Therapeutic Effects of HIF-1α on Bone Formation around Implants in Diabetic Mice Using Cell-Penetrating DNA-Binding Protein
Abstract
1. Introduction
2. Results
2.1. RNA Sequencing and Differentially Expressed Genes (DEGs)
2.2. Target Genes of HIF-1α in Bioinformatic Analysis
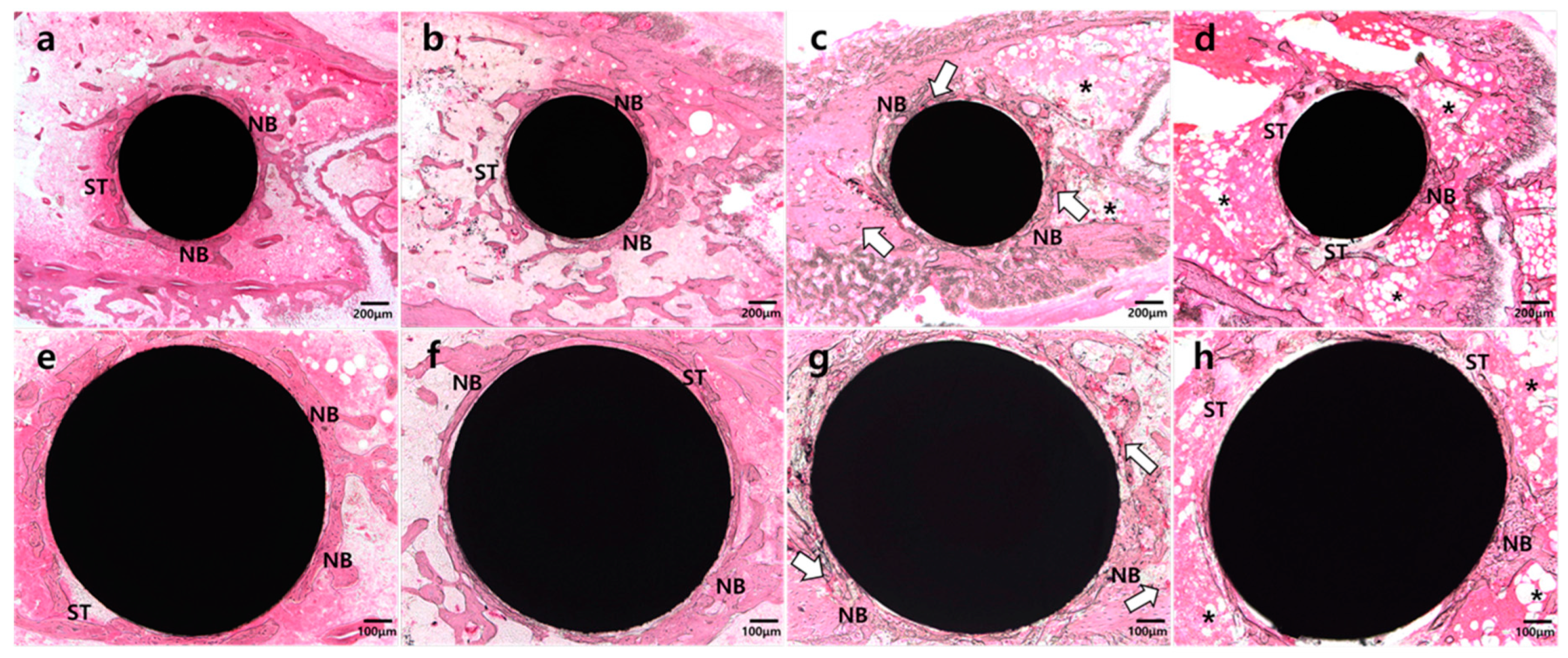
2.3. Histologic Analysis
2.4. Histomorphometric and Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Statements
4.2. Animal Models
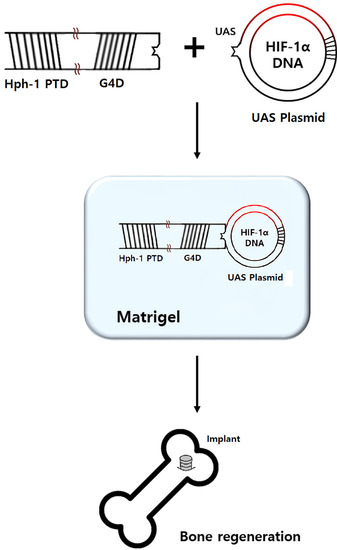
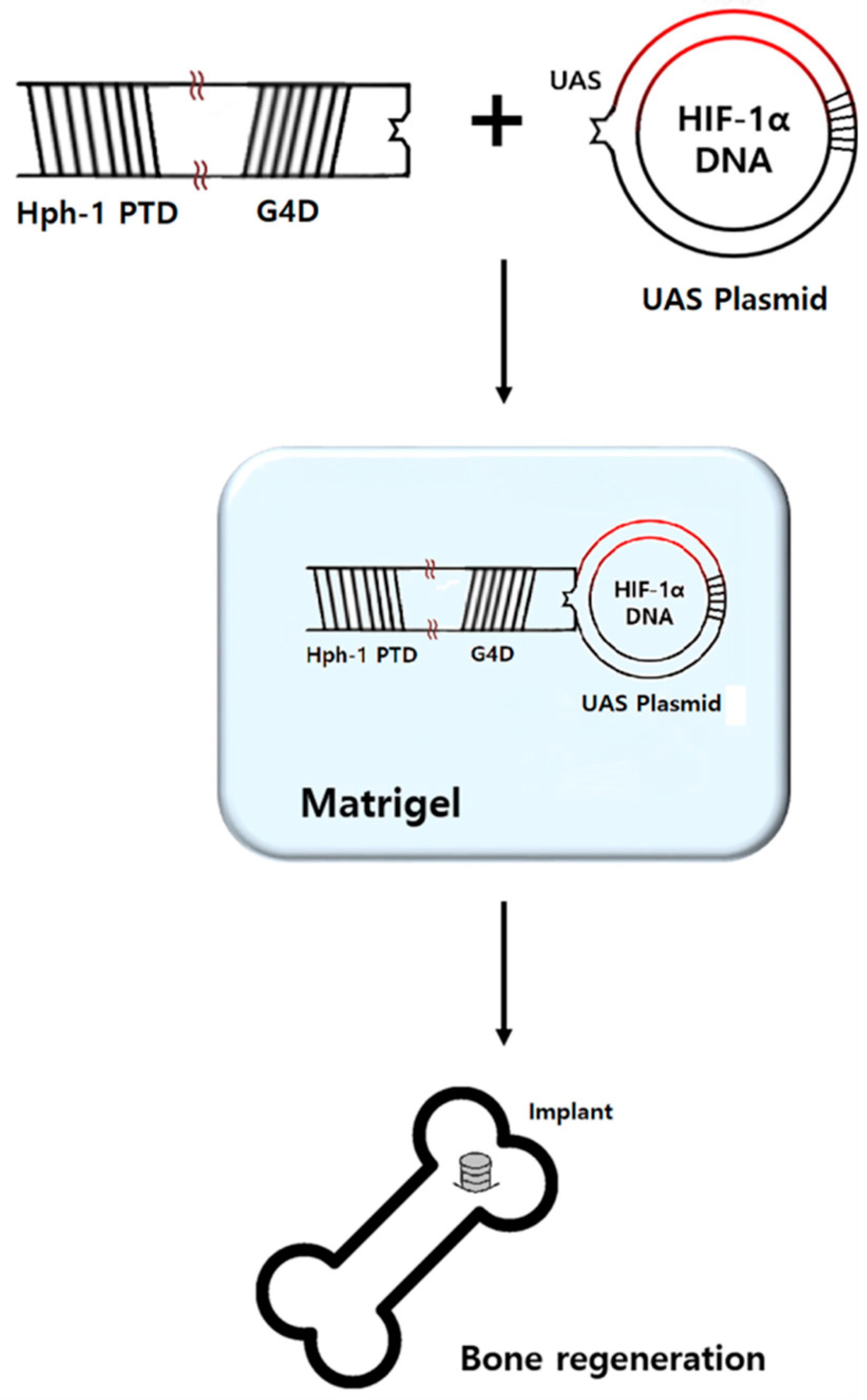
4.3. Preparation of HIF-1α Gene Construct and Hph-1-GAL4 DNA Binding Domain Protein
4.4. Preparation of HIF-1α Gel
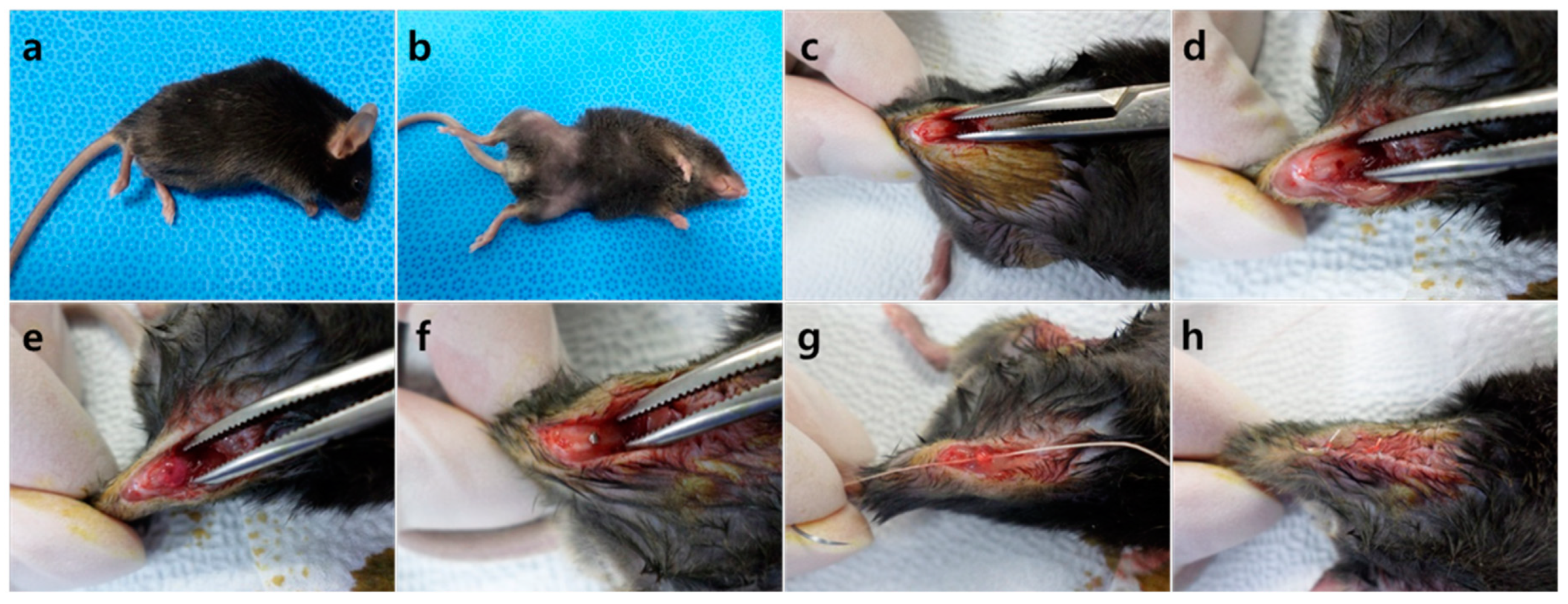
4.5. Surgical Procedure
4.6. RNA Sequencing
4.7. Identification of DEGs
4.8. Identification of Target Genes of HIF-1α
4.9. Histologic and Histomorphometric Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Millennium Research Group. U.S. Markets for Dental Implants; Millennium Research Group: Toronto, ON, Canada, 2006. [Google Scholar]
- Papaspyridakos, P.; Chen, C.-J.; Singh, M.; Weber, H.-P.; Gallucci, G.O. Success criteria in implant dentistry. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Moy, P.K.; Medina, D.; Shetty, V.; Aghaloo, T.L. Dental implant failure rates and associated risk factors. Int. J. Oral Maxillofac. Implants 2005, 20, 569–577. [Google Scholar] [PubMed]
- Alsaadi, G.; Quirynen, M.; Komarek, A.; van Steenberghe, D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J. Clin. Periodontol. 2007, 34, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Kopman, J.A.; Kim, D.M.; Rahman, S.S.; Arandia, J.A.; Karimbux, N.Y.; Fiorellini, J.P. Modulating the effects of diabetes on osseointegration with aminoguanidine and doxycycline. J. Periodontol. 2005, 76, 614–620. [Google Scholar] [CrossRef]
- Kwon, P.T.; Rahman, S.S.; Kim, D.M.; Kopman, J.A.; Karimbux, N.Y.; Fiorellini, J.P. Maintenance of osseointegration utilizing insulin therapy in a diabetic rat model. J. Periodontol. 2005, 76, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I. Osseointegrated implants in the treatment of edentulous jaw, experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. 1977, 1, 1–132. [Google Scholar]
- Garetto, L.P.; Chen, J.; Parr, J.A.; Roberts, W.E. Remodeling dynamics of bone supporting rigidly fixed titanium implants: A histomorphometric comparison in four species including humans. Implant Dent. 1995, 4, 235–243. [Google Scholar] [CrossRef]
- Wang, F.; Song, Y.-L.; Li, D.-H.; Li, C.-X.; Wang, Y.; Zhang, N.; Wang, B.-G. Type 2 diabetes mellitus impairs bone healing of dental implants in gk rats. Diabetes Res. Clin. Pract. 2010, 88, e7–e9. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.R.; Hale, J.E.; David Carmines, H.L.G.; Hurwitz, S.R. Biomechanical evaluation of early fracture healing in normal and diabetic rats. J. Orthop. Res. 2000, 18, 126–132. [Google Scholar] [CrossRef]
- Schlegel, K.; Prechtl, C.; Möst, T.; Seidl, C.; Lutz, R.; Wilmowsky, C. Osseointegration of slactive implants in diabetic pigs. Clin. Oral Implants Res. 2013, 24, 128–134. [Google Scholar] [CrossRef]
- Botusan, I.R.; Sunkari, V.G.; Savu, O.; Catrina, A.I.; Grünler, J.; Lindberg, S.; Pereira, T.; Ylä-Herttuala, S.; Poellinger, L.; Brismar, K. Stabilization of hif-1α is critical to improve wound healing in diabetic mice. Proc. Natl. Acad. Sci. USA 2008, 105, 19426–19431. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.-B.; Okamoto, K.; Pereira, T.; Brismar, K.; Poellinger, L. Hyperglycemia regulates hypoxia-inducible factor-1α protein stability and function. Diabetes 2004, 53, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ferguson, G.; Connell, P.; Walshe, T.; Murphy, R.; Birney, Y.A.; O’Brien, C.; Cahill, P.A. High glucose concentrations alter hypoxia-induced control of vascular smooth muscle cell growth via a hif-1α-dependent pathway. J. Mol. Cell. Cardiol. 2007, 42, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, A.; S. Tarnawski, A. Critical role of hypoxia sensor-HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr. Med. Chem. 2012, 19, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.G.; Zhao, X.; Yang, Q.; Li, Y.; Ge, C.; Zhao, G.; Franceschi, R.T. Physical and functional interactions between runx2 and hif-1α induce vascular endothelial growth factor gene expression. J. Cell. Biochem. 2011, 112, 3582–3593. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, H.; Ikeda, T.; Jeong, J.-H.; Saito, T.; Yano, F.; Jung, Y.-K.; Ohba, S.; Kawaguchi, H.; Chung, U.-I.; Choi, J.-Y. Analysis of the runx2 promoter in osseous and non-osseous cells and identification of hif2a as a potent transcription activator. Gene 2008, 416, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Zhang, Z.; He, J.; Zhu, S.; Wang, S.; Zhang, W.; Zhou, J.; Xu, Y.; Huang, Y.; Wang, Y.; et al. Repairing critical-sized calvarial defects with bmscs modified by a constitutively active form of hypoxia-inducible factor-1α and a phosphate cement scaffold. Biomaterials 2011, 32, 9707–9718. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; He, J.; Zhang, K.; Dai, J.; Zhang, W.; Wang, S.; Zhou, J.; Huang, Y.; Zhang, Z.; Jiang, X. The bone-forming effects of hif-1α-transduced bmscs promote osseointegration with dental implant in canine mandible. PLoS ONE 2012, 7, e32355. [Google Scholar] [CrossRef][Green Version]
- Kim, E.-S.; Yang, S.-W.; Hong, D.-K.; Kim, W.-T.; Kim, H.-G.; Lee, S.-K. Cell-penetrating DNA-binding protein as a safe and efficient naked DNA delivery carrier in vitro and in vivo. Biochem. Biophys. Res. Commun. 2010, 392, 9–15. [Google Scholar] [CrossRef]
- Jeon, M.; Shin, Y.; Jung, J.; Jung, U.-W.; Lee, J.-H.; Moon, J.-S.; Kim, I.; Shin, J.-S.; Lee, S.-K.; Song, J.S. Hif1a overexpression using cell-penetrating DNA-binding protein induces angiogenesis in vitro and in vivo. Mol. Cell. Biochem. 2018, 437, 99–107. [Google Scholar] [CrossRef]
- Corbett, S.; Hukkanen, M.; Batten, J.; McCarthy, I.; Polak, J.; Hughes, S. Nitric oxide in fracture repair: Differential localisation, expression and activity of nitric oxide synthases. J. Bone Joint Surg. Br. 1999, 81, 531–537. [Google Scholar] [CrossRef]
- Li, B.; Castano, A.P.; Hudson, T.E.; Nowlin, B.T.; Lin, S.-L.; Bonventre, J.V.; Swanson, K.D.; Duffield, J.S. The melanoma-associated transmembrane glycoprotein gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair. FASEB J. 2010, 24, 4767–4781. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Urano, T.; Ouchi, Y.; Inoue, S. Glucocorticoid-induced gene tripartite motif-containing 63 (trim63) promotes differentiation of osteoblastic cells. Endocr. J. 2010, 57, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Quint, P.; Weivoda, M.M.; Ruan, M.; Pederson, L.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Transforming growth factor beta 1 induces cxcl16 and leukemia inhibitory factor expression in osteoclasts to modulate migration of osteoblast progenitors. Bone 2013, 57, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Zins, S.R.; Amare, M.F.; Tadaki, D.K.; Elster, E.A.; Davis, T.A. Comparative analysis of angiogenic gene expression in normal and impaired wound healing in diabetic mice: Effects of extracorporeal shock wave therapy. Angiogenesis 2010, 13, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Rios, H.F.; Volk, S.L.; Sugai, J.V.; Jin, Q.; Giannobile, W.V. Gene expression dynamics during bone healing and osseointegration. J. Periodontol. 2011, 82, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Macey, L.R.; Kana, S.; Jingushi, S.; Terek, R.; Borretos, J.; Bolander, M. Defects of early fracture-healing in experimental diabetes. J. Bone Joint Surg. Am. 1989, 71, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Wu, Y.; Ye, D.; Wang, S.; Zou, D.; Zhang, X.; Kaplan, D.L.; Jiang, X. Vegf and bmp-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur. Cell. Mater. 2014, 27, 1–12. [Google Scholar] [CrossRef]
- Lin, G.H.; Lim, G.; Chan, H.L.; Giannobile, W.V.; Wang, H.L. Recombinant human bone morphogenetic protein 2 outcomes for maxillary sinus floor augmentation: A systematic review and meta-analysis. Clin. Oral Implants Res. 2016, 27, 1349–1359. [Google Scholar] [CrossRef]
- Jeon, O.; Song, S.J.; Yang, H.S.; Bhang, S.-H.; Kang, S.-W.; Sung, M.A.; Lee, J.H.; Kim, B.-S. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochem. Biophys. Res. Commun. 2008, 369, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.B.E.; Raque, G.H.; Glassman, S.D.; Campbell, M.; Vitaz, T.; Harpring, J.; Shields, C.B. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine 2006, 31, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, J.; Brewer, E.; Tu, Q.; Yu, L.; Tang, J.; Krebsbach, P.; Wieland, M.; Chen, J. Osterix enhances bmsc-associated osseointegration of implants. J. Dent. Res. 2009, 88, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Slate, A.R.; Bandyopadhyay, S.; Francis, K.P.; Papich, M.G.; Karolewski, B.; Hod, E.A.; Prestia, K.A. Efficacy of enrofloxacin in a mouse model of sepsis. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 381–386. [Google Scholar] [PubMed]
- Tubbs, J.T.; Kissling, G.E.; Travlos, G.S.; Goulding, D.R.; Clark, J.A.; King-Herbert, A.P.; Blankenship-Paris, T.L. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 185–191. [Google Scholar] [PubMed]
- Lu, H.; Kraut, D.; Gerstenfeld, L.C.; Graves, D.T. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 2003, 144, 346–352. [Google Scholar] [CrossRef]
- Wingender, E. The transfac project as an example of framework technology that supports the analysis of genomic regulation. Brief. Bioinform. 2008, 9, 326–332. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| 2-Fold; p-Value < 0.05; FDR < 0.1 | Up | Down |
|---|---|---|
| Group NH and NP | 94 | 1 |
| Group DH and DP | 201 | 15 |
| Gene Symbol | Fold Change (log2X) | Molecule Type |
|---|---|---|
| CACNA1S | 1.07 | Calcium channel |
| CCL2 | 1.31 | Chemokine |
| CCL5 | 1.49 | Chemokine |
| CD274 | 1.70 | Ligand |
| CXCL16 | 1.11 | Chemokine |
| COBL | 1.46 | Cordon bleu |
| DES | 1.20 | Enzyme |
| GPNMB | 1.06 | ECM |
| IL2RA | 1.76 | Binding protein |
| JSRP1 | 1.05 | Membrane protein |
| MARCO | 2.88 | Binding protein |
| MIA | −1.03 | Structural protein |
| MURC | 1.12 | Structural protein |
| MYH14 | 1.14 | Enzyme |
| MYL3 | −1.54 | Structural protein |
| NOS2 | 1.41 | Enzyme |
| OAS2 | 1.21 | Enzyme |
| PRKAG3 | 1.50 | Protein kinase |
| TGTP1 | 2.16 | GTPase |
| TRIM63 | 1.93 | Ubiquitin protein ligase |
| TTN | 1.05 | ECM |
| Gene Symbol | Functions of Target Gene |
|---|---|
| NOS2 | Mediate increased blood flow Reparative collagen accumulation |
| GPNMB | Inducing differentiation and mineralization of hBMSCs into osteoblasts Increasing endothelial cell proliferation and migration, resulting in capillary tube formation |
| CXCL16 | Recruitment of osteoclasts to restore the bone lost during the resorptive phase of bone turnover |
| CCL2 | Consistent up-regulation during implant healing |
| CCL5 | Promoting neovascularization and eventual wound repair |
| TRIM63 | Mediating the glucocorticoid-induced promotion of osteoblastic differentiation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.-M.; Shin, J.-S.; Kim, I.-K.; Kim, J.-H.; Moon, J.-S.; Lee, S.-K.; Lee, J.-H. Therapeutic Effects of HIF-1α on Bone Formation around Implants in Diabetic Mice Using Cell-Penetrating DNA-Binding Protein. Molecules 2019, 24, 760. https://doi.org/10.3390/molecules24040760
Oh S-M, Shin J-S, Kim I-K, Kim J-H, Moon J-S, Lee S-K, Lee J-H. Therapeutic Effects of HIF-1α on Bone Formation around Implants in Diabetic Mice Using Cell-Penetrating DNA-Binding Protein. Molecules. 2019; 24(4):760. https://doi.org/10.3390/molecules24040760
Chicago/Turabian StyleOh, Sang-Min, Jin-Su Shin, Il-Koo Kim, Jung-Ho Kim, Jae-Seung Moon, Sang-Kyou Lee, and Jae-Hoon Lee. 2019. "Therapeutic Effects of HIF-1α on Bone Formation around Implants in Diabetic Mice Using Cell-Penetrating DNA-Binding Protein" Molecules 24, no. 4: 760. https://doi.org/10.3390/molecules24040760
APA StyleOh, S.-M., Shin, J.-S., Kim, I.-K., Kim, J.-H., Moon, J.-S., Lee, S.-K., & Lee, J.-H. (2019). Therapeutic Effects of HIF-1α on Bone Formation around Implants in Diabetic Mice Using Cell-Penetrating DNA-Binding Protein. Molecules, 24(4), 760. https://doi.org/10.3390/molecules24040760