109 ± 8 | 6.6 | 105 ± 8 | 4.5 |
| 200 | 103 ± 6 | 4.9 | 102 ± 5 | 6.8 | |
| 3000 | 98 ± 7 | 2.4 | 103 ± 3 | 4.3 | |
| IS | 2000 | 101 ± 8 | 3.2 | 101 ± 6 | 3.8 |
| Cecum | 10 | 103 ± 6 | 2.8 | 106 ± 8 | 4.5 |
| 200 | 101 ± 7 | 4.5 | 102 ± 4 | 5.6 | |
| 3000 | 103 ± 6 | 5.1 | 104 ± 4 | 4.5 | |
| IS | 2000 | 109 ± 7 | 3.6 | 106 ± 5 | 6.5 |
| Jejunum | 10 | 101 ± 6 | 5.5 | 107 ± 5 | 7.5 |
| 200 | 104 ± 3 | 7.4 | 99 ± 8 | 6.4 | |
| 3000 | 99 ± 4 | 8.6 | 97 ± 9 | 6.9 | |
| IS | 2000 | 102 ± 10 | 9.2 | 104 ± 6 | 5.8 |
| Colon | 10 | 97 ± 9 | 8.3 | 108 ±3 | 5.5 |
| 200 | 94 ± 7 | 7.4 | 101 ±8 | 5.4 | |
| 3000 | 106 ± 8 | 9.9 | 104 ± 10 | 6.7 | |
| IS | 2000 | 98 ± 7 | 6.3 | 107 ± 7 | 8.2 |
| Urinary | 10 | 101 ± 9 | 6.2 | 102 ± 8 | 9.1 |
| 200 | 99 ± 7 | 6.7 | 106 ± 6 | 6.8 | |
| 3000 | 104 ± 7 | 5.9 | 109 ± 4 | 7.4 | |
| IS | 2000 | 103 ± 7 | 6.4 | 101 ± 8 | 6.8 |
| fecal | 10 | 101 ± 6 | 7.2 | 102 ± 9 | 9.3 |
| 200 | 107 ± 3 | 6.8 | 105 ± 2 | 9.4 | |
| 3000 | 102 ± 10 | 9.1 | 106 ± 9 | 8.6 | |
| IS | 2000 | 98 ± 9 | 7.7 | 102 ± 7 | 5.4 |
| Biosamples | Analyte Concentration (ng/mL) | Short-term (25 °C, 24 h) | Freeze-thaw Cycles | Long-term (−70 °C, 4 Weeks) | |||
|---|---|---|---|---|---|---|---|
| RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | ||
| Plasma | 10 | 5.1 | 7.9 | 6.3 | 7.7 | 5.8 | 7.8 |
| 200 | 6.8 | 7.6 | 7.9 | 4.1 | 7.3 | 8.4 | |
| 3000 | 1.5 | 5.4 | 1.7 | 5.4 | 2 | 5.1 | |
| Heart | 10 | 3.9 | 4.3 | 3.5 | 8.7 | 3.3 | 8 |
| 200 | −0.7 | 2.8 | −0.3 | 0.5 | −0.7 | 0.3 | |
| 3000 | 6.3 | 7.1 | 6.3 | 4.2 | 6.5 | 4.6 | |
| Liver | 10 | 2.9 | 5.1 | 3.3 | 6.5 | 3.4 | 6.3 |
| 200 | 3.3 | 4.7 | 3.4 | 5.6 | 3.6 | 5.4 | |
| 3000 | 6.1 | 5.3 | 1.9 | 2.2 | 2.1 | 3.8 | |
| Spleen | 10 | 3.7 | 1.4 | 3.6 | 4.7 | 3.3 | 4.9 |
| 200 | 7.2 | −1.8 | 7.9 | −1.5 | 7.1 | 5.2 | |
| 3000 | 5.9 | 7.1 | 5.6 | 3.5 | 5.7 | 3.6 | |
| Lung | 10 | 3.3 | 4.6 | 2.5 | 2.7 | 2.9 | 4.4 |
| 200 | 2.1 | 2.9 | 3.1 | 5.2 | 3.5 | 5.4 | |
| 3000 | 1.3 | 1.1 | 7.6 | 9.4 | 7.5 | 9.6 | |
| Kidney | 10 | 3.4 | 5.1 | 3.7 | 4.5 | 3.5 | 4.3 |
| 200 | 0.9 | 2.1 | 2.3 | 3.3 | 1.9 | 2.7 | |
| 3000 | 3.2 | 6.3 | 10.1 | 7.6 | 9.9 | 7.3 | |
| Large intestine | 10 | 1.9 | 3.1 | 6.3 | 9.3 | 6.9 | 10.2 |
| 200 | 5.7 | 5.4 | 2.4 | 4 | 2.2 | 7.1 | |
| 3000 | −0.9 | 4.1 | 3.1 | 4.1 | 3.5 | 4.5 | |
| Small intestine | 10 | 1.3 | 3.1 | 4.9 | 3.1 | 4.6 | 3.2 |
| 200 | 3.7 | 1.5 | 3.2 | 7.3 | 3.6 | 7.4 | |
| 3000 | 5.3 | 3.2 | 3.9 | 1.3 | 3.8 | 1.2 | |
| Bladder | 10 | 4.1 | 6.3 | 3.9 | 2.1 | 3.6 | 1.7 |
| 200 | 1.9 | 3.4 | 5.1 | 2 | 5.1 | 1.9 | |
| 3000 | 3.1 | 1.8 | 3.5 | 7.1 | 3.3 | 6.9 | |
| Brain | 10 | 1.1 | 5.1 | 4.9 | 7.3 | 5.3 | 7.3 |
| 200 | 3.3 | 4.5 | 6.5 | 5.1 | 6.6 | 9.3 | |
| 3000 | 8.1 | 5.5 | 4.1 | 6.5 | 4.1 | 6.2 | |
| Ileum | 10 | 2.9 | 4.1 | −3.5 | 4.1 | 3.2 | 4.5 |
| 200 | 1.7 | 1.2 | 5.2 | 7.3 | −5.1 | 7.1 | |
| 3000 | 3.1 | 4.5 | 3.3 | 5.1 | 3.5 | 4.9 | |
| Cecum | 10 | 6.7 | 5.5 | 7.2 | 5.5 | 6.4 | 2.1 |
| 200 | 3.1 | 7.2 | 3.9 | 6.3 | 3.2 | 7.5 | |
| 3000 | −5.1 | 4.8 | −4.9 | 4.5 | −6.4 | 3.8 | |
| Jejunum | 10 | 7.2 | 3.6 | 6.1 | 7.2 | 5.6 | 7.2 |
| 200 | 7.5 | 4.8 | 7.3 | 4.9 | 7.3 | 4.8 | |
| 3000 | −5.3 | 4.6 | −5.4 | 4.8 | −5.6 | 5.1 | |
| Colon | 10 | 5.4 | 3.2 | 6.3 | 4.5 | 4.8 | 3.7 |
| 200 | 6.8 | 5.9 | 7.2 | 8.9 | 2.2 | 5.4 | |
| 3000 | 7.3 | 8.4 | −4.3 | 6.5 | 2.3 | 5.5 | |
| Urinary | 10 | 6.6 | 2.1 | 8.1 | 7.2 | 8.4 | 6.6 |
| 200 | 5.3 | 4.5 | 4.2 | 3.4 | −1.2 | 5.9 | |
| 3000 | −5.6 | 7.2 | −5.5 | 6.8 | −3.3 | 4.5 | |
| Fecal | 10 | 3.2 | 5.7 | 4.8 | 3.6 | 3.3 | 3.9 |
| 200 | 2.9 | 4.1 | 3.6 | 3.9 | 4.1 | 3.7 | |
| 3000 | 4.8 | 5.1 | 4.7 | 4.5 | 4.3 | 4.6 | |
| Parameters | i.v. | i.m. | p.o. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 25 | 75 | 10 | 25 | 75 | 10 | 25 | 75 | |
| Cmax (μg/mL) * | 7.5 ± 0.4 | 16.0 ± 0.5 | 65.1 ± 0.8 | 0.5 ± 0.1 | 0.7 ± 0.1 | 1.8 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.1 |
| Tmax (h) | 0.08 (0.08–1.00) | 0.08 (0.08–1.00) | 0.083 (0.08–1.00) | 0.50 (0.17–4.00) | 0.50 (0.17–4.00) | 0.50 (0.17–4.00) | 2.00 (0.17–6.00) | 2.00 (0.17–6.00) | 2.00 (0.17–6.00) |
| t1/2 (h) | 1.7 ± 0.1 | 1.7 ± 0.4 | 1.9 ± 0.5 | 3.5 ± 0.0 | 3.2 ± 0.2 | 3.3 ± 0.3 | 4.6 ± 0.1 | 4.4 ± 0.2 | 4.7 ± 0.4 |
| CL (L/h/kg)* | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.03 ± 0.0 | – | – | – | – | – | – |
| MRT0–t (h) | 1.0 ± 0.2 | 1.3 ± 0.7 | 1.5 ± 0.4 | 4.4 ± 0.3 | 4.8 ± 0.3 | 4.2 ± 0.8 | 12.8 ± 0.1 | 9.8 ± 0.2 | 6.9 ± 0.4 |
| Vss (L/kg)* | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.03 ± 0.0 | – | – | – | – | – | – |
| AUC0→t (μg·h/mL)* | 2.2 ± 0.2 | 5.4 ± 0.2 | 36.0 ± 0.3 | 1.5 ± 0.1 | 2.6 ± 0.2 | 5.7 ± 0.2 | 0.5 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.0 |
| CLR(0–t) (mL/min) | – | – | 97.6 ± 9.6 | – | – | 46.2 ± 3.8 | – | – | 12.2 ± 1.3 |
| F (%) | – | – | – | 68.16 | 48.52 | 15.84 | 22.87 | 24.26 | 4.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Liu, Y.; Yi, Y.; Liang, J.; Wu, Q.; Shang, R. A Validated HPLC-MS/MS Assay for 14-O-[(4,6-Diaminopyrimidine-2-yl)thioacetyl] Mutilin in Biological Samples and Its Pharmacokinetic, Distribution and Excretion via Urine and Feces in Rats. Molecules 2019, 24, 790. https://doi.org/10.3390/molecules24040790
Fu Y, Liu Y, Yi Y, Liang J, Wu Q, Shang R. A Validated HPLC-MS/MS Assay for 14-O-[(4,6-Diaminopyrimidine-2-yl)thioacetyl] Mutilin in Biological Samples and Its Pharmacokinetic, Distribution and Excretion via Urine and Feces in Rats. Molecules. 2019; 24(4):790. https://doi.org/10.3390/molecules24040790
Chicago/Turabian StyleFu, Yunxing, Yu Liu, Yunpeng Yi, Jianping Liang, Qingfeng Wu, and Ruofeng Shang. 2019. "A Validated HPLC-MS/MS Assay for 14-O-[(4,6-Diaminopyrimidine-2-yl)thioacetyl] Mutilin in Biological Samples and Its Pharmacokinetic, Distribution and Excretion via Urine and Feces in Rats" Molecules 24, no. 4: 790. https://doi.org/10.3390/molecules24040790
APA StyleFu, Y., Liu, Y., Yi, Y., Liang, J., Wu, Q., & Shang, R. (2019). A Validated HPLC-MS/MS Assay for 14-O-[(4,6-Diaminopyrimidine-2-yl)thioacetyl] Mutilin in Biological Samples and Its Pharmacokinetic, Distribution and Excretion via Urine and Feces in Rats. Molecules, 24(4), 790. https://doi.org/10.3390/molecules24040790




