Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Essential Oils from AgNPs and AuNPs Elicited Lavandula angustifolia In Vitro Cultures
Abstract
:1. Introduction
2. Results and Discussion
3. Material and Methods
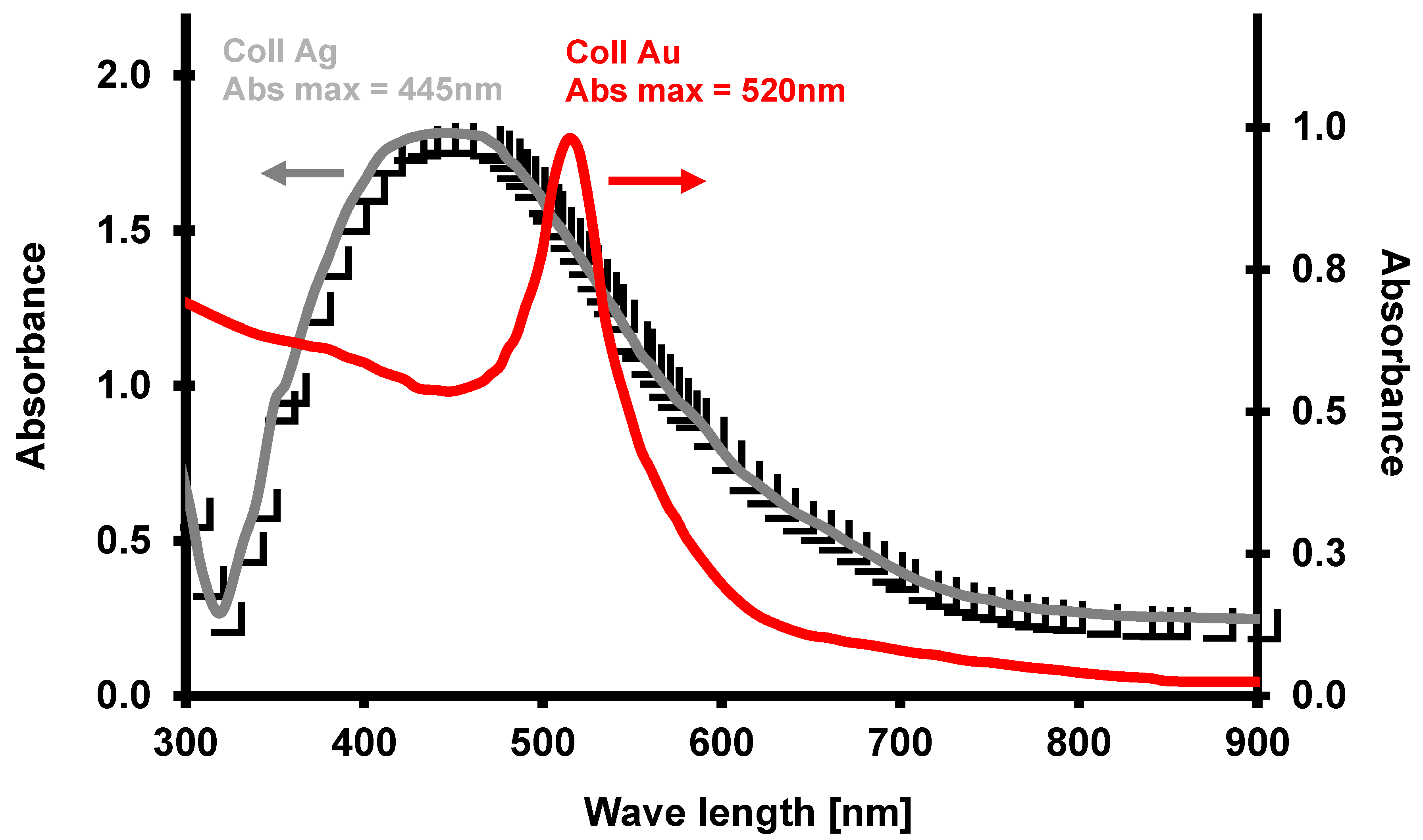
3.1. Nanoparticles
3.2. In Vitro Cultures
3.3. Extraction of Essential Oils
3.4. Gas Chromatography/Mass Spectrometry (GC-MS) Analyses of Essential Oils
Author Contributions
Funding
Conflicts of Interest
References
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Penyala, N.R.; Pena-Mendez, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? Review. J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar]
- Kim, Y.K.; Lee, Y.S.; Jeong, D.H.; Cho, M.H. Antimicrobial effect of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N., Jr.; Ross, L.; Rao, Y.S.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ionsand silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 25, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.; Ghanti, F. Taxanes content and cytotoxity of hazel cells extract after elicitation with silver nanoparticles. Plant Psychol. Chem. 2016, 110, 178–184. [Google Scholar]
- Zhao, J.; Hu, Q.; Guo, Y.Q.; Zhu, W.H. Elicitor-induced indole alkaloid biosynthesis in Catharanthus roseus cell cultures is related to Ca? Influx and the oxidative burst. Plant Sci. 2001, 161, 423–431. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekaa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.; Quigg, A.; Santschi, P.H.; Sigg, I. Environmental behaviour and ecotoxicity of engineered nanoparticles to algae, plants and fungi. Ecotoxicol. 2008, 17, 372–386. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tiss. Organ Cult. 2017, 129, 195–207. [Google Scholar]
- Ramachandra, R.S.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef]
- Shakeran, Z.; Keyhanfari, M.; Asghari, G.; Ghanadian, M. Improvement of atropine production by diferent biotic and abiotic elicitors in hairy root cultures of Datura metel. Turk. J. Biol. 2015, 39, 111–118. [Google Scholar] [CrossRef]
- Raei, M.; Angaji, A.A.; Omidi, M.; Khodayari, M. Effect of abiotic elicitors on tissue culture of Aloe vera. Inter. J. Biosc. 2014, 5, 74–81. [Google Scholar]
- Zhang, C.; Yan, Q.; Cheuk, W.; Wu, J. Enhancement of Tanshinone Production in Salvia miltiorrhiza hairy root culture by Ag elicitation and nutrient feeding. Planta Med. 2004, 70, 147–151. [Google Scholar] [PubMed]
- Zhang, B.; Zheng, L.P.; Wan Wen, W.J. Stimulation of Artemisinin Production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr. Nanosc. 2013, 9, 363–370. [Google Scholar] [CrossRef]
- Pitta-Alvarez, S.I.; Spollansky, T.C.; Giulietti, A.M. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enz. Microb. Technol. 2000, 26, 252–258. [Google Scholar] [CrossRef]
- Jamshidi, M.; Ghanati, F.; Rezaei, A.; Bemani, E. Change of antioxidant enzymes activity of hazel (Corylus avellana L.) cells by AgNPs. Cytotech. 2016, 68, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Fazal, H.; Abbasi, B.H.; Ahmad, N. Optimization of adventitious root culture for production of biomass and secondary metabolites in Prunella vulgaris L. Appl. Biochem Biotechnol. 2014, 174, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical composition and biological activity of essential oils of Origanum vulgare subsp. vulgare L. under different growth conditions. Molecules 2013, 18, 14948–14960. [Google Scholar] [CrossRef]
- Mancini, E.; Camele, I.; Elshafie, H.S.; De Martino, L.; Pellegrino, C.; Grulova, D. Chemical Composition and Biological Activity of the Essential Oil of Origanum vulgare ssp. hirtum from Different Areas in the Southern Apennines (Italy). Chem. Biodiv. 2014, 11, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Smigielski, K.; Prusinowska, R.; Stobiecka, A.; Kunicka-Styczyñska, A.; Gruska, R. Biological Properties and Chemical Composition of Essential Oils from Flowers and Aerial Parts of Lavender (Lavandula angustifolia). J. Essent. Oil Bear. Pl. 2018, 21, 1303–1314. [Google Scholar] [CrossRef]
- Wesolowska, A.; Grzeszczuk, M.; Kulpa, D. GC-MS analysis of the essential oil from flowers of Chrysanthemum coronarium L. propagated conventionally and derived from in vitro cultures. Acta Chromat. 2015, 27, 525–539. [Google Scholar] [CrossRef]
- Wesolowska, A.; Grzeszczuk, M.; Wilas, J.; Kulpa, D. Gas Chromatography-Mass Spectrometry (GC-MS) analysis of indole alkaloids isolated from Catharanthus roseus (L.) G. don cultivated conventionally and derived from in vitro cultures. Not. Bot. Hort. Agrobot. Cluj-Napoca. 2016, 44, 100–106. [Google Scholar] [CrossRef]
- Hatami, M.; Hatamzadeh, A.; Ghasemnezhad, M.; Sajidi, R.H. Variations of the Phytochemical Compounds in Rosescented Geranium Plant Exposed to Nanosilver Particles. J. Essent. Oil Bear. Pl. 2016. 19, 1747–1753.
- Ghanati, F.; Bakhtiarian, S. Changes of natural compounds of Artemisia annua L. by methyl jasmonate and silver nanoparticles. Adv. Envir. Biol. 2013, 7, 2251–2258. [Google Scholar]
- Kara, N.; Baydar, H. Determination of lavender and lavandin cultivars (Lavandula sp.) containing high quality essential oil in Isparta, Turkey. Turk. J. Field Crops. 2013, 18, 58–65. [Google Scholar]
- Zheljazkov, V.; Astatkie, T.; Hristov, A. Lavender and hyssop productivity, oil content and bioactivity as function of harvest time and drying. Ind. Crops Prod. 2012, 36, 222–228. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Hassani, A.; Vojodi, L.; Asl, B.H.; Rostami, A. Essential oil constituents of Lavandula officinalis Chaix. from Northwest Iran. Chemija. 2011, 22, 167–171. [Google Scholar]
- Verma, R.S.; Rahman, L.U.; Chanotiya, C.S.; Verma, R.K.; Chauhan, A.; Yadav, A.; Singh, A.; Yadav, A.K. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid hills of Uttarakhand, India. J. Serb. Chem. Soc. 2010, 75, 343–348. [Google Scholar] [CrossRef]
- Śmigielski, K.; Prusinowska, R.; Raj, A.; Sikora, M.; Wolińska, K.; Gruska, R. Effect of drying on the composition of essential oil from Lavandula angustifolia. J. Ess. Oil Bearing Plants. 2011, 14, 532–542. [Google Scholar] [CrossRef]
- Mostefa, M.B.; Kabouche, A.; Abaza, I.; Aburjai, T.; Touzani, R.; Kabouche, Z. Chemotypes investigation of Lavandula essential oils growing at different North African soils. J. Mater. Environ. Sci. 2014, 5, 1896–1901. [Google Scholar]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Andrys, D.; Adaszyńska-Skwirzyńska, M.; Kulpa, D. Jasmonic acid changes the composition of essential oil isolated from narrow-leaved lavender propagated in in vitro cultures. Nat. Prod. Res. 2018, 32, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Andrys, D.; Kulpa, D. In Vitro Propagation Affects the Composition of Narrow-Leaved Lavender Essential Oils. Acta Chrom. 2018, 30, 225–230. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L.). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Cherneva, E.; Pavlovic, V.; Smelcerovic, A.; Yancheva, D. The effect of camphor and borneol on rat thymocyte viability and oxidative stress. Molecules 2012, 17, 10258–10266. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Sakr, S.; Mang, S.M.; Belviso, S.; De Feo, V.; Camele, I. Antimicrobial activity and chemical composition of three essential oils extracted from Mediterranean aromatic plants. J. Med. Food. 2016, 19, 1096–1103. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Love, J.N.; Sammon, M.; Smereck, J. Are one or two dangerous? Camphor exposure in toddlers. J. Emerg. Med. 2004, 27, 49–54. [Google Scholar] [CrossRef]
- Nakahashi, H.; Miyazawa, M. Biotransformation of (−)-camphor by Salmonella typhimurium OY1002/2A6 expressing human CYP2A6 and NADPH-P450 reductase. J. Oleo Sci. 2011, 60, 545–548. [Google Scholar] [CrossRef]
- Dai, J.P.; Chen, J.; Bei, Y.F.; Han, B.X.; Wang, S. Influence of borneol on primary mice oral fibroblasts: A penetration enhancer may be used in oral submucous fibrosis. J. Oral. Pathol. Med. 2009, 38, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Black pepper and its pungent principle-piperine:a review of diverse physiological effects. Critical Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Khan, M.; Guo, B.; Bokhari, S.A.; Khan, M.A. Efficient regeneration and antioxidative enzyme activities in Brassica rapa var. turnip. Plant Cell Tiss. Organ Cult. 2011, 105, 337–344. [Google Scholar] [CrossRef]
- Bhat, P.; Bhat, A. Silver nanoparticles for enhancement of accumulation of capsaicin in suspension culture of Capsicum sp. J. Exp. Scien. 2016, 7, 1–6. [Google Scholar]
- Hemm, M.R.; Rider, S.D.; Ogas, J.; Murry, D.J.; Chapple, C. Light induces phenylpropanoid metabolism in Arabidopsis roots. Plant J. 2004, 38, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Guo, C.; Wang, Y.; Ouyang, F. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua. Proc. Biochem. 2002, 38, 581–585. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Liu, F.K.; Ker, C.J.; Chang, Y.C.; Ko, F.H.; Chu, T.C.; Dai, B.T. Microwave heating for the preparation of nanometer gold particles. Jpn. J Appl. Physic. 2003, 42, 4152–4158. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Andrys, D.; Kulpa, D.; Grzeszczuk, M.; Bihun, M.; Dobrowolska, A. Antioxidant and antimicrobial activities of Lavandula angustifolia Mill. field-grown and propagated in vitro. Folia Hort. 2017, 29, 161–180. [Google Scholar] [CrossRef]
- European Pharmacopoeia 5.0; EDQM: Strasbourg, France, 2005; p. 1894.
- Hassanpouraghdam, M.B.; Hassani, A.; Shalamzari, M.S. Menthone-and estragole-rich essential oil of cultivated Ocimum basilicum L. from Northwest Iran. Chemija 2010, 21, 59–62. [Google Scholar]
- Rosas, J.F.; Zoghbi, M.G.B.; Andrade, E.H.A.; van den Berg, M.E. Chemical composition of a methyl-(E)-cinnamate Ocimum micranthum Willd. from the Amazon. Flavour Fragr. J. 2005, 20, 161–163. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Medium [mg·dm−3] | Essential Oil Content (%w/w) |
|---|---|
| 0—control | 1.15 |
| 50 Au | 0.95 |
| 10 Au | 0.81 |
| 50 Ag | 0.82 |
| 10 Ag | 1.27 |
| Compound | RI | Control | 50 mg·dm−3 Au | 10 mg·dm−3 Au | 50 mg·dm−3 Ag | 10 mg·dm−3 Ag | |
|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 933 | 1.46a | 0.99b | 1.03b | 1.11b | 0.64c |
| 2 | β-Pinene | 977 | 3.14a | 2.56b | 2.25b | 2.53b | 1.93b |
| 3 | p-Cymene | 1025 | 1.39a | 1.18b | 1.17b | 0.91c | 0.92c |
| 4 | 1,8-Cineole | 1031 | 4.49a | 4.58a | 2.80b | 2.95b | 2.95b |
| 5 | trans-Pinocarveol | 1140 | 1.61a | 1.65a | 1.54b | 1.42c | 1.14d |
| 6 | Camphor | 1145 | 2.75a | 2.79a | 2.41b | 2.06c | 2.05c |
| 7 | Pinocarvone | 1164 | 1.32a | 1.36a | 1.32a | 1.16b | 0.90c |
| 8 | Borneol | 1170 | 16.00a | 16.46a | 12.78b | 12.14b | 12.99b |
| 9 | Myrtenol | 1198 | 2.25a | 2.35a | 1.94a | 2.13a | 1.85a |
| 10 | Geranylacetate | 1385 | 1.20a | 1.38a | 1.14a | 0.59b | 1.41a |
| 11 | α-Santalene | 1422 | 1.90bc | 1.42d | 2.16b | 2.64a | 1.74c |
| 12 | γ-Cadinene | 1518 | 4.97c | 4.54d | 5.09c | 6.08a | 5.36b |
| 13 | Caryophylleneoxide | 1589 | 9.12c | 8.54c | 11.06b | 12.23a | 8.79c |
| 14 | τ-Cadinol | 1648 | 12.96c | 14.35b | 13.65bc | 14.17b | 16.63a |
| 16 | α-Cadinol | 1662 | 1.33a | 1.13b | 1.35a | 1.36a | 1.08c |
| 17 | Cadalene | 1675 | 1.87d | 1.58e | 2.34b | 2.53a | 2.03c |
| 18 | cis-14-nor-Muurol-5-en-4-one | 1693 | 2.68c | 3.72b | 3.72b | 3.37c | 4.45a |
| 19 | (E,E)-Farnesol | 1720 | 1.20d | 1.43b | 1.32c | 1.45b | 1.57a |
| 20 | Bisabolol oxide A | 1750 | 1.97c | 2.26b | 2.23b | 2.13b | 2.60a |
 compounds with a significantly lower content as compared with the control oil;
compounds with a significantly lower content as compared with the control oil;  compounds with a significantly higher content as compared with the control oil; a, b, c—values followed by the same letter are not significantly different at p ≤ 0.05 according to the LSD (least significant differences) Tukey test.
compounds with a significantly higher content as compared with the control oil; a, b, c—values followed by the same letter are not significantly different at p ≤ 0.05 according to the LSD (least significant differences) Tukey test.| No. | Compound | RI | Control | 50 mg·dm−3 Au | 10 mg·dm−3 Au | 50 mg·dm−3 Ag | 10 mg·dm−3 Ag | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | MH | α-Thujene | 927 | 0.09 | ±0.01 | ||||||||
| 2. | MH | α-Pinene | 933 | 1.46 | ±0.16 | 0.99 | ±0.10 | 1.03 | ±0.01 | 1.11 | ±0.18 | 0.64 | ±0.04 |
| 3. | MH | Camphene | 948 | 1.08 | ±0.16 | 0.78 | ±0.07 | 0.85 | ±0.04 | 0.77 | ±0.11 | 0.48 | ±0.03 |
| 4. | MH | Thuja-2,4(10)-diene | 954 | 0.09 | ±0.01 | ||||||||
| 5. | MH | β-Thujene | 971 | 0.33 | ±0.02 | 0.24 | ±0.01 | 0.28 | ±0.01 | 0.20 | ±0.00 | 0.21 | ±0.01 |
| 6. | MH | Sabinene | 974 | 0.31 | ±0.01 | 0.25 | ±0.03 | 0.23 | ±0.01 | 0.23 | ±0.03 | 0.21 | ±0.01 |
| 7. | MH | β-Pinene | 977 | 3.14 | ±0.26 | 2.56 | ±0.26 | 2.25 | ±0.08 | 2.53 | ±0.46 | 1.93 | ±0.09 |
| 8. | MH | δ-3-Carene | 1010 | 0.93 | ±0.08 | 0.72 | ±0.06 | 0.77 | ±0.03 | 0.78 | ±0.10 | 0.59 | ±0.03 |
| 9. | MH | m-Cymene | 1022 | 0.52 | ±0.05 | 0.45 | ±0.04 | 0.49 | ±0.02 | 0.33 | ±0.01 | 0.32b | ±0.01 |
| 10. | MH | p-Cymene | 1025 | 1.39 | ±0.13 | 1.18 | ±0.10 | 1.17 | ±0.07 | 0.91 | ±0.06 | 0.92c | ±0.04 |
| 11. | MH | D-Limonene | 1029 | 0.81 | ±0.11 | 0.33 | ±0.47 | 0.64 | ±0.02 | 0.58 | ±0.04 | 0.27a | ±0.38 |
| 12. | OM | 1,8-Cineole | 1031 | 4.49 | ±0.26 | 4.58 | ±0.01 | 2.80 | ±0.12 | 2.95 | ±0.43 | 2.95b | ±0.33 |
| 13. | MH | γ-Terpinene | 1060 | 0.10 | ±0.01 | 0.08 | ±0.01 | ||||||
| 14. | OM | cis-Sabinenehydrate | 1068 | 0.070 | ±0.00 | 0.09 | ±0.00 | ||||||
| 15. | MH | α-Terpinolene | 1091 | 0.24 | ±0.01 | 0.23 | ±0.00 | 0.27 | ±0.02 | 0.15 | ±0.06 | ||
| 16. | OM | Linalool | 1101 | 0.65 | ±0.03 | 0.77 | ±0.05 | 0.55 | ±0.04 | 0.30 | ±0.06 | 0.51b | ±0.01 |
| 17. | O | α-Pineneoxide | 1110 | 0.210 | ±0.01 | ||||||||
| 18. | OM | Fenchol | 1114 | 0.21 | ±0.01 | 0.26 | ±0.01 | 0.16 | ±0.01 | 0.19 | ±0.00 | ||
| 19. | O | 3-Octanol acetate | 1122 | 0.15 | ±0.00 | 0.15 | ±0.01 | 0.20 | ±0.01 | 0.13 | ±0.01 | ||
| 20. | OM | α-Campholenal | 1127 | 0.23 | ±0.00 | 0.22 | ±0.01 | 0.24 | ±0.01 | 0.21 | ±0.01 | 0.20 | ±0.01 |
| 21. | OM | 1,2-Dihydrolinalool | 1136 | 0.24 | ±0.01 | 0.21 | ±0.01 | 0.26 | ±0.01 | 0.20 | ±0.01 | 0.18 | ±0.00 |
| 22. | OM | trans-Pinocarveol | 1140 | 1.61 | ±0.04 | 1.65 | ±0.10 | 1.54 | ±0.08 | 1.42 | ±0.05 | 1.14 | ±0.02 |
| 23. | OM | Camphor | 1145 | 2.75 | ±0.08 | 2.79 | ±0.21 | 2.41 | ±0.13 | 2.06 | ±0.13 | 2.05 | ±0.03 |
| 24. | OM | Pinocarvone | 1164 | 1.32 | ±0.01 | 1.36 | ±0.08 | 1.32 | ±0.08 | 1.16 | ±0.01 | 0.90 | ±0.00 |
| 25. | OM | Borneol | 1170 | 16.00 | ±0.58 | 16.46 | ±1.51 | 12.78 | ±0.44 | 12.14 | ±1.20 | 12.99 | ±0.16 |
| 26. | OM | Terpinen-4-ol | 1179 | 0.69 | ±0.04 | 0.64 | ±0.04 | 0.62 | ±0.01 | 0.60 | ±0.06 | 0.48 | ±0.00 |
| 27. | OM | p-Cymen-8-ol | 1184 | 0.80 | ±0.04 | 0.84 | ±0.01 | 0.99 | ±0.06 | 0.57 | ±0.07 | 0.40 | ±0.02 |
| 28. | OM | Cryptone | 1187 | 0.55 | ±0.25 | 0.71 | ±0.03 | 0.77 | ±0.04 | 0.52 | ±0.06 | 0.35 | ±0.01 |
| 29. | OM | α-Terpineol | 1193 | 0.66 | ±0.01 | 0.65 | ±0.04 | 0.51 | ±0.00 | 0.52 | ±0.06 | 0.41 | ±0.01 |
| 30. | OM | Myrtenol | 1198 | 2.25 | ±0.06 | 2.35 | ±0.11 | 1.94 | ±0.06 | 2.13 | ±0.22 | 1.85 | ±0.03 |
| 31. | OM | Verbenone | 1210 | 0.78 | ±0.06 | 0.69 | ±0.08 | 0.61 | ±0.02 | 0.51 | ±0.07 | 0.45 | ±0.01 |
| 32. | OM | cis-Carveol | 1221 | 0.18 | ±0.01 | 0.18 | ±0.00 | 0.24 | ±0.01 | 0.08 | ±0.11 | ||
| 33. | OM | trans-Carveol | 1224 | 0.20 | ±0.00 | 0.23 | ±0.01 | 0.23 | ±0.00 | 0.09 | ±0.12 | ||
| 34. | OM | Bornylformate | 1229 | 0.86 | ±0.01 | 0.85 | ±0.04 | 0.63 | ±0.00 | 0.62 | ±0.02 | 0.91 | ±0.01 |
| 35. | OM | d-Carvone | 1247 | 0.24 | ±0.00 | 0.24 | ±0.01 | 0.32 | ±0.01 | 0.10 | ±0.14 | 0.20 | ±0.02 |
| 36. | OM | Geraniol | 1254 | 0.46 | ±0.01 | 0.43 | ±0.01 | 0.40 | ±0.01 | 0.33 | ±0.02 | 0.31 | ±0.01 |
| 37. | OM | α-Citral | 1272 | 0.12 | ±0.01 | 0.12 | ±0.00 | 0.11 | ±0.01 | ||||
| 38. | OM | Bornylacetate | 1287 | 0.32 | ±0.01 | 0.29 | ±0.00 | 0.32 | ±0.04 | 0.27 | ±0.00 | 0.38 | ±0.03 |
| 39. | OM | Lavandulylacetate | 1292 | 0.20 | ±0.00 | 0.16 | ±0.00 | 0.21 | ±0.01 | 0.19 | ±0.01 | 0.18 | ±0.01 |
| 40. | OM | Piperitenone | 1341 | 0.11 | ±0.01 | 0.12 | ±0.01 | 0.14 | ±0.00 | 0.05 | ±0.06 | 0.12 | ±0.01 |
| 41. | OM | Nerylacetate | 1367 | 0.10 | ±0.01 | ||||||||
| 42. | OM | Geranylacetate | 1385 | 1.20 | ±0.06 | 1.38 | ±0.07 | 1.14 | ±0.06 | 0.59 | ±0.28 | 1.41 | ±0.27 |
| 43. | SH | α-Cedrene | 1416 | 0.40 | ±0.06 | 0.39 | ±0.04 | 0.45 | ±0.04 | 0.47 | ±0.01 | 0.45 | ±0.04 |
| 44. | SH | α-Santalene | 1422 | 1.90 | ±0.04 | 1.42 | ±0.02 | 2.16 | ±0.01 | 2.64 | ±0.00 | 1.74 | ±0.04 |
| 45. | SH | α-Bergamotene | 1438 | 0.28 | ±0.01 | 0.25 | ±0.01 | 0.33 | ±0.01 | 0.38 | ±0.02 | 0.29 | ±0.00 |
| 46. | SH | Aromadendrene | 1448 | 0.11 | ±0.01 | 0.08 | ±0.00 | 0.14 | ±0.00 | 0.14 | ±0.01 | 0.12 | ±0.01 |
| 47. | SH | β-Santalene | 1450 | 0.11 | ±0.01 | 0.12 | ±0.01 | 0.15 | ±0.01 | 0.10 | ±0.00 | ||
| 48. | SH | trans-β-Bergamotene | 1460 | 0.15 | ±0.01 | 0.09 | ±0.04 | 0.16 | ±0.06 | ||||
| 49. | SH | β-Chamigrene | 1463 | 0.11 | ±0.01 | 0.09 | ±0.00 | 0.11 | ±0.00 | 0.14 | ±0.01 | 0.12 | ±0.00 |
| 50. | SH | Di-epi-α-Cedrene | 1470 | 0.13 | ±0.00 | 0.14 | ±0.01 | 0.15 | ±0.00 | 0.15 | ±0.01 | 0.15 | ±0.01 |
| 51. | SH | cis-β-Farnesene | 1488 | 0.10 | ±0.00 | 0.12 | ±0.06 | 0.19 | ±0.05 | 0.15 | ±0.04 | 0.16 | ±0.01 |
| 52. | SH | β-Bisabolene | 1511 | 0.07 | ±0.09 | ||||||||
| 53. | SH | γ-Cadinene | 1518 | 4.97 | ±0.06 | 4.54 | ±0.11 | 5.09 | ±0.03 | 6.08 | ±0.03 | 5.36 | ±0.05 |
| 54. | SH | β-Sesquiphellandrene | 1522 | 0.39 | ±0.01 | 0.44 | ±0.04 | 0.56 | ±0.01 | 0.50 | ±0.01 | 0.52 | ±0.01 |
| 55. | SH | δ-Cadinene | 1526 | 0.45 | ±0.01 | 0.42 | ±0.03 | 0.49 | ±0.01 | 0.54 | ±0.01 | 0.50 | ±0.02 |
| 56. | SH | trans-Calamenene | 1533 | 0.24 | ±0.00 | 0.24 | ±0.03 | 0.33 | ±0.01 | 0.32 | ±0.03 | 0.35 | ±0.01 |
| 57. | SH | Cadina-1,4-diene | 1536 | 0.53 | ±0.01 | 0.60 | ±0.06 | 0.59 | ±0.03 | 0.53 | ±0.02 | 0.60 | ±0.01 |
| 58. | SH | α-Cadinene | 1543 | 0.19 | ±0.01 | 0.13 | ±0.18 | ||||||
| 59. | SH | α-Calacorene | 1547 | 0.36 | ±0.01 | 0.52 | ±0.06 | 0.51 | ±0.03 | 0.45 | ±0.05 | 0.48 | ±0.01 |
| 60. | SH | Germacrene B | 1557 | 0.89 | ±0.02 | 0.82 | ±0.07 | 1.14 | ±0.03 | 1.23 | ±0.05 | 0.86 | ±0.01 |
| 61. | SH | β-Calacorene | 1563 | 0.10 | ±0.01 | 0.11 | ±0.03 | 0.06 | ±0.08 | 0.13 | ±0.01 | ||
| 62. | OS | Nerolidol | 1569 | 0.51 | ±0.01 | 0.64 | ±0.07 | 0.62 | ±0.02 | 0.60 | ±0.02 | 0.66 | ±0.01 |
| 63. | O | (Z)-3-Hexenyl benzoate | 1579 | 0.63 | ±0.01 | 0.76 | ±0.08 | 0.74 | ±0.04 | 0.73 | ±0.01 | 0.76 | ±0.01 |
| 64. | OS | Caryophylleneoxide | 1589 | 9.12 | ±0.16 | 8.54 | ±0.31 | 11.06 | ±0.13 | 12.23 | ±0.21 | 8.79 | ±0.07 |
| 65. | O | Hexadecane | 1600 | 0.26 | ±0.01 | 0.30 | ±0.05 | 0.32 | ±0.01 | 0.29 | ±0.01 | 0.36 | ±0.01 |
| 66. | OS | Humuleneepoxide | 1605 | 0.21 | ±0.00 | 0.22 | ±0.04 | 0.30 | ±0.01 | 0.28 | ±0.01 | 0.26 | ±0.01 |
| 67. | OS | Humuleneepoxide II | 1613 | 0.65 | ±0.02 | 0.69 | ±0.06 | 0.87 | ±0.01 | 0.89 | ±0.04 | 0.80 | ±0.01 |
| 68. | OS | epi-Cubenol | 1619 | 1.55 | ±0.04 | 1.73 | ±0.12 | 1.70 | ±0.04 | 1.77 | ±0.08 | 2.01 | ±0.01 |
| 69. | OS | γ-Eudesmol | 1628 | 0.25 | ±0.01 | 0.28 | ±0.04 | 0.32 | ±0.03 | 0.29 | ±0.01 | 0.20 | ±0.02 |
| 70. | OS | Isospathulenol | 1638 | 0.17 | ±0.04 | 0.24 | ±0.02 | 0.25 | ±0.03 | 0.18 | ±0.01 | ||
| 71. | OS | Caryophylla-4(12),8(13)-dien-5β-ol | 1642 | 0.30 | ±0.00 | 0.47 | ±0.04 | 0.26 | ±0.37 | 0.62 | ±0.12 | ||
| 72. | OS | τ-Cadinol | 1648 | 12.96 | ±0.69 | 14.35 | ±0.64 | 13.65 | ±0.14 | 14.17 | ±0.69 | 16.63 | ±0.06 |
| 73. | OS | α-Muurolol | 1655 | 0.44 | ±0.02 | 0.47 | ±0.06 | 0.54 | ±0.01 | 0.53 | ±0.00 | 0.59 | ±0.00 |
| 74. | OS | α-Eudesmol | 1659 | 0.46 | ±0.03 | 0.51 | ±0.06 | 0.55 | ±0.01 | 0.53 | ±0.07 | 0.69 | ±0.01 |
| 75. | OS | α-Cadinol | 1662 | 1.33 | ±0.04 | 1.13 | ±0.06 | 1.35 | ±0.06 | 1.36 | ±0.02 | 1.08 | ±0.04 |
| 76. | OS | 9-Cedranone | 1667 | 1.13 | ±0.04 | 1.29 | ±0.16 | 1.33 | ±0.01 | 1.37 | ±0.06 | 1.41 | ±0.07 |
| 77. | O | Cadalene | 1675 | 1.87 | ±0.05 | 1.58 | ±0.16 | 2.34 | ±0.04 | 2.53 | ±0.18 | 2.03 | ±0.01 |
| 78. | OS | α-Bisabolol | 1681 | 0.77 | ±0.04 | 0.87 | ±0.09 | 0.96 | ±0.03 | 0.92 | ±0.04 | 0.91 | ±0.00 |
| 79. | OS | epi-α-Bisabolol | 1691 | 0.69 | ±0.03 | 0.38 | ±0.53 | ||||||
| 80. | OS | cis-14-nor-Muurol-5-en-4-one | 1693 | 2.68 | ±0.08 | 3.72 | ±0.22 | 3.72 | ±0.05 | 3.37 | ±0.7 | 4.45 | ±0.03 |
| 81. | O | Heptadecane | 1703 | 0.28 | ±0.05 | 0.30 | ±0.01 | 0.13 | ±0.18 | 0.31 | ±0.00 | ||
| 82. | O | 5-Ethyl-5-methylpentadecane | 1709 | 0.27 | ±0.01 | 0.31 | ±0.06 | 0.38 | ±0.01 | 0.33 | ±0.03 | 0.41 | ±0.01 |
| 83. | O | Pentadecanal | 1714 | 0.46 | ±0.04 | 0.53 | ±0.07 | 0.57 | ±0.01 | 0.52 | ±0.01 | 0.68 | ±0.01 |
| 84. | OS | (E,E)-Farnesol | 1720 | 1.20 | ±0.07 | 1.43 | ±0.14 | 1.32 | ±0.04 | 1.45 | ±0.04 | 1.57 | ±0.02 |
| 85. | O | 5-Phenyldodecane | 1733 | 0.50 | ±0.05 | 0.55 | ±0.13 | 0.68 | ±0.02 | 0.62 | ±0.03 | 0.81 | ±0.01 |
| 86. | OS | Bisabolol oxide A | 1750 | 1.97 | ±0.12 | 2.26 | ±0.27 | 2.23 | ±0.03 | 2.13 | ±0.12 | 2.60 | ±0.03 |
| 87. | OS | (E)-α-Atlantone | 1777 | 0.12 | ±0.02 | 0.19 | ±0.03 | 0.19 | ±0.00 | 0.22 | ±0.03 | 0.23 | ±0.05 |
| 88. | O | Octadecane | 1805 | 0.13 | ±0.02 | 0.20 | ±0.03 | 0.27 | ±0.02 | ||||
| 89. | DT | Phytane | 1811 | 0.21 | ±0.00 | ||||||||
| 90. | O | Diisobutylphthalate | 1872 | 0.24 | ±0.01 | 0.25 | ±0.08 | 0.33 | ±0.01 | ||||
| 91. | DT | m-Camphorene | 1957 | 0.14 | ±0.01 | 0.26 | ±0.02 | ||||||
| 92. | O | Eicosane | 2003 | 0.12 | ±0.08 | ||||||||
| 93. | O | Octadecanal | 2021 | 0.20 | ±0.03 | ||||||||
| 94. | O | 1-Octadecanol | 2088 | 0.29 | ±0.03 | 0.66 | ±0.08 | ||||||
| 95. | O | 1-Tricosene | 2296 | 0.31 | ±0.16 | ||||||||
| 96. | O | Tricosane | 2300 | 0.25 | ±0.13 | 0.44 | ±0.09 | ||||||
| 97. | O | 2-Heneicosanone | 2307 | 0.66 | ±0.20 | 0.84 | ±0.21 | 2.22 | ±0.35 | ||||
| Total identified [No.] | 82 | 81 | 81 | 83 | 83 | ||||||||
| Total identified [%] | 99.29 | 99.95 | 99.95 | 99.69 | 99.72 | ||||||||
| Monoterpene hydrocarbnons (MH) | 10.40 | 7.90 | 7.98 | 7.59 | 5.57 | ||||||||
| Oxygenated monoterpenes (OM) | 37.19 | 38.23 | 31.34 | 27.77 | 28.65 | ||||||||
| Sesquiterpene hydrocarbons (SH) | 11.22 | 10.27 | 12.36 | 14.35 | 12.06 | ||||||||
| Oxygenated sesquiterpenes (OS) | 36.34 | 38.96 | 41.21 | 43.36 | 43.06 | ||||||||
| Diterpenes (DT) | - | - | 0.14 | - | 0.47 | ||||||||
| Other (O) | 4.14 | 4.59 | 6.92 | 6.62 | 9.91 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesołowska, A.; Jadczak, P.; Kulpa, D.; Przewodowski, W. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Essential Oils from AgNPs and AuNPs Elicited Lavandula angustifolia In Vitro Cultures. Molecules 2019, 24, 606. https://doi.org/10.3390/molecules24030606
Wesołowska A, Jadczak P, Kulpa D, Przewodowski W. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Essential Oils from AgNPs and AuNPs Elicited Lavandula angustifolia In Vitro Cultures. Molecules. 2019; 24(3):606. https://doi.org/10.3390/molecules24030606
Chicago/Turabian StyleWesołowska, Aneta, Paula Jadczak, Danuta Kulpa, and Włodzimierz Przewodowski. 2019. "Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Essential Oils from AgNPs and AuNPs Elicited Lavandula angustifolia In Vitro Cultures" Molecules 24, no. 3: 606. https://doi.org/10.3390/molecules24030606
APA StyleWesołowska, A., Jadczak, P., Kulpa, D., & Przewodowski, W. (2019). Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Essential Oils from AgNPs and AuNPs Elicited Lavandula angustifolia In Vitro Cultures. Molecules, 24(3), 606. https://doi.org/10.3390/molecules24030606








