Korean Thistle (Cirsium japonicum var. maackii (Maxim.) Matsum.): A Potential Dietary Supplement against Diabetes and Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. α-Glucosidase and BACE1 Inhibitory Activity of Parts/Extracts and Solvent Soluble Fractions of C. maackii
2.2. Pro-Oxidant Activity of Different Compounds from C. maackii
2.3. Anti-Oxidant Activity of Different Compounds from C. maackii
2.4. Anti-Diabetic and Anti-AD Activity of Different Compounds from C. maackii
2.5. Enzyme Kinetic Analysis of Compounds with α-Glucosidase and BACE1
2.6. Molecular Docking Simulation of α-Glucosidase and BACE1 Inhibition
2.7. Absorption, Distribution, Metabolism, and Excretion (ADME) Properties of Different Compounds from Korean Thistle, C. maackii
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Extraction, Fractionation, and Isolation
4.4. In Vitro α-Glucosidase Inhibitory Activity Assay
4.5. In Vitro Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitory Activity Assay
4.6. In Vitro BACE1 Enzyme Assay
4.7. Pro-Oxidant Assay
4.8. 2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
4.9. Peroxynitrite (ONOO−) Scavenging Activity
4.10. Kinetic Parameters of Active Compounds towards α-Glucosidase and BACE1 Inhibition
4.11. α-Glucosidase and BACE1 Molecular Docking Simulations
4.12. Pharmacokinetic Profile of Active Compounds from C. maackii
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes—Evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Janson, J.; Laedtke, T.; Parisi, J.E.; O’Brien, P.; Petersen, R.C.; Butler, P.C. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 2004, 53, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Lee, J.H.; Lee, I.S.; Lee, H.S.; Park, K.H.; Chiba, S.; Kim, D. Two potent competitive inhibitors discriminating α-glucosidase family I from family II. Carbohydr. Res. 2004, 339, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-Glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Truscheit, E.; Frommer, W.; Junge, B.; Müller, L.; Schmidt, D.D.; Wingender, W. Chemistry and biochemistry of microbial α-glucosidase inhibitors. Angew. Chem. Int. Ed. Engl. 1981, 20, 744–761. [Google Scholar] [CrossRef]
- Alzheimer’s Association, 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018, 14, 367–429. [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7. [Google Scholar] [CrossRef]
- Vardy, E.R.; Catto, A.J.; Hooper, N.M. Proteolytic mechanisms in amyloid-β metabolism: Therapeutic implications for Alzheimer’s disease. Trends Mol. Med. 2005, 11, 464–472. [Google Scholar] [CrossRef]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; Luo, Y. β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Type 2 diabetes mellitus and Alzheimer’s disease. World J. Diabetes 2014, 5, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Lhotsky, A.; Wang, Y.; Forno, G.D.; An, Y.; Metter, E.J.; Ferrucci, L.; O’brien, R.; Zonderman, A.B. Association of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer’s disease. Am. J. Epidemiol. 2008, 168, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, D.; Leng, S.X. Link between type 2 diabetes and Alzheimer’s disease: From epidemiology to mechanism and treatment. Clin. Interv. Aging 2015, 10, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G. Illustrated Natural Drugs Encyclopedia; Namsandang: Seoul, Korea, 1984; p. 37. [Google Scholar]
- Liu, S.; Luo, X.; Li, D.; Zhang, J.; Qiu, D.; Liu, W.; She, L.; Yang, Z. Tumor inhibition and improved immunity in mice treated with flavone from Cirsium japonicum DC. Int. Immunopharmacol. 2006, 6, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
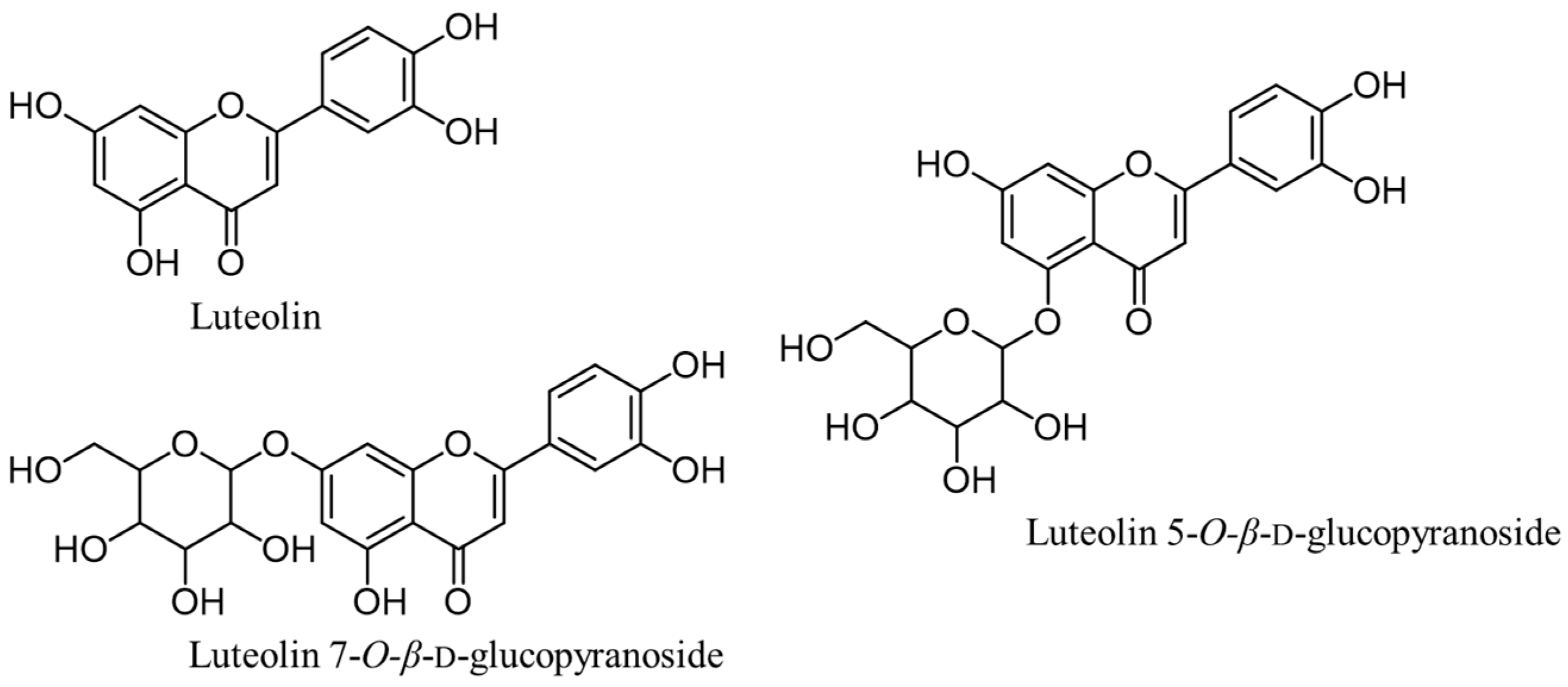
- Jung, H.A.; Jin, S.E.; Min, B.S.; Kim, B.W.; Choi, J.S. Anti-inflammatory activity of Korean thistle Cirsium maackii and its major flavonoid, luteolin 5-O-glucoside. Food Chem. Toxicol. 2012, 50, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Abdul, Q.A.; Byun, J.S.; Joung, E.J.; Gwon, W.G.; Lee, M.S.; Kim, H.R.; Choi, J.S. Protective effects of flavonoids isolated from Korean milk thistle Cirsium japonicum var. maackii (Maxim.) Matsum on tert-butyl hydroperoxide-induced hepatotoxicity in HepG2 cells. J. Ethnopharmacol. 2017, 209, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, H.Y.; Shibamoto, T.; Jang, T.S.; Lee, S.C.; Shim, J.S.; Hahm, D.H.; Lee, H.J.; Lee, S.; Kang, K.S. Beneficial effects of a medicinal herb, Cirsium japonicum var. maackii, extract and its major component, cirsimaritin on breast cancer metastasis in MDA-MB-231 breast cancer cells. Bioorg. Med. Chem. Lett. 2017, 27, 3968–3973. [Google Scholar] [CrossRef]
- Jung, H.A.; Park, J.J.; Min, B.S.; Jung, H.J.; Islam, M.N.; Choi, J.S. Inhibition of advanced glycation endproducts formation by Korean thistle, Cirsium maackii. Asian Pac. J. Trop. Dis. 2015, 8, 1–5. [Google Scholar] [CrossRef]
- Jung, H.A.; Kim, Y.S.; Choi, J.S. Quantitative HPLC analysis of two key flavonoids and inhibitory activities against aldose reductase from different parts of the Korean thistle, Cirsium maackii. Food Chem. Toxicol. 2009, 47, 2790–2797. [Google Scholar] [CrossRef]
- Lee, J.; Rodriguez, J.P.; Lee, K.H.; Park, J.Y.; Kang, K.S.; Hahm, D.H.; Huh, C.K.; Lee, S.C.; Lee, S. Determination of flavonoids from Cirsium japonicum var. maackii and their inhibitory activities against aldose reductase. Appl. Biol. Chem. 2017, 60, 487–496. [Google Scholar] [CrossRef]
- Sims-Robinson, C.; Kim, B.; Rosko, A.; Feldman, E.L. How does diabetes accelerate Alzheimer disease pathology? Nat. Rev. Neurol. 2010, 6, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Santos, M.S.; Seiça, R.; Oliveira, C.R. Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. J. Neurol. Sci. 2007, 257, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid β-protein. J. Alzheimer’s Dis. 2001, 3, 75–80. [Google Scholar] [CrossRef]
- Watson, G.S.; Craft, S. The role of insulin resistance in the pathogenesis of Alzheimer’s disease. CNS Drugs 2003, 17, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Comparative molecular docking studies of lupeol and lupenone isolated from Pueraria lobata that inhibits BACE1: Probable remedies for Alzheimer’s disease. Asian Pac. J. Trop. Dis. 2017, 10, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, G.L. A qualitative approach to enzyme inhibition. Biochem. Mol. Biol. Educ. 2009, 37, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Lee, J.H.; Lee, M.H.; Lee, B.W.; Kwon, H.S.; Park, C.H.; Shim, K.B.; Kim, H.T.; Baek, I.Y.; Jang, D.S. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012, 135, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, G.; Pan, J.; Wang, Y. α-Glucosidase inhibition by luteolin: Kinetics, interaction and molecular docking. Int. J. Biol. Macromol. 2014, 64, 213–223. [Google Scholar] [CrossRef]
- Li, H.; Song, F.; Xing, J.; Tsao, R.; Liu, Z.; Liu, S. Screening and structural characterization of α-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MSn and SORI-CID FTICR MS. J. Am. Soc. Mass Spectrom. 2009, 20, 1496–1503. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, C.S.; Son, K.H. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci. Biotechnol. Biochem. 2000, 64, 2458–2461. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Iwabuchi, K.; Mimaki, Y. Chemical constituents of the aerial parts of Scutellaria lateriflora and their alpha-glucosidase inhibitory activities. Nat. Prod. Commun. 2012, 7, 471–474. [Google Scholar] [PubMed]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.; Sousa, J.L.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure–activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. Inhibition kinetics of flavonoids on yeast α-glucosidase merged with docking simulations. Protein Pept. Lett. 2010, 17, 1270–1279. [Google Scholar] [CrossRef]
- Choi, S.H.; Hur, J.M.; Yang, E.J.; Jun, M.; Park, H.J.; Lee, K.B.; Moon, E.; Song, K.S. β-Secretase (BACE1) inhibitors from Perilla frutescens var. acuta. Arch. Pharmacal Res. 2008, 31, 183–187. [Google Scholar] [CrossRef]
- Liu, R.; Gao, M.; Qiang, G.F.; Zhang, T.T.; Lan, X.; Ying, J.; Du, G.H. The anti-amnesic effects of luteolin against amyloid β25–35 peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience 2009, 162, 1232–1243. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Douglas Shytle, R.; Bai, Y.; Tian, J.; Hou, H.; Mori, T.; Zeng, J.; Obregon, D.; Town, T.; Tan, J. Flavonoid-mediated presenilin-1 phosphorylation reduces Alzheimer’s disease β-amyloid production. J. Cell. Mol. Med. 2009, 13, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.S.; Cheng, H.Y.; Hsieh, M.T.; Wu, C.R.; Lin, Y.C.; Peng, W.H. The ameliorating effects of luteolin on beta-amyloid-induced impairment of water maze performance and passive avoidance in rats. Am. J. Chin. Med. 2010, 38, 279–291. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Hsieh, M.T.; Tsai, F.S.; Wu, C.R.; Chiu, C.S.; Lee, M.M.; Xu, H.X.; Zhao, Z.Z.; Peng, W.H. Neuroprotective effect of luteolin on amyloid β protein (25–35)-induced toxicity in cultured rat cortical neurons. Phytother. Res. 2010, 24, S102–S108. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Le, J.; Abraham, M.H.; Hersey, A.; Eddershaw, P.J.; Luscombe, C.N.; Boutina, D.; Beck, G.; Sherborne, B.; Cooper, I.; Platts, J.A. Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure–activity relationship (QSAR) with the Abraham descriptors. J. Pharm. Sci. 2001, 90, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Yee, S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—Fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Furubayashi, T.; Kataoka, M.; Sakane, T.; Sezaki, H.; Tokuda, H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur. J. Pharm. Sci. 2000, 10, 195–204. [Google Scholar] [CrossRef]
- Irvine, J.D.; Takahashi, L.; Lockhart, K.; Cheong, J.; Tolan, J.W.; Selick, H.E.; Grove, J.R. MDCK (Madin-Darby canine kidney) cells: A tool for membrane permeability screening. J. Pharm. Sci. 1999, 88, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef]
- Spencer, J.P.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar] [CrossRef]
- Kure, A.; Nakagawa, K.; Kondo, M.; Kato, S.; Kimura, F.; Watanabe, A.; Shoji, N.; Hatanaka, S.; Tsushida, T.; Miyazawa, T. Metabolic fate of luteolin in rats: Its relationship to anti-inflammatory effect. J. Agric. Food Chem. 2016, 64, 4246–4254. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.T.; Fujita, K.; Hosoya, T.; Imai, S.; Shimoi, K. Absorption and metabolism of luteolin and its glycosides from the extract of Chrysanthemum morifolium flowers in rats and Caco-2 cells. J. Agric. Food Chem. 2015, 63, 7693–7699. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef]
- Wagle, A.; Seong, S.H.; Joung, E.J.; Kim, H.R.; Jung, H.A.; Choi, J.S. Discovery of a highly potent tyrosinase inhibitor, luteolin 5-O-β-d-glucopyranoside, isolated from Cirsium japonicum var. maakii (Maxim.) Matsum., Korean thistle: Kinetics and computational molecular docking simulation. ACS Omega 2018, 3, 17236–17245. [Google Scholar] [CrossRef]
- Agrawal, P.K. Carbon-13 NMR of Flavonoids; Elsevier Science Publishers: Amsterdam, The Netherlands, 1989; Volume 118, pp. 134–320. [Google Scholar]
- Choi, J.S.; Young, H.S.; Kim, B.W. Hypoglycemic and hypolipemic effects of Ixeris dentata in diabetic rats. Arch. Pharm. Res. 1990, 13, 269–273. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.D.; Song, Y.W.; Liu, W.A. A microplate based screening method for α-glucosidase inhibitors. Chin. J. Clin. Pharm. Ther. 2005, 10, 1128–1134. [Google Scholar]
- Cui, L.; Na, M.; Oh, H.; Bae, E.Y.; Jeong, D.G.; Ryu, S.E.; Kim, S.; Kim, B.Y.; Oh, W.K.; Ahn, J.S. Protein tyrosine phosphatase 1B inhibitors from Morus root bark. Bioorg. Med. Chem. Lett. 2006, 16, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Ali, M.Y.; Choi, R.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Kinetics and molecular docking studies of fucosterol and fucoxanthin, BACE1 inhibitors from brown algae Undaria pinnatifida and Ecklonia stolonifera. Food Chem. Toxicol. 2016, 89, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D.; Chuang, D.Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Paudel, P.; Jung, H.A.; Choi, J.S. Anthraquinone and naphthopyrone glycosides from Cassia obtusifolia seeds mediate hepatoprotection via Nrf2-mediated HO-1 activation and MAPK modulation. Arch. Pharmacal Res. 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199. [Google Scholar] [CrossRef]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic. Biol. Med. 1994, 16, 149–156. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Cornish-Bowden, A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and noncompetitive inhibitors. Biochem. J. 1974, 137, 143–144. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors and commercial sources. |
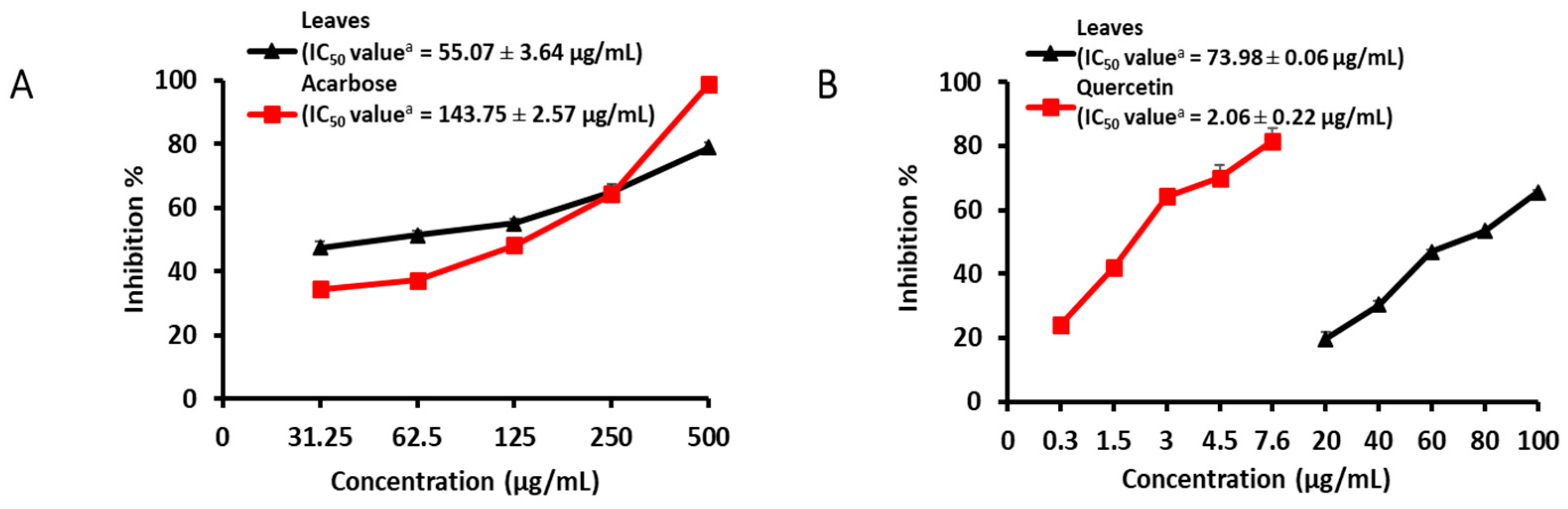



| Samples | α-Glucosidase | BACE1 | ||
|---|---|---|---|---|
| Inhibition (%), Mean ± SD a | IC50 Value b | Inhibition (%), Mean ± SD a | IC50 Value b | |
| Aerial part | 70.21 ± 0.50 *** | 375.66 ± 3.21 | 81.33 ± 1.44 *** | 41.43 ± 0.23 |
| MeOH extract stem | 47.61 ± 1.29 ** | >1000 | 16.84 ± 3.37 * | 178.79 ± 2.41 |
| MeOH extract root | 16.66 ± 0.44 * | >1000 | 36.82 ± 4.47 ** | >100 |
| MeOH extract flower | 9.93 ± 1.65 * | >1000 | 52.11 ± 0.66 *** | 91.44 ± 0.84 |
| MeOH extract leaves | 78.98 ± 1.75 *** | 55.07 ± 3.64 | 65.47 ± 0.65 *** | 73.98 ± 0.06 |
| CH2Cl2 fraction | 11.59 ± 5.39 | – | 79.08 ± 0.36 *** | 25.77 ± 0.57 |
| EtOAc fraction | 75.65 ± 3.61 *** | 32.79 ± 1.63 | 93.01 ± 0.68 *** | 39.88 ± 1.66 |
| n-BuOH fraction | 16.80 ± 2.01 * | – | 35.7 ± 4.99 * | 150.53 ± 3.95 |
| H2O fraction | 3.1 ± 1.7 | – | NA | – |
| Acarbose c/Quercetin c | 99.04 ± 0.20 *** | 143.75 ± 2.57 | 70.01 ± 3.91 *** | 2.06 ± 0.22 |
| Control | 0.00 ± 5.45 | 0.00 ± 5.60 | ||
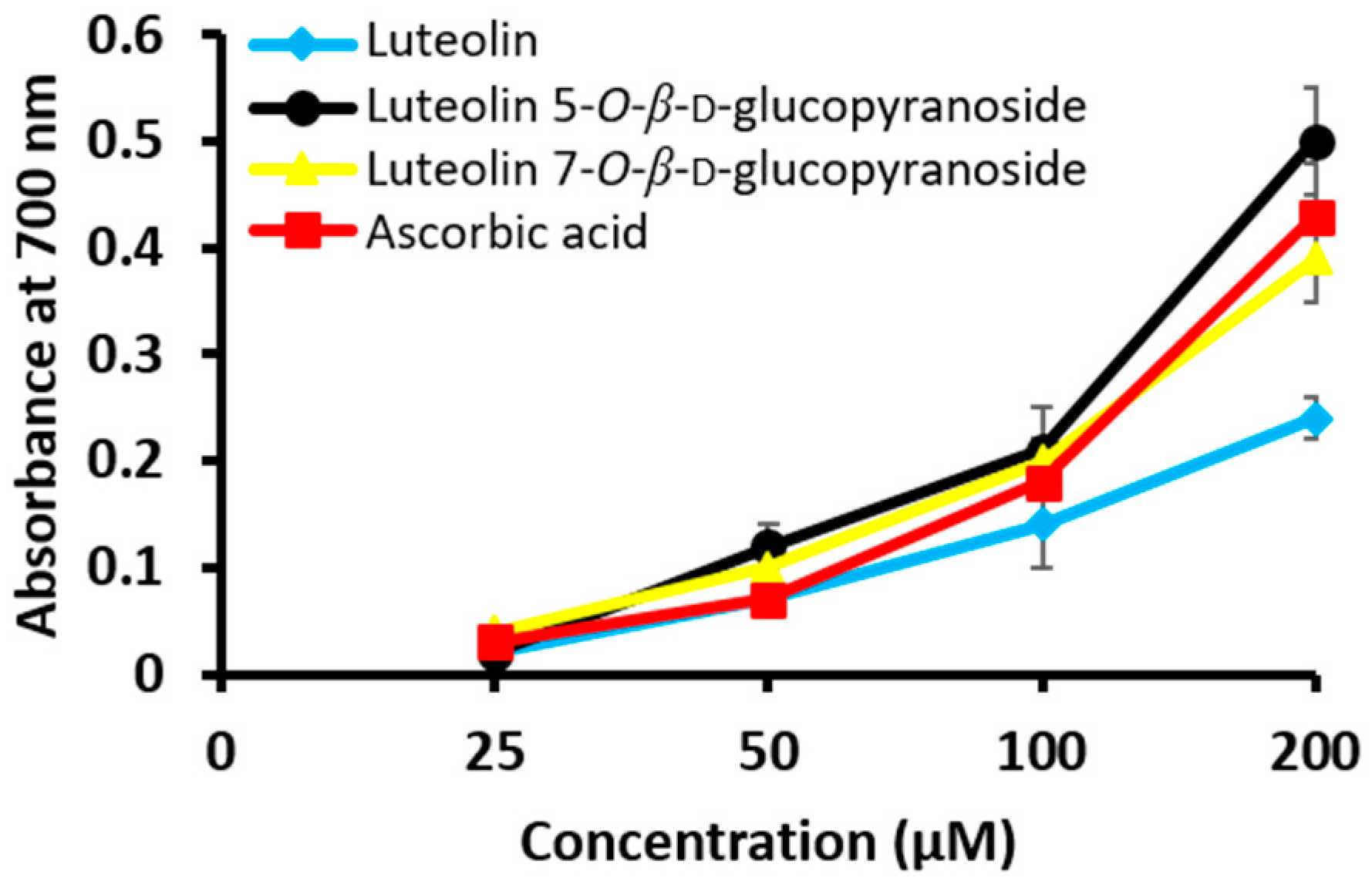
| Compounds | DPPH | ONOO− | ||
|---|---|---|---|---|
| Inhibition (%), Mean ± SD a | EC50 Value b | Inhibition (%), Mean ± SD a | EC50 Value b | |
| Luteolin | 76.65 ± 0.73 ** | 3.05 ± 0.06 | 85.21 ± 1.61 ** | 0.86 ± 0.04 |
| Luteolin 5-O-β-d-gluco-pyranoside | 52.43 ± 0.53 ** | 4.76 ± 0.05 | 79.23 ± 4.95 * | 0.50 ± 0.03 |
| Luteolin 7-O-β-d-gluco-pyranoside | 58.97 ± 1.95 ** | 4.79 ± 0.19 | 80.23 ± 4.22 ** | 2.51 ± 0.05 |
| Ascorbic acid c | 18.72 ± 0.44 ** | 21.35 ± 0.09 | – | – |
| Penicillamine c | – | – | 56.57 ± 2.03 * | 2.28 ± 0.02 |
| Control | 0.00 ± 1.18 | 0.00 ± 7.68 | ||
| Compounds | α-Glucosidase | PTP1B | BACE1 | ||
|---|---|---|---|---|---|
| Inhibition (%), Mean ± SD a | IC50 Value b (Ki c, Inhibition Mode d) | IC50 Value b | Inhibition (%), Mean ± SD a | IC50 Value b (Ki c, Inhibition Mode d) | |
| Luteolin | 69.12 ± 1.20 *** | 51.27 ± 1.23 (52.04, NC) | >400 | 73.73 ± 0.57 *** | 13.75 ± 0.26 (14.76, NC) |
| Luteolin 5-O-β-d-glucopyranoside | 14.02 ± 1.45 * | 270.03 ± 4.69 (271.80, M) | >400 | 17.53 ± 1.65 * | 969.97 ± 7.83 |
| Luteolin 7-O-β-d-glucopyranoside | 25.53 ± 1.21 * | 248.77 ± 2.56 (251.20, C) | >400 | NA | – |
| Acarbose e | 40.36 ± 0.40 ** | 267.27 ± 2.29 | – | – | – |
| Ursolic acid e | – | – | 16.73 ± 0.52 | – | – |
| Quercetin e | – | – | – | 67.56 ± 4.10 *** | 6.30 ± 0.05 |
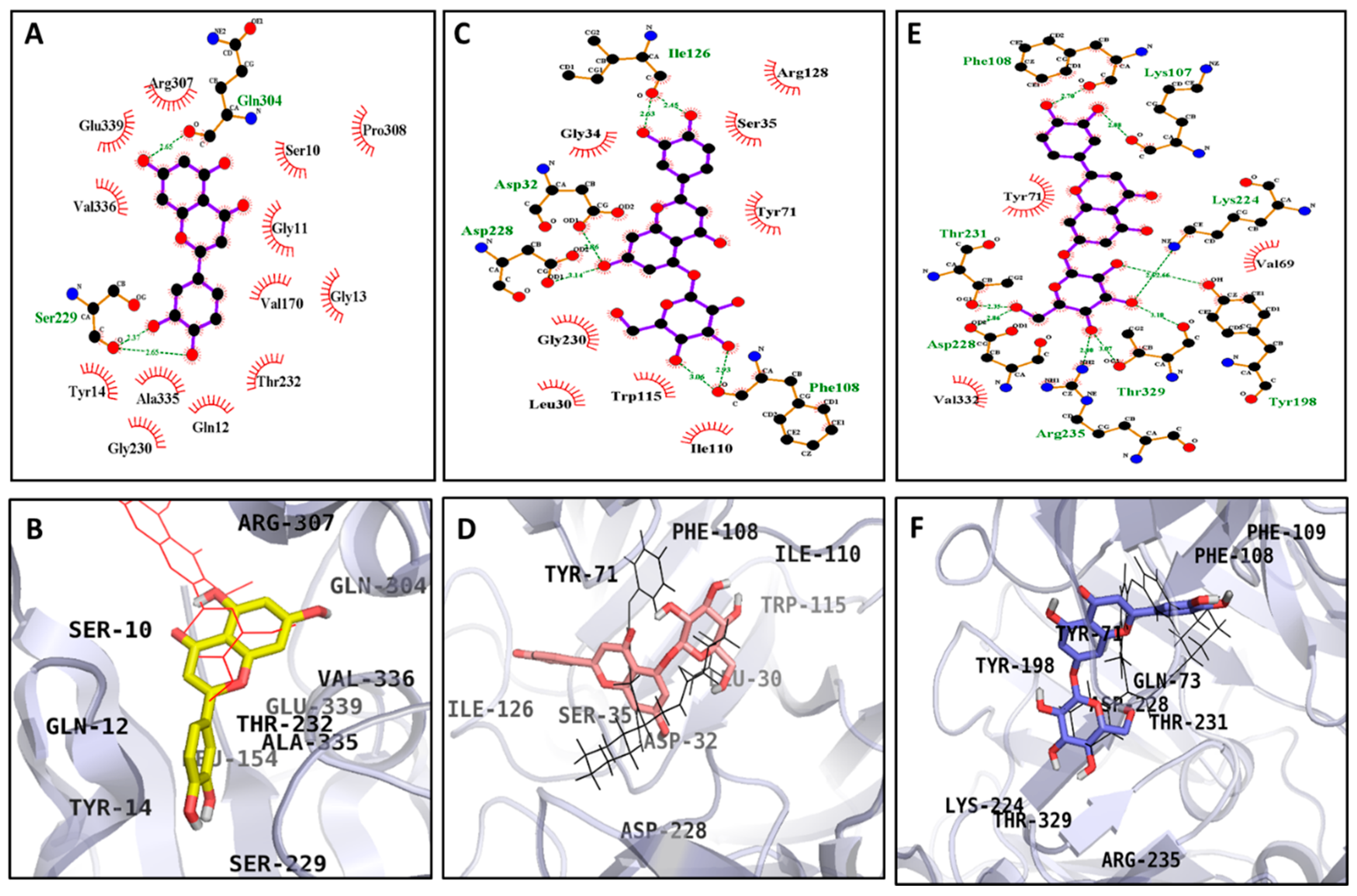
| Compounds | Docking Score (kcal/mol) | No. of H-Bond | H-Bond Interacting Residues | Hydrophobic Interacting Residues |
|---|---|---|---|---|
| Luteolin (Allosteric) | −7.25 | 5 | Glu271, Ile272, Ser298, Thr274 | Gly269, Arg270, Glu296, Asn259, Ile262, Val266, Arg263 |
| Luteolin 5-O-β-d-glucopyranoside (Catalytic) | −7.22 | 7 | His112, Asp215, Gln182, Tyr158, Glu411, Asp307, Gln353 | Asp69, Tyr72, Phe159, Arg315, His280, Asp352, Phe303, Arg442, Val109, Phe178 |
| Luteolin 5-O-β-d-glucopyranoside (Allosteric) | −6.98 | 6 | Thr290, Ser298, Ile272, Arg270, His295 | Asp341, Cys342, Trp15, Asn259, Ile262, Arg263, Glu271, Thr274, Leu297, Ala292 |
| Luteolin 7-O-β-d-glucopyranoside (Catalytic) | −7.21 | 6 | Asp69, Arg442, Pro312, Asp242, His280 | Phe159, Glu277, Asp352, Phe314, Phe303, Arg315, Leu313, Tyr158, Gln279, Val216, Asp215, Phe178 |
| Acarbose a (Catalytic inhibitor) | −8.59 | 17 | Gln182, Asp69, Asp215, Arg213, Glu277, Asp352, Arg442, Asp307, His280, Asp242, Ser240, Tyr158 | Lys156, Arg315, Gln279, Phe178, Phe303, Gln353, Tyr72, Val216, His351, Glu411 |
| BIP b (Allosteric inhibitor) | −6.89 | 2 | Glu296, His295 | Asp341, Cys342, Ala292, Arg294, Leu297, Ser291, Asn259, Thr290, Ser298, Trp15, Lys16, Trp343 |
| Compounds | Docking Score (kcal/mol) | No. of H-Bond | H-Bond Interacting Residues | Hydrophobic Interacting Residues |
|---|---|---|---|---|
| Luteolin (Allosteric) | −7.18 | 3 | Ser299, Gln304 | Ser10, Gly11, Gln12, Gly13, Pro308, Val170, Thr232, Gly230, Ala335, Tyr14, Val336, Glu339, Arg307 |
| Luteolin 5-O-β-d-glucopyranoside (Catalytic) | −6.02 | 6 | Asp32, Asp228, Ile126, Phe108 | Leu30, Gly34, Ser35, Tyr71, Ile110, Trp115, Gly230, Arg128 |
| Luteolin 7-O-β-d-glucopyranoside (Catalytic) | −5.47 | 9 | Lys107, Phe108, Lys224, Tyr198, Thr329, Arg235, Asp228, Thr231 | Tyr71, Val69, Val332 |
| QUD a (Catalytic inhibitor) | −9.3 | 4 | Asp32, Asp228, Gly230 | Lys75, Gly74, Leu30, Thr231, Val69, Tyr198, Ile226, Thr329, Gly34, Ser35, Arg235, Tyr71, Ile118, Lys107 |
| PMF b (Allosteric inhibitor) | −6.15 | 1 | Ser10 | Ala168, Glu339, Val170, Thr232, Gly11, Gln304, Gly156, Ala335, Arg307, Pro308, Ala157 |
| Compounds | Molecular Weight (g/mol) | % HIA a | Plasma Protein Binding | MDCK b | Caco-2 Cell |
|---|---|---|---|---|---|
| Luteolin | 286.24 | 79.43 | 99.71 | 36.52 | 4.54 |
| Luteolin 5-O-β-d-glucopyranoside | 448.38 | 25.17 | 68.05 | 0.70 | 3.12 |
| Luteolin 7-O-β-d-glucopyranoside | 448.38 | 25.16 | 73.28 | 0.75 | 4.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagle, A.; Seong, S.H.; Shrestha, S.; Jung, H.A.; Choi, J.S. Korean Thistle (Cirsium japonicum var. maackii (Maxim.) Matsum.): A Potential Dietary Supplement against Diabetes and Alzheimer’s Disease. Molecules 2019, 24, 649. https://doi.org/10.3390/molecules24030649
Wagle A, Seong SH, Shrestha S, Jung HA, Choi JS. Korean Thistle (Cirsium japonicum var. maackii (Maxim.) Matsum.): A Potential Dietary Supplement against Diabetes and Alzheimer’s Disease. Molecules. 2019; 24(3):649. https://doi.org/10.3390/molecules24030649
Chicago/Turabian StyleWagle, Aditi, Su Hui Seong, Srijan Shrestha, Hyun Ah Jung, and Jae Sue Choi. 2019. "Korean Thistle (Cirsium japonicum var. maackii (Maxim.) Matsum.): A Potential Dietary Supplement against Diabetes and Alzheimer’s Disease" Molecules 24, no. 3: 649. https://doi.org/10.3390/molecules24030649
APA StyleWagle, A., Seong, S. H., Shrestha, S., Jung, H. A., & Choi, J. S. (2019). Korean Thistle (Cirsium japonicum var. maackii (Maxim.) Matsum.): A Potential Dietary Supplement against Diabetes and Alzheimer’s Disease. Molecules, 24(3), 649. https://doi.org/10.3390/molecules24030649





