A Convenient, Rapid, Sensitive, and Reliable Spectrophotometric Assay for Adenylate Kinase Activity
Abstract
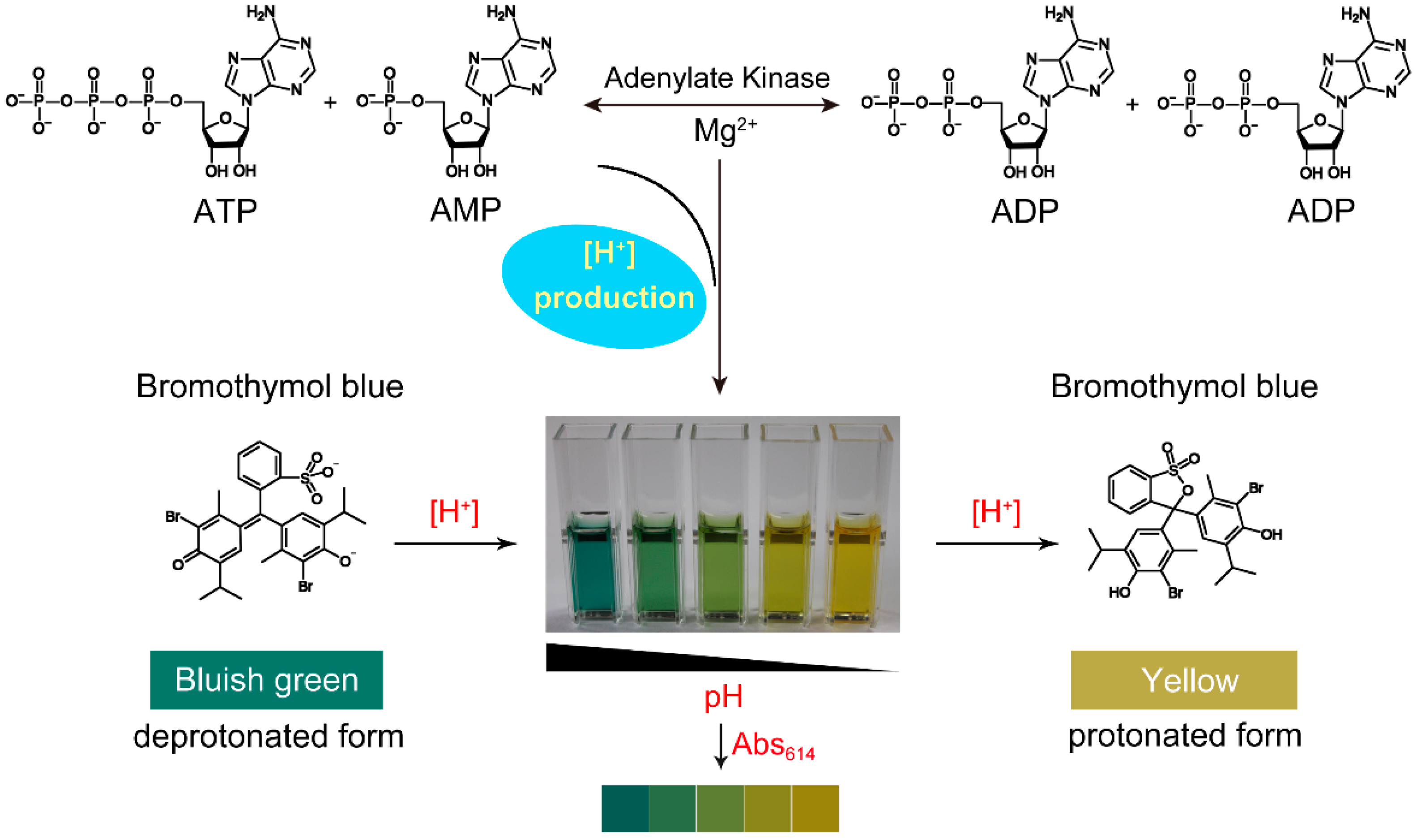
1. Introduction
2. Results
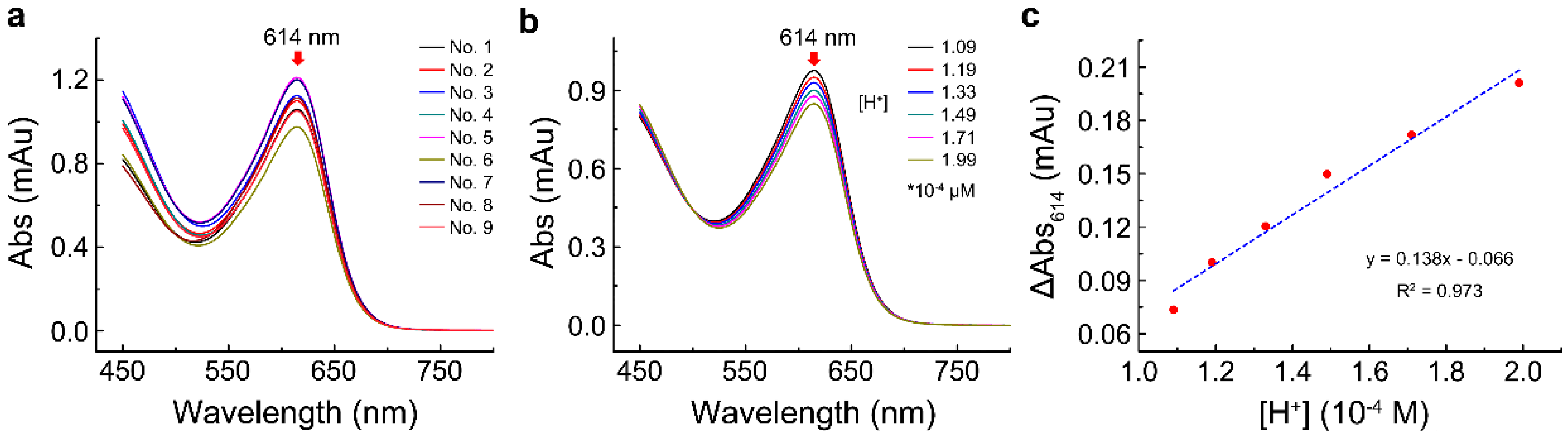
2.1. The Maximum Absorption Wavelength of Reaction Mixture
2.2. Optimization of Adk Activity Assay
2.3. Effect of H+ on the Absorbance of Bromothymol Blue
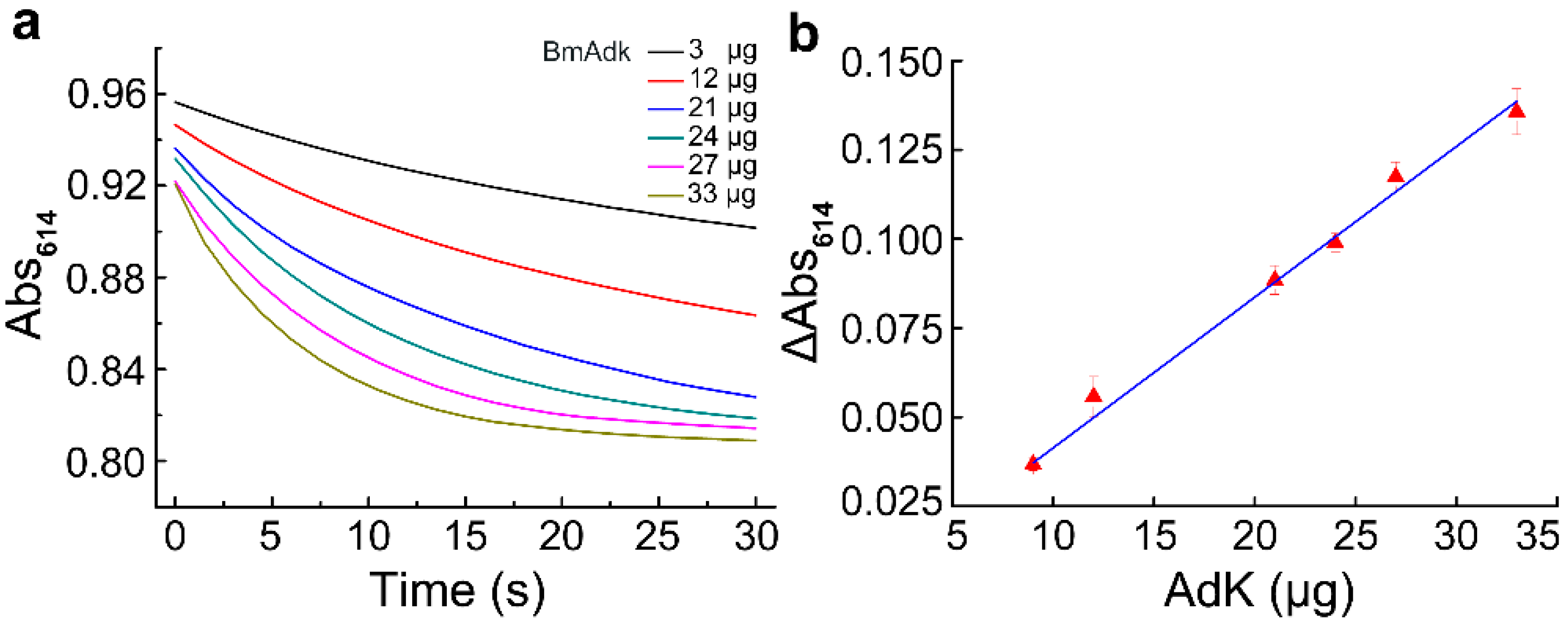
2.4. Effect of Adk Contents on the Reaction Velocity
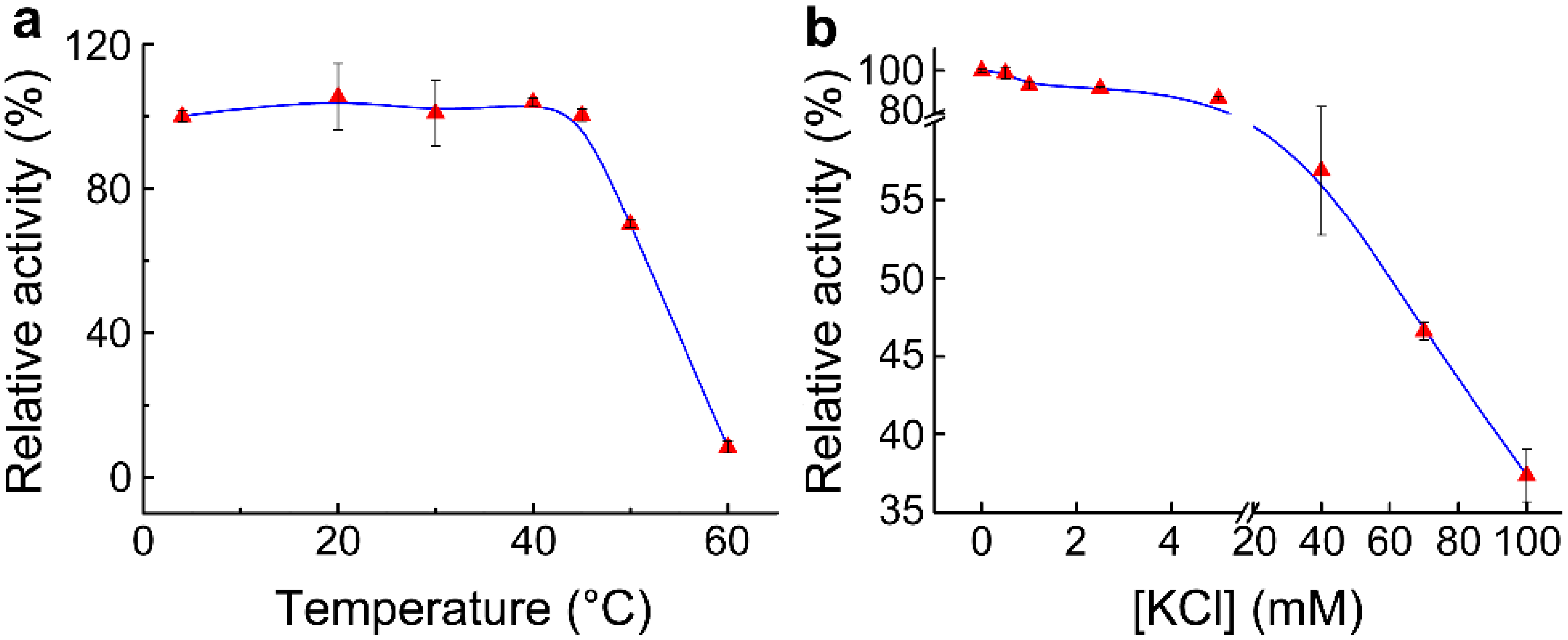
2.5. Effect of Temperature and KCl on Adk Activity
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Adk Preparation and Concentration Determination
3.3. Adk Activity Assay
3.4. Orthogonal Design
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Adk | Adenylate kinase |
| ATP | adenosine 5′-triphosphates |
| ADP | adenosine 5′-diphosphates |
| AMP | adenosine 5′-monophosphate |
| NADPH | reduced nicotinamide adenine dinucleotide phosphate |
| Abs | absorbance |
References
- Krishnamurthy, H.; Lou, H.F.; Kimple, A.; Vieille, C.; Cukier, R.I. Associative mechanism for phosphoryl transfer: A molecular dynamics simulation of Escherichia coli adenylate kinase complexed with its substrates. Proteins 2005, 58, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Rogne, P.; Rosselin, M.; Grundstrom, C.; Hedberg, C.; Sauer, U.H.; Wolf-Watz, M. Molecular mechanism of ATP versus GTP selectivity of adenylate kinase. Proc. Natl. Acad. Sci. USA 2018, 115, 3012–3017. [Google Scholar] [CrossRef]
- Oshima, K.; Saiki, N.; Tanaka, M.; Imamura, H.; Niwa, A.; Tanimura, A.; Nagahashi, A.; Hirayama, A.; Okita, K.; Hotta, A.; et al. Human AK2 links intracellular bioenergetic redistribution to the fate of hematopoietic progenitors. Biochem. Biophys. Res. Commun. 2018, 497, 719–725. [Google Scholar] [CrossRef]
- Wujak, M.; Czarnecka, J.; Gorczycka, M.; Hetmann, A. Human adenylate kinases-classification, structure, physiological and pathological importance. Postep. Hig. Med. Dosw. 2015, 69, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Bucher, T.; Pfleiderer, G. Pyruvate Kinase from Muscle. Method Enzymol. 1955, 1, 435–440. [Google Scholar]
- Colowick, S.P.; Kalckar, H.M. The role of myokinase in transphosphorylations I. The enzymatic phosphorylation of hexoses by adenyl pyrophosphate. J. Biol. Chem. 1943, 148, 117–126. [Google Scholar]
- Chiu, C.S.; Su, S.; Russell, P.J. Adenylate kinase from baker’s yeast. I. Purification and intracellular location. Biochimica Biophys. Acta 1967, 132, 361–369. [Google Scholar] [CrossRef]
- Oliver, I.T. Spectrophotometric Method for the Determination of Creatine Phosphokinase and Myokinase. Biochem. J. 1955, 61, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Haslam, R.J.; Mills, D.C.B. Adenylate Kinase of Human Plasma Erythrocytes and Platelets in Relation to Degradation of Adenosine Diphosphate in Plasma. Biochem. J. 1967, 103, 773. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pan, J.; Zhou, H.M. A direct continuous PH-spectrophotometric assay for arginine kinase activity. Protein Peptide Lett. 2002, 9, 545–552. [Google Scholar]
- Dhale, M.A.; Mohan-Kumari, H.P. A comparative rapid and sensitive method to screen L-asparaginase producing fungi. J. Microbiol. Meth. 2014, 102, 66–68. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, T.; Hemelsoet, K.; Van Speybroeck, V.; De Clerck, K. Substituent effects on absorption spectra of pH indicators: An experimental and computational study of sulfonphthaleine dyes. Dyes Pigments 2014, 102, 241–250. [Google Scholar] [CrossRef]
- Zhu, J.J.; Chew, D.A.S.; Lv, S.N.; Wu, W.W. Optimization method for building envelope design to minimize carbon emissions of building operational energy consumption using orthogonal experimental design (OED). Habitat. Int. 2013, 37, 148–154. [Google Scholar] [CrossRef]
- Colowick, S.P. Adenylate Kinase (Myokinase, Adp Phosphomutase). Method Enzymol. 1955, 2, 598–604. [Google Scholar]
- Bowen, W.J.; Kerwin, T.D. The Kinetics of Myokinase. 1. Studies of the Effects of Salts and Ph and of the State of Equilibrium. Arch. Biochem. Biophys. 1954, 49, 149–159. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, Y.; Zhao, P.; Wei, S.; Li, Z.; Chang, H.; He, H. Biochemical characterization and functional analysis of the POU transcription factor POU-M2 of Bombyx mori. Int. J. Biol. Macromol. 2016, 86, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Bernstei, L.H.; Horenstein, J.M.; Sybers, H.D.; Russell, P.J. Adenylate Kinase in Human Tissue. 2. Serum Adenylate Kinase and Myocardial-Infarction. J. Mol. Cell Cardiol. 1973, 5, 71–85. [Google Scholar] [CrossRef]
- Russell, P.J.; Horenstein, J.M.; Goins, L.; Jones, D.; Laver, M. Adenylate Kinase in Human Tissues. 1. Organ Specificity of Adenylate Kinase Isoenzymes. J. Biol. Chem. 1974, 249, 1874–1879. [Google Scholar]
- Kalckar, H.M. The role of myokinase in transphosphorylations II. The enzymatic action of myokinase on adenine nucleotides. J. Biol. Chem. 1943, 148, 127–137. [Google Scholar]
- Moon, S.; Kim, J.; Bae, E. Structural analyses of adenylate kinases from Antarctic and tropical fishes for understanding cold adaptation of enzymes. Sci. Rep. 2017, 7, 16027. [Google Scholar] [CrossRef]
- Slater, E.C. A Method of Measuring the Yield of Oxidative Phosphorylation. Biochem. J. 1953, 53, 521–530. [Google Scholar] [CrossRef]
- Palella, T.D.; Andres, C.M.; Fox, I.H. Human Placental Adenosine Kinase-Kinetic Mechanism and Inhibition. J. Biol. Chem. 1980, 255, 5264–5269. [Google Scholar] [PubMed]
Sample Availability: Not available. |
| Level | A | B | C | D |
|---|---|---|---|---|
| ATP (mM) | AMP (mM) | Bromothymol Blue (mM) | Glycine–NaOH (mM) | |
| 1 | 2.0 | 1.0 | 0.0930 | 0.1 |
| 2 | 2.5 | 1.5 | 0.1084 | 0.3 |
| 3 | 3.0 | 2.0 | 0.1238 | 0.5 |
| No. | Combination | Factor | ΔAbs a (0–30 s) | |||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| 1 | A1B1C1D1 | 2.0 | 1.0 | 0.0930 | 0.1 | 0.0914 ± 0.0020 |
| 2 | A1B2C2D2 | 2.0 | 1.5 | 0.1084 | 0.3 | 0.0432 ± 0.0045 |
| 3 | A1B3C3D3 | 2.0 | 2.0 | 0.1238 | 0.5 | 0.0112 ± 0.0017 |
| 4 | A2B1C2D3 | 2.5 | 1.0 | 0.1084 | 0.5 | 0.0404 ± 0.0017 |
| 5 | A2B2C3D1 | 2.5 | 1.5 | 0.1238 | 0.1 | 0.0221 ± 0.0020 |
| 6 | A2B3C1D2 | 2.5 | 2.0 | 0.0930 | 0.3 | 0.0606 ± 0.0022 |
| 7 | A3B1C3D2 | 3.0 | 1.0 | 0.1238 | 0.3 | 0.0341 ± 0.0009 |
| 8 | A3B2C1D3 | 3.0 | 1.5 | 0.0930 | 0.5 | 0.0705 ± 0.0049 |
| 9 | A3B3C2D1 | 3.0 | 2.0 | 0.1084 | 0.1 | 0.0382 ± 0.0017 |
| T1 | 0.1458 | 0.1659 | 0.2225 | 0.1517 | ||
| T2 | 0.1231 | 0.1358 | 0.1218 | 0.1379 | ||
| T3 | 0.1428 | 0.1100 | 0.0674 | 0.1221 | ||
| t1 | 0.0486 | 0.0553 | 0.0742 | 0.0506 | ||
| t2 | 0.0410 | 0.0453 | 0.0406 | 0.0460 | ||
| t3 | 0.0476 | 0.0367 | 0.0225 | 0.0407 | ||
| Range (R) | 0.0076 | 0.0186 | 0.0517 | 0.0099 | ||
| Order | C ˃ B ˃ D ˃ A | |||||
| Optimal level | A1 | B1 | C1 | D1 | ||
| Optimal combination | A1B1C1D1 | |||||
| Level | A | B | C | D |
|---|---|---|---|---|
| ATP (mM) | AMP (mM) | Bomothymol Blue (mM) | Glycine–NaOH (mM) | |
| 1 | 2.0 | 1.0 | 0.0930 | 0.1 |
| 2 | 2.0 | 0.8 | 0.0775 | 0.1 |
| 3 | 2.0 | 0.6 | 0.0620 | 0.1 |
| Run | Combination | Factor | ΔAbs b (0–30 s) | |||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| 1 | AB1C1D | 2.0 | 1.0 | 0.0930 | 0.1 | 0.0914 ± 0.0020 |
| 2 | AB1C2D | 2.0 | 1.0 | 0.0775 | 0.1 | 0.0926 ± 0.0030 |
| 3 | AB1C3D | 2.0 | 1.0 | 0.0620 | 0.1 | 0.0764 ± 0.0030 |
| 4 | AB2C1D | 2.0 | 0.8 | 0.0930 | 0.1 | 0.0914 ± 0.0020 |
| 5 | AB2C2D | 2.0 | 0.8 | 0.0775 | 0.1 | 0.0930 ± 0.0018 |
| 6 | AB2C3D | 2.0 | 0.8 | 0.0620 | 0.1 | 0.0680 ± 0.0041 |
| 7 | AB3C1D | 2.0 | 0.6 | 0.0930 | 0.1 | 0.0983 ± 0.0028 |
| 8 | AB3C2D | 2.0 | 0.6 | 0.0775 | 0.1 | 0.0850 ± 0.0054 |
| 9 | AB3C3D | 2.0 | 0.6 | 0.0620 | 0.1 | 0.0604 ± 0.0001 |
| pH | 6.47 | 6.54 | 6.73 | 6.79 | 6.85 | 7.07 | 7.21 | 7.28 |
|---|---|---|---|---|---|---|---|---|
| Abs | 0.8145 | 0.9197 | 0.9438 | 1.0375 | 1.0847 | 1.2385 | 1.3850 | 1.4188 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Wang, Y.; Li, Y.; Ding, C.; Cai, R.; Tao, G.; Zhao, P.; Xia, Q.; He, H. A Convenient, Rapid, Sensitive, and Reliable Spectrophotometric Assay for Adenylate Kinase Activity. Molecules 2019, 24, 663. https://doi.org/10.3390/molecules24040663
Song K, Wang Y, Li Y, Ding C, Cai R, Tao G, Zhao P, Xia Q, He H. A Convenient, Rapid, Sensitive, and Reliable Spectrophotometric Assay for Adenylate Kinase Activity. Molecules. 2019; 24(4):663. https://doi.org/10.3390/molecules24040663
Chicago/Turabian StyleSong, Kai, Yejing Wang, Yu Li, Chaoxiang Ding, Rui Cai, Gang Tao, Ping Zhao, Qingyou Xia, and Huawei He. 2019. "A Convenient, Rapid, Sensitive, and Reliable Spectrophotometric Assay for Adenylate Kinase Activity" Molecules 24, no. 4: 663. https://doi.org/10.3390/molecules24040663
APA StyleSong, K., Wang, Y., Li, Y., Ding, C., Cai, R., Tao, G., Zhao, P., Xia, Q., & He, H. (2019). A Convenient, Rapid, Sensitive, and Reliable Spectrophotometric Assay for Adenylate Kinase Activity. Molecules, 24(4), 663. https://doi.org/10.3390/molecules24040663






