Efficient Synthesis of Furfural from Biomass Using SnCl4 as Catalyst in Ionic Liquid
Abstract
1. Introduction
2. Results and Discussion
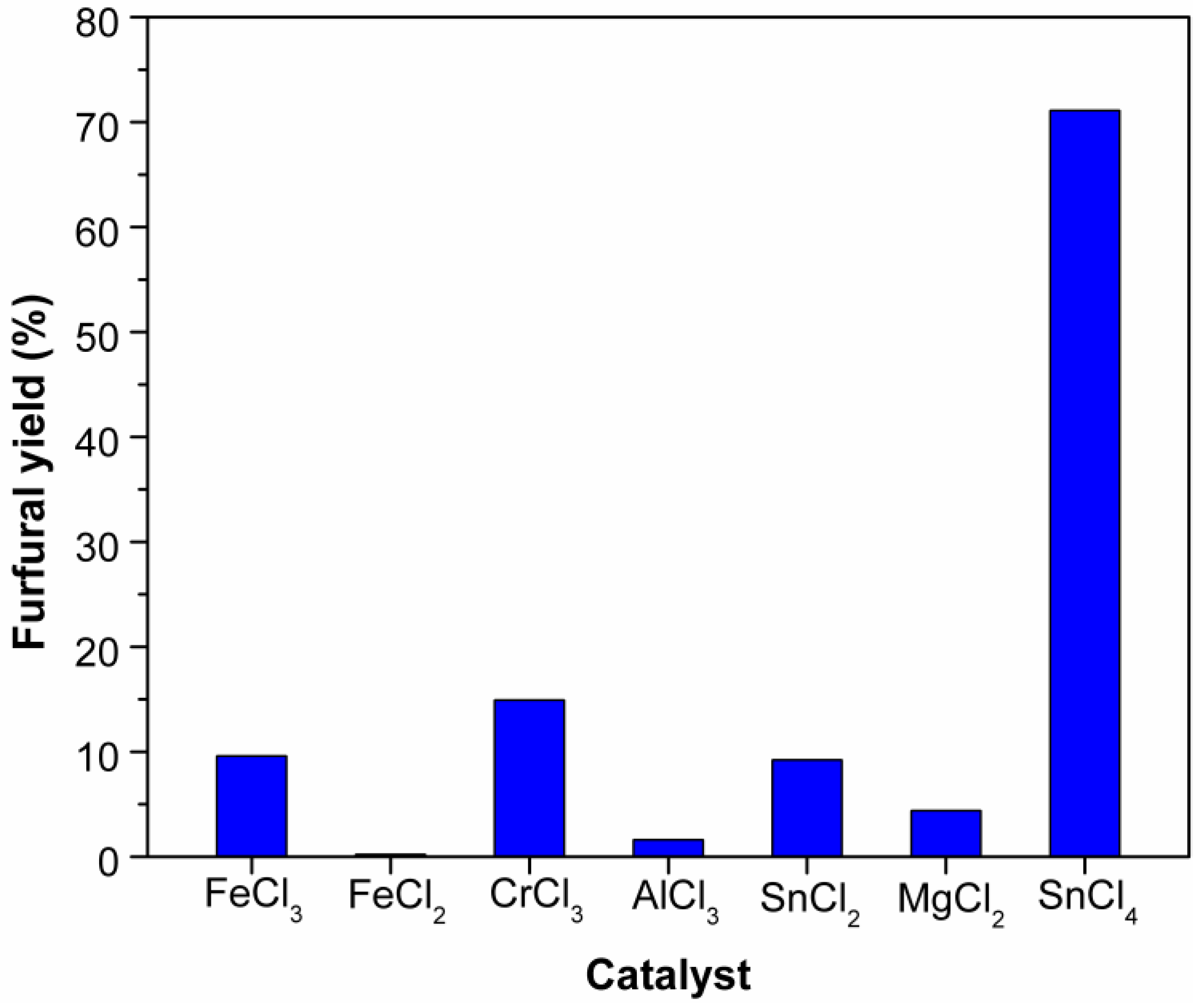
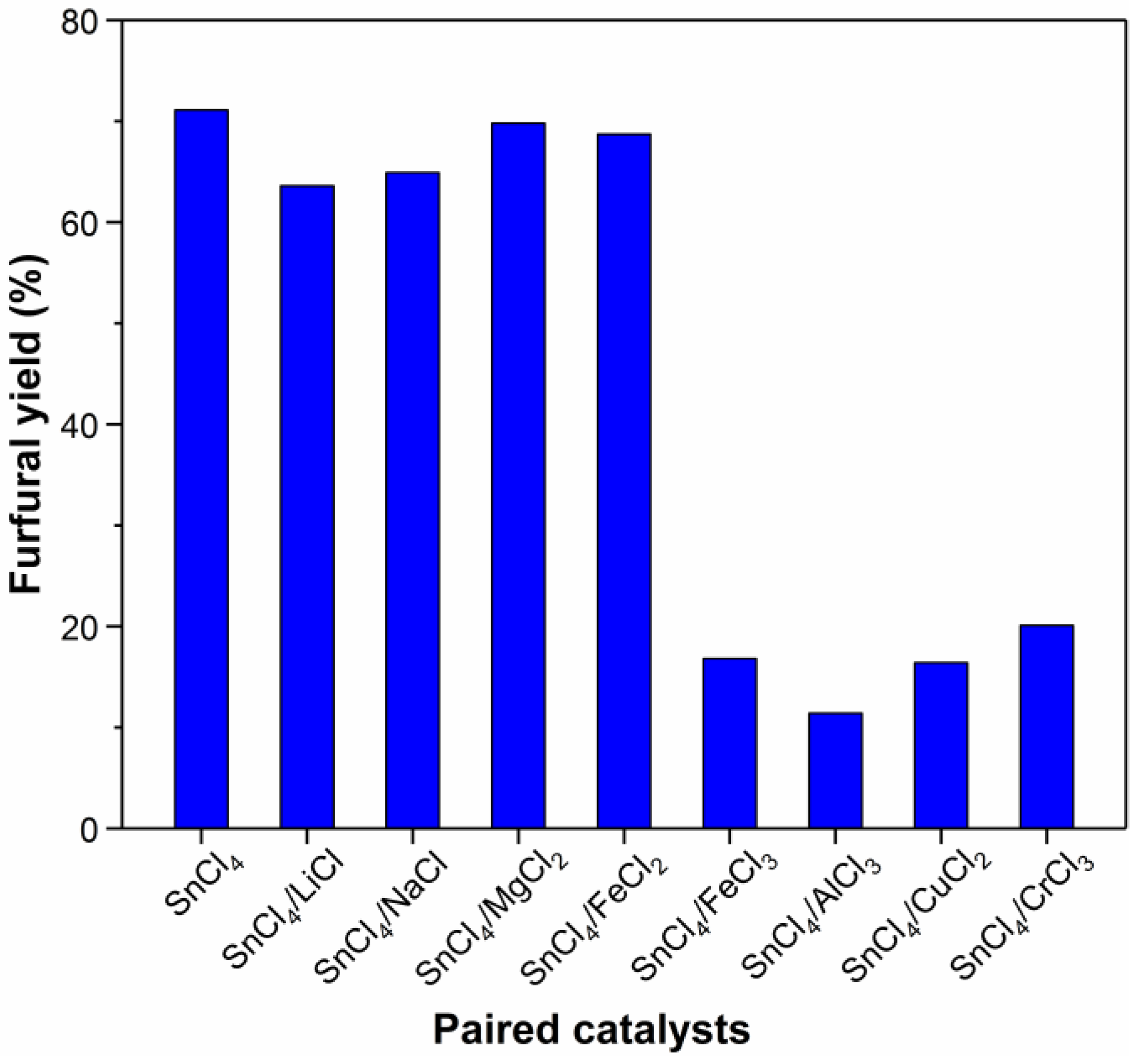
2.1. Effect of Catalysts on the Dehydration of Xylose into Furfural
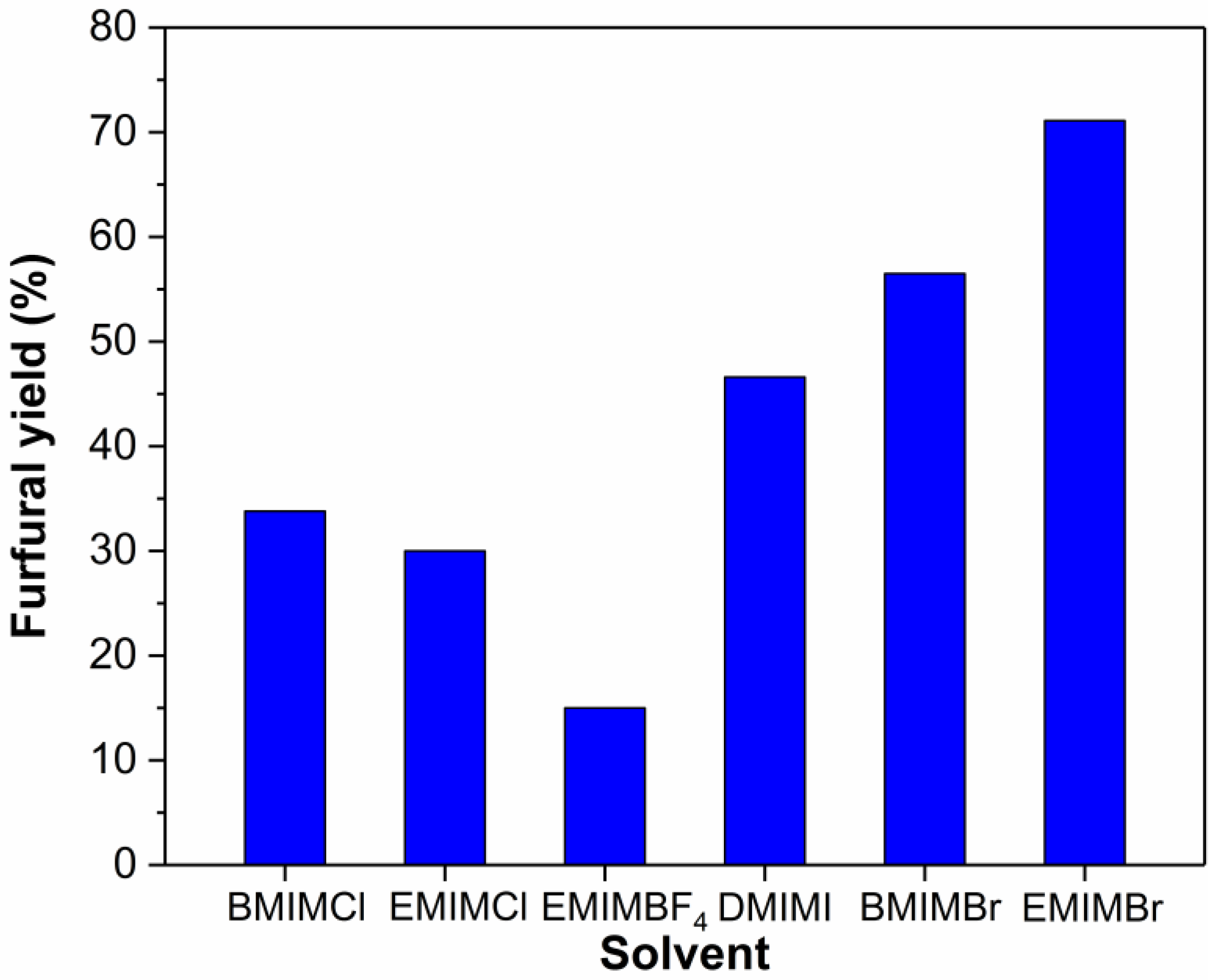
2.2. Effect of Solvents on the Dehydration of Xylose into Furfural
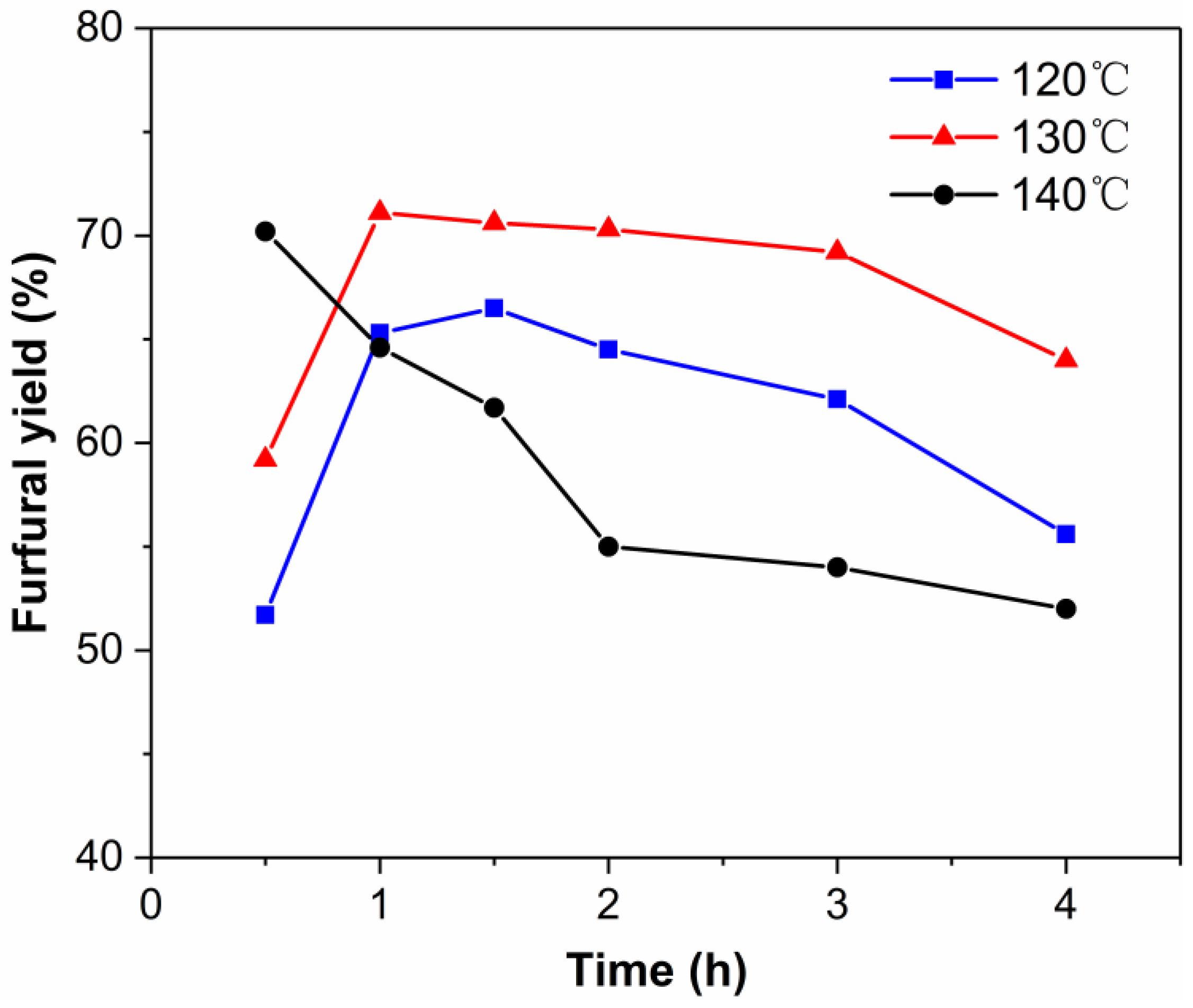
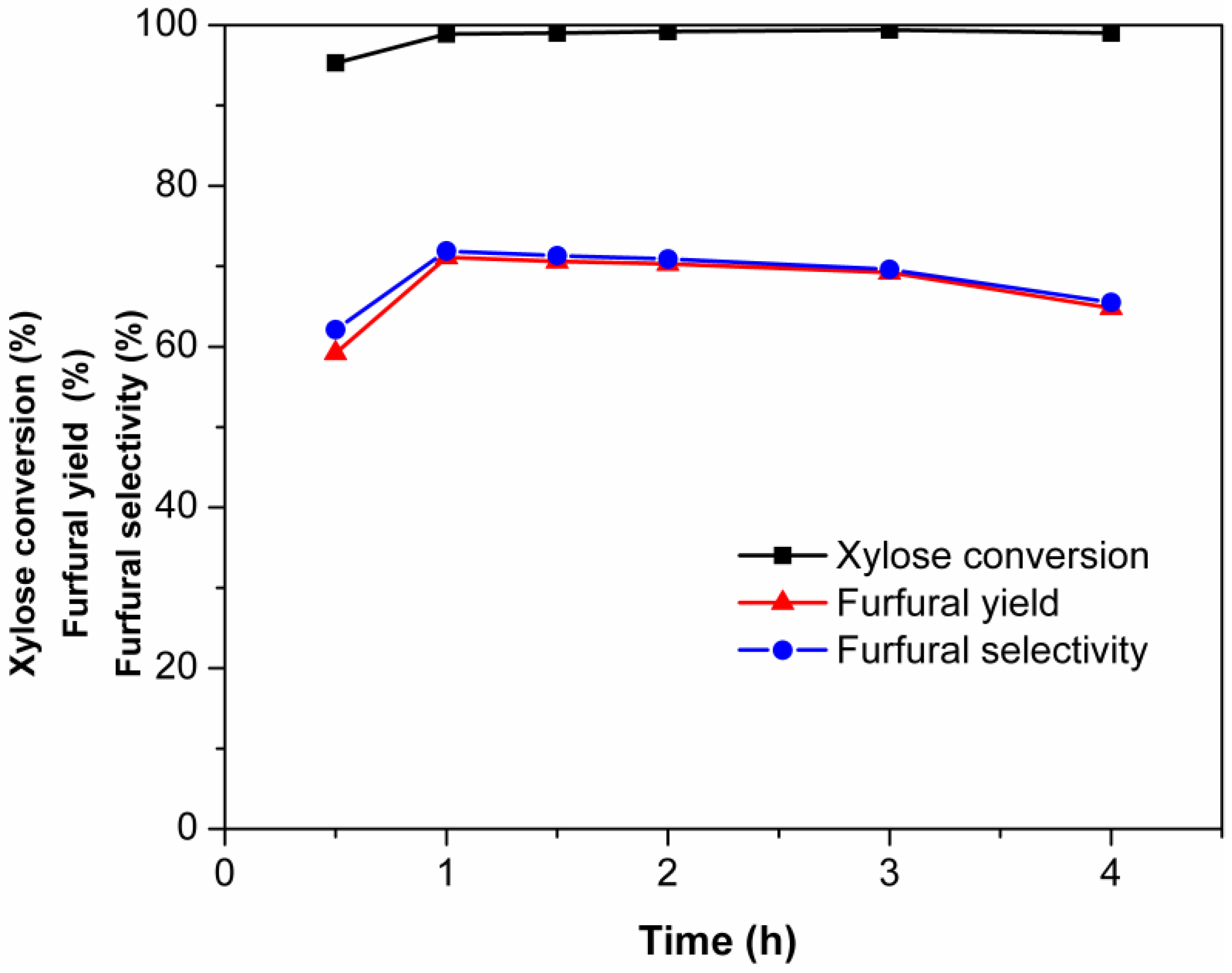
2.3. Effect of Reaction Temperature on the Dehydration of Xylose into Furfural
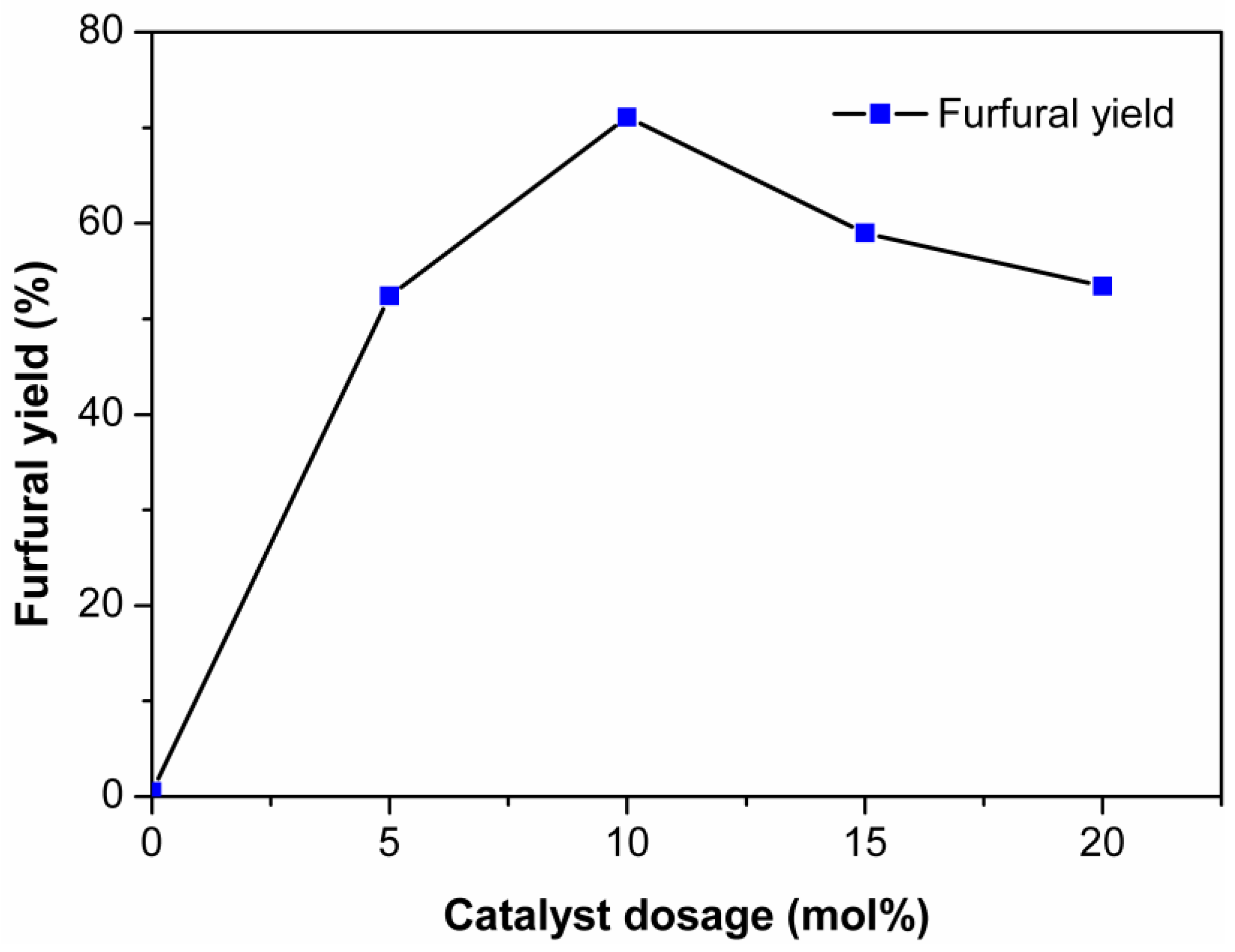
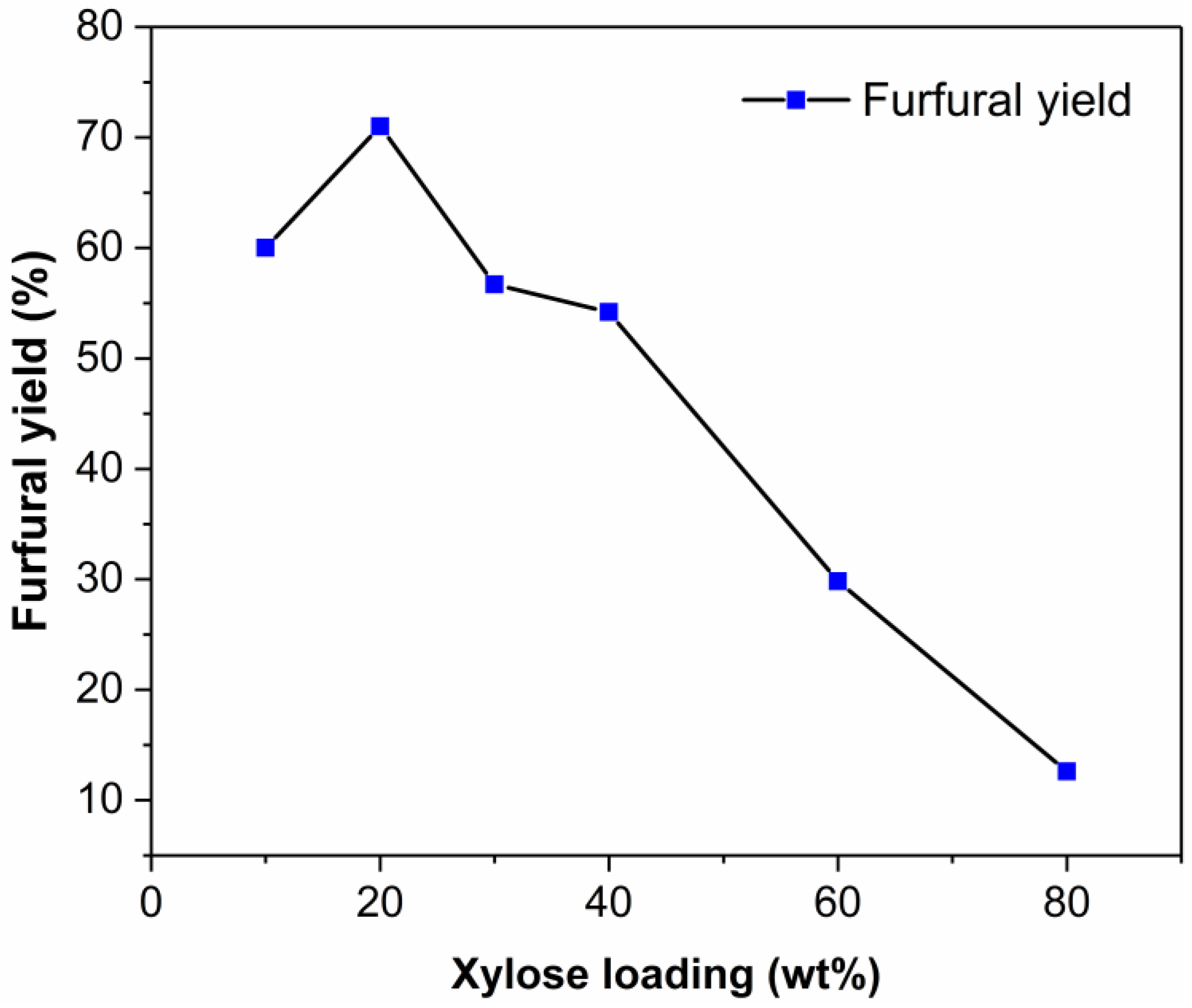
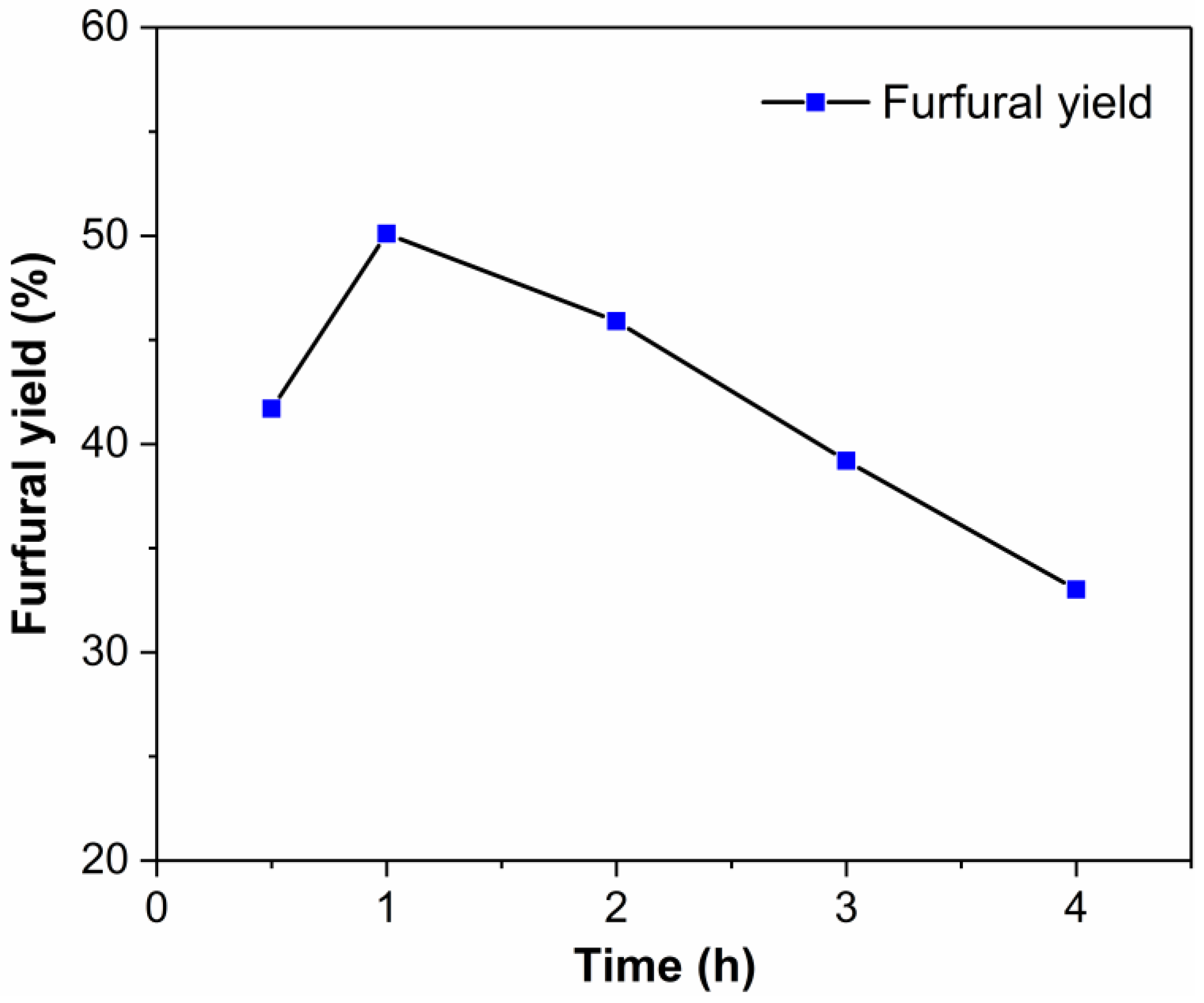
2.4. Effect of Initial Xylose Loading on the Dehydration of Xylose into Furfural
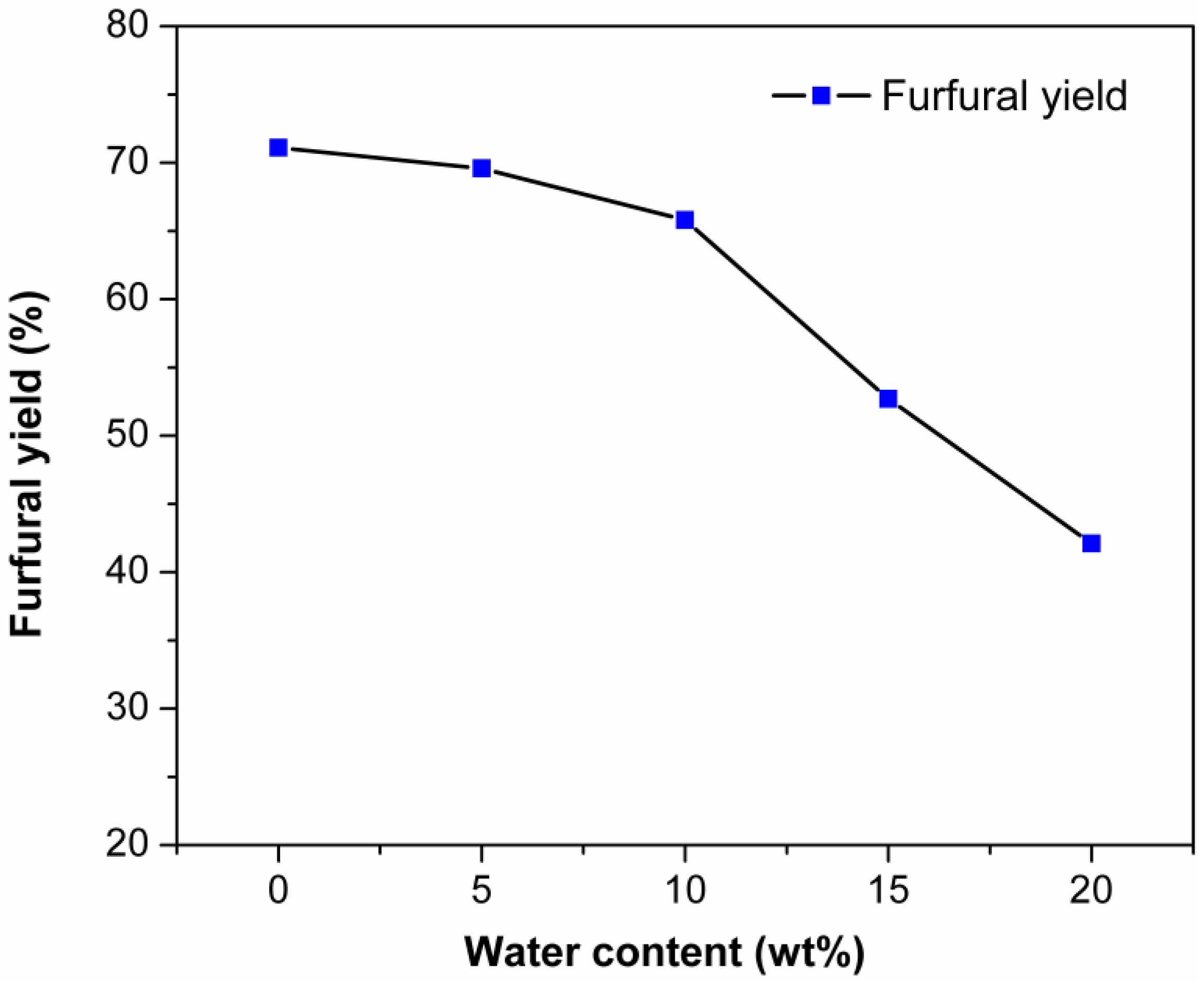
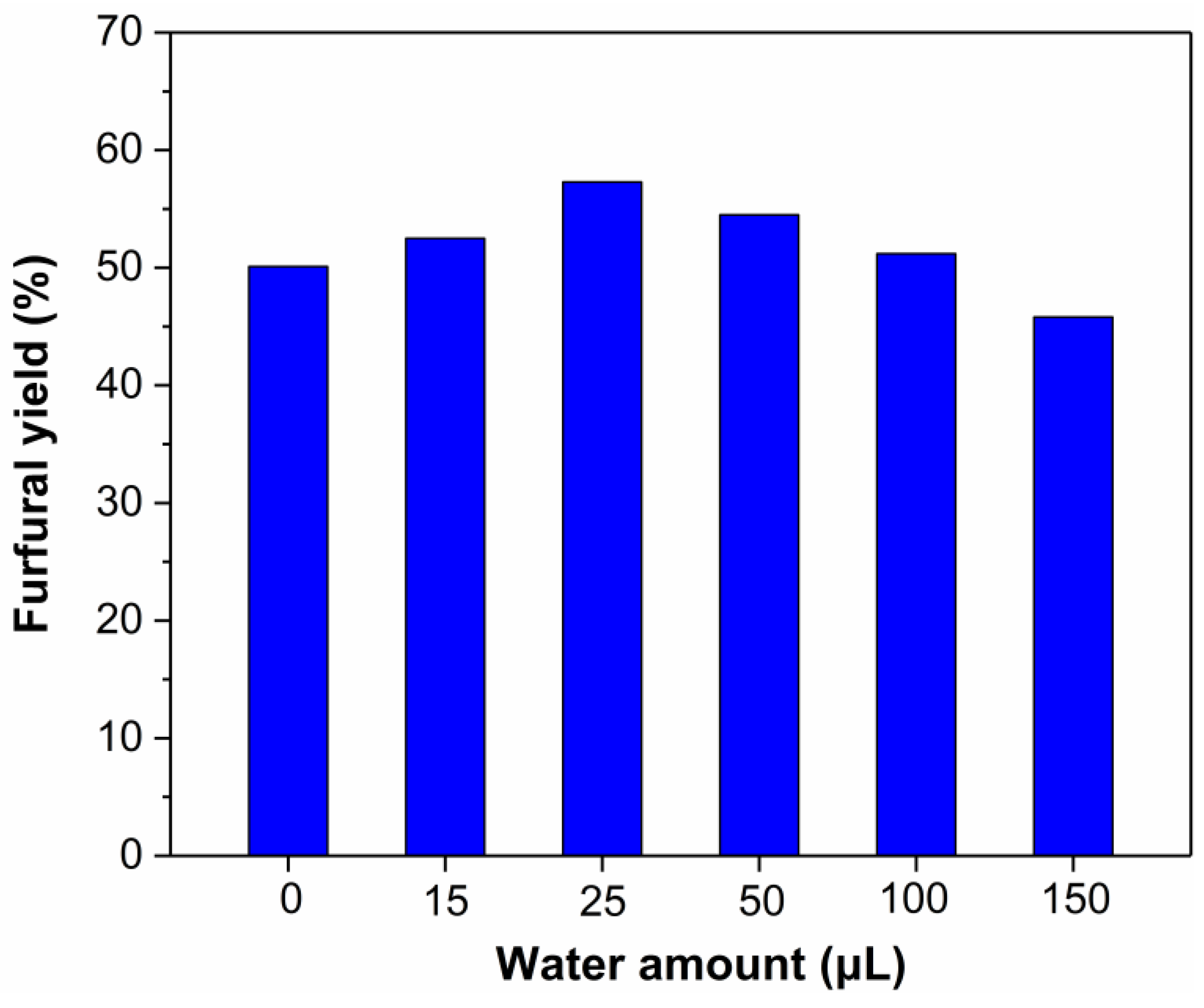
2.5. Effect of Water Content on the Dehydration of Xylose into Furfural
2.6. Conversion of Xylan into Furfural
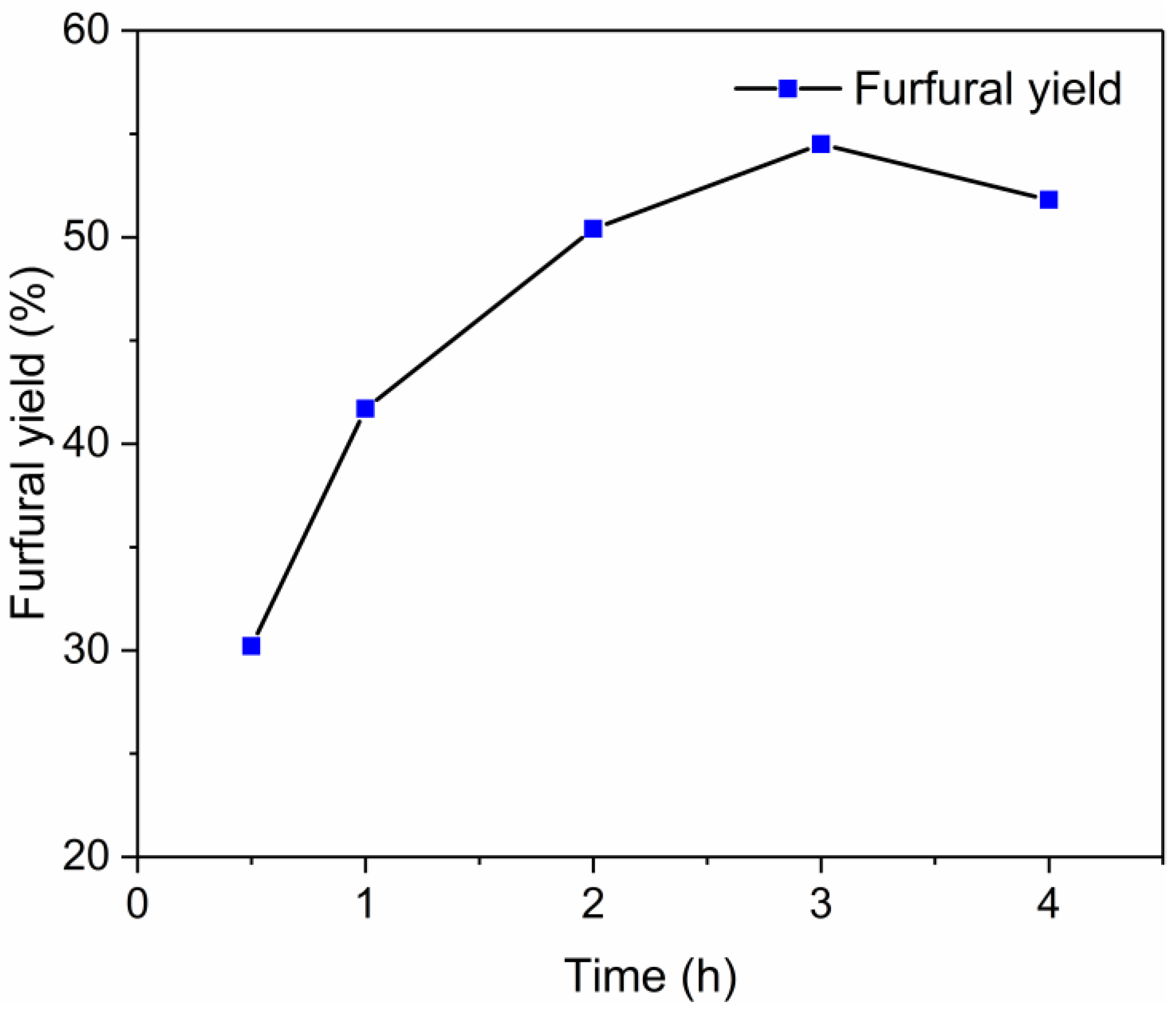
2.7. Conversion of Corn Stalk into Furfural
3. Materials and Methods
3.1. Materials
3.2. Conversion of Xylose and Xylan into Furfural
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lozano, F.J.; Lozano, R. Assessing the potential sustainability benefits of agricultural residues: Biomass conversion to syngas for energy generation or to chemicals production. J. Clean. Prod. 2018, 172, 4162–4169. [Google Scholar] [CrossRef]
- Gandarias, I.; García-Fernández, S.; Obregón, I.; Agirrezabal-Telleria, I.; Arias, P.L. Production of 2-methylfuran from biomass through an integrated biorefinery approach. Fuel Process. Technol. 2018, 178, 336–343. [Google Scholar] [CrossRef]
- Di Gioia, M.; Nardi, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable deep eutectic solvent for selective and scalable conversion of furfural into cyclopentenone derivatives. Molecules 2018, 23, 1891. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, C.; Bonari, E.; Licursi, D.; Nassi o Di Nasso, N.; Raspolli Galletti, A. Hydrothermal conversion of giant reed to furfural and levulinic acid: Optimization of the process under microwave irradiation and investigation of distinctive agronomic parameters. Molecules 2015, 20, 19760–19781. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yabushita, M.; Komanoya, T.; Hara, K.; Fujita, I.; Fukuoka, A. High-yielding one-pot synthesis of glucose from cellulose using simple activated carbons and trace hydrochloric acid. ACS Catal. 2013, 3, 581–587. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, X.; Xu, J.; Shi, M.; Wang, F.; Chen, C.; Xu, J. Depolymerization of cellulose to glucose by oxidation-hydrolysis. Green Chem. 2015, 17, 1519–1524. [Google Scholar] [CrossRef]
- Rizal, N.; Ibrahim, M.; Zakaria, M.; Abd-Aziz, S.; Yee, P.; Hassan, M. Pre-treatment of oil palm biomass for fermentable sugars production. Molecules 2018, 23, 1381. [Google Scholar] [CrossRef]
- Peleteiro, S.; Garrote, G.; Santos, V.; Parajó, J.C. Furan manufacture from softwood hemicelluloses by aqueous fractionation and further reaction in a catalyzed ionic liquid: A biorefinery approach. J. Clean. Prod. 2014, 76, 200–203. [Google Scholar] [CrossRef]
- Parejas, A.; Montes, V.; Hidalgo-Carrillo, J.; Sánchez-López, E.; Marinas, A.; Urbano, F. Microemulsion and sol-gel synthesized ZrO2-MgO catalysts for the liquid-phase dehydration of xylose to furfural. Molecules 2017, 22, 2257. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Liu, S.; Abrahamson, L.P.; Scott, G.M. Biorefinery: Ensuring biomass as a sustainable renewable source of chemicals, materials, and energy. Biomass Bioenergy 2012, 39, 1–4. [Google Scholar] [CrossRef]
- Werpy, T.A.; Holladay, J.E.; White, J.F. Top value added chemicals from biomass: I. results of screening for potential candidates from sugars and synthesis gas. Synth. Fuels 2004. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many by-Products; Elsevier Science: Amsterdam, The Netherlands, 2000; pp. 323–358. [Google Scholar]
- Casoni, A.I.; Hoch, P.M.; Volpe, M.A.; Gutierrez, V.S. Catalytic conversion of furfural from pyrolysis of sunflower seed hulls for producing bio-based furfuryl alcohol. J. Clean. Prod. 2018, 178, 237–246. [Google Scholar] [CrossRef]
- Lv, G.; Chen, S.; Zhu, H.; Li, M.; Yang, Y. Determination of the crucial functional groups in graphene oxide for vanadium oxide nanosheet fabrication and its catalytic application in 5-hydroxymethylfurfural and furfural oxidation. J. Clean. Prod. 2018, 196, 32–41. [Google Scholar] [CrossRef]
- Rogowski, J.; Andrzejczuk, M.; Berlowska, J.; Binczarski, M.; Kregiel, D.; Kubiak, A.; Modelska, M.; Szubiakiewicz, E.; Stanishevsky, A.; Tomaszewska, J.; et al. WxC-β-SiC nanocomposite catalysts used in aqueous phase hydrogenation of furfural. Molecules 2017, 22, 2033. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárova, K.; Liptaj, T.; Prónayová, N.; Soták, T. Bio-derived fuel additives from furfural and cyclopentanone. Fuel Process. Technol. 2015, 138, 564–569. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajo, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Mamman, A.S.; Lee, J.M.; Kim, Y.C.; Hwang, I.T.; Park, N.J.; Hwang, Y.K.; Chang, J.S.; Hwang, J.S. Furfural: Hemicellulose/xylosederived biochemical. Biofuels Bioprod. Biorefin. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Binder, J.B.; Blank, J.J.; Cefali, A.V.; Raines, R.T. Synthesis of furfural from xylose and xylan. ChemSusChem 2010, 3, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, C.W.; Abu-Omar, M.M. Synthesis of furfural from xylose, xylan, and biomass using alcl3⋅6 h2o in biphasic media via xylose isomerization to xylulose. ChemSusChem 2012, 5, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, H.; Wang, P.; Dong, H.; Peng, X. Conversion of xylan, d-xylose and lignocellulosic biomass into furfural using AlCl3 as catalyst in ionic liquid. Bioresour. Technol. 2013, 130, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Dussan, K.; Leahy, J.J. Enhancing the conversion of D-xylose into furfural at low temperatures using chloride salts as co-catalysts: Catalytic combination of AlCl3 and formic acid. Chem. Eng. J. 2017, 323, 278–286. [Google Scholar] [CrossRef]
- Ershova, O.; Nieminen, K.; Sixta, H. The role of various chlorides on xylose conversion to furfural: Experiments and kinetic modeling. Chemcatchem 2017, 9, 3031–3040. [Google Scholar] [CrossRef]
- Gupta, N.K.; Fukuoka, A.; Nakajima, K. Amorphous Nb2O5 as a selective and reusable catalyst for furfural production from xylose in biphasic water and toluene. ACS Catal. 2017, 7, 2430–2436. [Google Scholar] [CrossRef]
- Kaiprommarat, S.; Kongparakul, S.; Reubroycharoen, P.; Guan, G.; Samart, C. Highly efficient sulfonic MCM-41 catalyst for furfural production: Furan-based biofuel agent. Fuel 2016, 174, 189–196. [Google Scholar] [CrossRef]
- Nakajima, K.; Hirata, J.; Kim, M.; Gupta, N.K.; Murayama, T.; Yoshida, A.; Hiyoshi, N.; Fukuoka, A.; Ueda, W. Facile formation of lactic acid from a triose sugar in water over niobium oxide with a deformed orthorhombic phase. ACS Catal. 2017, 8, 283–290. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Liu, L.; Chen, Y.; Huang, F.; Zhang, S.; Li, W.; Ju, M. Tin phosphate as a heterogeneous catalyst for efficient dehydration of glucose into 5-hydroxymethylfurfural in ionic liquid. Appl. Catal. B 2018, 224, 183–193. [Google Scholar] [CrossRef]
- Le Guenic, S.; Gergela, D.; Ceballos, C.; Delbecq, F.; Len, C. Furfural Production from D-Xylose and Xylan by using stable Nafion NR50 and NaCl in a microwave-assisted biphasic reaction. Molecules 2016, 21, 1102. [Google Scholar] [CrossRef]
- Sievers, C.; Musin, I.; Marzialetti, T.; Olarte, M.B.; Agrawal, P.K.; Jones, C.W. Acid-catalyzed conversion of sugars and furfurals in an ionic-liquid phase. ChemSusChem 2009, 2, 665–671. [Google Scholar] [CrossRef]
- Enslow, K.R.; Bell, A.T. SnCl4-catalyzed isomerization/dehydration of xylose and glucose to furanics in water. Catal. Sci. Technol. 2015, 5, 2839–2847. [Google Scholar] [CrossRef]
- Vom, S.T.; Grande, P.M.; Leitner, W.; de María, P.D. Iron-catalyzed furfural production in biobased biphasic systems: From pure sugars to direct use of crude xylose effluents as feedstock. Chemsuschem 2011, 4, 1592–1594. [Google Scholar]
- Ma, S.; Li, P.; Zhu, T.; Chang, H.; Lin, L. Reaction extraction of furfural from pentose solutions in a modified Scheibel column. Chem. Eng. Process. 2014, 83, 71–78. [Google Scholar] [CrossRef]
- Rivas, S.; Gonzálezmuñoz, M.J.; Santos, V.; Parajó, J.C. Production of furans from hemicellulosic saccharides in biphasic reaction systems. Holzforschung 2013, 67, 923–929. [Google Scholar] [CrossRef]
- Qing, Q.; Guo, Q.; Zhou, L.; Wan, Y.; Xu, Y.; Ji, H.; Gao, X.; Zhang, Y. Catalytic conversion of corncob and corncob pretreatment hydrolysate to furfural in a biphasic system with addition of sodium chloride. Bioresour. Technol. 2016, 226, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Li, W.; Ju, M.; Liu, L.; Chen, Y.; Yang, Q. One-pot synthesis of sulfonated graphene oxide for efficient conversion of fructose into HMF. RSC Adv. 2016, 6, 104016–104024. [Google Scholar] [CrossRef]
- Ahmad, T.; Olsson, K.T.O.; Kenne, L. The formation of 2-furaldehyde and formic acid from pentoses in slightly acidic deuterium oxide studied by 1H NMR spectroscopy. Carbohydr. Res. 1995, 276, 309–320. [Google Scholar] [CrossRef]
- Siewping, T.; Yi, G.S.; Zhang, Y.G. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026. [Google Scholar]
- Peleteiro, S.; Velasco, G.G.; Santos, V.; Liñares, J.C.P. Conversion of hexoses and pentoses into furans in an ionic liquid. Afinidad 2014, 71, 202–206. [Google Scholar]
- Da Costa Lopes, A.M.; Bogel-Łukasik, R. Acidic ionic liquids as sustainable approach of cellulose and lignocellulosic biomass conversion without additional catalysts. ChemSusChem 2015, 8, 947–965. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Lopes, A.M.; João, K.G.; Bogel-Łukasik, E.; Roseiro, L.B.; Bogel-Łukasik, R. Pretreatment and Fractionation of Wheat Straw Using Various Ionic Liquids. J. Agric. Food Chem. 2013, 61, 7874–7882. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Ray, M.J.; To, T.Q.; Leak, D.J.; Murphy, R.J.; Welton, T. Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid-water mixtures. Green Chem. 2011, 13, 2489–2499. [Google Scholar] [CrossRef]
- Hou, Q.; Li, W.; Zhen, M.; Liu, L.; Chen, Y.; Yang, Q.; Huang, F.; Zhang, S.; Ju, M. An ionic liquid-organic solvent biphasic system for efficient production of 5-hydroxymethylfurfural from carbohydrates at high concentrations. RSC Adv. 2017, 7, 47288–47296. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, Y.H.; Zhu, S.Z.; Pan, H.; Huang, Y.B. Insight into aluminum sulfate-catalyzed xylan conversion into furfural in a γ-valerolactone/water biphasic solvent under microwave conditions. ChemSusChem 2017, 10, 4066–4079. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Ren, J.; Sun, R.; Zheng, J.; Sun, G.; Liu, S. An efficient process for dehydration of xylose to furfural catalyzed by inorganic salts in water/dimethyl sulfoxide system. Chin. J. Catal. 2014, 35, 741–747. [Google Scholar] [CrossRef]
- Su, Y.; Brown, H.M.; Huang, X.; Zhou, X.D.; Amonette, J.E.; Zhang, Z.C. Single-step conversion of cellulose to 5-hydroxymethylfurfural (HMF), a versatile platform chemical. Appl. Catal. A 2009, 361, 117–122. [Google Scholar] [CrossRef]
- Chatterjee, A.; Hu, X.; Lam, F.L.Y. A dual acidic hydrothermally stable MOF-composite for upgrading xylose to furfural. Appl. Catal. A 2018, 566, 130–139. [Google Scholar] [CrossRef]
- Caes, B.R.; Palte, M.J.; Raines, R.T. Organocatalytic conversion of cellulose into a platform chemical. Chem. Sci. 2013, 4, 196–199. [Google Scholar] [CrossRef]
- García-Sancho, C.; Fúnez-Núñez, I.; Moreno-Tost, R.; Santamaría-González, J.; Pérez-Inestrosa, E.; Fierro, J.L.G.; Maireles-Torres, P. Beneficial effects of calcium chloride on glucose dehydration to 5-hydroxymethylfurfural in the presence of alumina as catalyst. Appl. Catal. B 2017, 206, 617–625. [Google Scholar] [CrossRef]
- Chang, G.Y.; Zhang, S.; Pan, X. Effective conversion of biomass into bromomethylfurfural, furfural, and depolymerized lignin in lithium bromide molten salt hydrate of a biphasic system. RSC Adv. 2016, 7, 300–308. [Google Scholar]
- D’Anna, F.; Marullo, S.; Vitale, P.; Rizzo, C.; Meo, P.L.; Noto, R. Ionic liquid binary mixtures: Promising reaction media for carbohydrate conversion into 5-hydroxymethylfurfural. Appl. Catal. A 2014, 482, 287–293. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Wu, Y.; Song, X.; Gao, L.; Li, W.; Das, L.; Xiao, G. Efficient production of furfural from xylose and wheat straw by bifunctional chromium phosphate catalyst in biphasic systems. Fuel Process. Technol. 2018, 175, 90–96. [Google Scholar] [CrossRef]
- Peleteiro, S.; Lopes, A.M.D.C.; Garrote, G.; Parajó, J.C.; Bogel-Lukasik, R. Simple and efficient furfural production from xylose in media containing 1-butyl-3-methylimidazolium Hydrogen Sulfate. Ind. Eng. Chem. Res. 2015, 54, 8368–8373. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Aresta, M.; Di Bitonto, L.; Pastore, C. Organic carbonates: Efficient extraction solvents for the synthesis of HMF in aqueous media with cerium phosphates as catalysts. ChemSusChem 2016, 9, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wrigstedt, P.; Keskiväli, J.; Leskelä, M.; Repo, T. The role of salts and bronsted acids in lewis acid-catalyzed aqueous-phase glucose dehydration to 5-hydroxymethylfurfural. Chemcatchem 2015, 7, 501–507. [Google Scholar] [CrossRef]
- Gupta, D.; Ahmad, E.; Pant, K.K.; Saha, B. Efficient utilization of potash alum as a green catalyst for production of furfural, 5-hydroxymethylfurfural and levulinic acid from mono-sugars. RSC Adv. 2017, 7, 41973–41979. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Lin, H.; Chen, J.; Zhu, L.; Luo, Z. Conversion of C5 carbohydrates into furfural catalyzed by a Lewis acidic ionic liquid in renewable γ-valerolactone. Green Chem. 2017, 19, 3869–3879. [Google Scholar] [CrossRef]
- O’Neill, R.; Ahmad, M.N.; Vanoye, L.; Aiouache, F. Kinetics of aqueous phase dehydration of xylose into furfural catalyzed by ZSM-5 Zeolite. Ind. Eng. Chem. Res. 2009, 48, 4300–4306. [Google Scholar] [CrossRef]
- Antaljr, M.J.; Richards, M.G.N. Mechanism of formation of 2-furaldehyde from d-xylose. Carbohydr. Res. 1991, 217, 71–85. [Google Scholar] [CrossRef]
- Yang, W.; Li, P.; Bo, D.; Chang, H. The optimization of formic acid hydrolysis of xylose in furfural production. Carbohydr. Res. 2012, 357, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Yemis, O.; Mazza, G. Acid-catalyzed conversion of xylose, xylan and straw into furfural by microwave-assisted reaction. Bioresour. Technol. 2011, 102, 7371–7378. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.S.; Lima, S.; Pillinger, M.; Valente, A.A. Modified versions of sulfated zirconia as catalysts for the conversion of xylose to furfural. Catal. Lett. 2007, 114, 151–160. [Google Scholar] [CrossRef]
- Choudhary, V.; Sandler, S.I.; Vlachos, D.G. Conversion of xylose to furfural using lewis and bronsted acid catalysts in aqueous media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárová, K. Terephthalic acid from waste PET: An efficient and reusable catalyst for xylose conversion into furfural. Catal. Today 2018, 324, 27–32. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Bogel-Lukasik, R. Highly efficient and selective CO2-adjunctive dehydration of xylose to furfural in aqueous media with THF. Green Chem. 2016, 18, 2331–2334. [Google Scholar] [CrossRef]
- Enslow, K.R.; Bell, A.T. The role of metal halides in enhancing the dehydration of xylose to furfural. ChemCatChem 2015, 7, 479–489. [Google Scholar] [CrossRef]
- Lam, E.; Majid, E.; Leung, A.C.; Chong, J.H.; Mahmoud, K.A.; Luong, J.H. Synthesis of Furfural from Xylose by Heterogeneous and Reusable Nafion Catalysts. ChemSusChem 2011, 4, 535–541. [Google Scholar] [CrossRef]
- Tao, F.T.; Song, H.S.; Chou, L.C. Efficient process for the conversion of xylose to furfural with acidic. Can. J. Chem 2011, 89, 83–87. [Google Scholar] [CrossRef]
- Chheda, J.N.; Románleshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Lange, J.P.; Van Der Heide, E.; Van Buijtenen, J.; Price, R. Furfural-a promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, Z.K. Microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquid. Bioresour. Technol. 2010, 101, 1111–1114. [Google Scholar] [CrossRef]
- Sen, S.M.; Binder, J.B.; Raines, R.T.; Maravelias, C.T. Conversion of biomass to sugars via ionic liquid hydrolysis: Process synthesis and economic evaluation. Biofuels, Bioprod. Biorefin. 2012, 6, 444–452. [Google Scholar] [CrossRef]
- Swatloski, R.P. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Chen, S.S.; Ok, Y.S.; Poon, C.S. Valorization of food waste into hydroxymethylfurfural: Dual role of metal ions in successive conversion steps. Bioresour. Technol. 2016, 219, 338–347. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; Xu, Z.; Liu, Q.; Ma, Q.; Jameel, H.; Chang, H.M.; Ma, L. Catalytic conversion of xylose and corn stalk into furfural over carbon solid acid catalyst in γ-valerolactone. Bioresour. Technol. 2016, 209, 108–114. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Matuchaki, M.D.D.J.; Andreaus, J.; Bogel-Lukasik, R. A green and efficient approach to selective conversion of xylose and biomass hemicellulose into furfural in aqueous media using high-pressure CO2 as a sustainable catalyst. Green Chem. 2016, 18, 2985–2994. [Google Scholar] [CrossRef]
- Carvalho, A.V.; da Costa Lopes, A.M.; Bogel-Łukasik, R. Relevance of the acidic 1-butyl-3-methylimidazolium hydrogen sulphate ionic liquid in the selective catalysis of the biomass hemicellulose fraction. RSC Adv. 2015, 5, 47153–47164. [Google Scholar] [CrossRef]
- Bado-Nilles, A.; Diallo, A.-O.; Marlair, G.; Pandard, P.; Chabot, L.; Geffard, A.; Len, C.; Porcher, J.-M.; Sanchez, W. Coupling of OECD standardized test and immunomarkers to select the most environmentally benign ionic liquids option-Towards an innovative “safety by design” approach. J. Hazard. Mater. 2015, 283, 202–210. [Google Scholar] [CrossRef]
- Peric, B.; Sierra, J.; Martí, E.; Cruañas, R.; Garau, M.A.; Arning, J.; Bottin-Weber, U.; Stolte, S. (Eco)toxicity and biodegradability of selected protic and aprotic ionic liquids. J. Hazard. Mater. 2013, 261, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.-O.; Fayet, G.; Len, C.; Marlair, G. Evaluation of Heats of Combustion of Ionic Liquids through Use of Existing and Purpose-Built Models. Ind. Eng. Chem. Res. 2012, 51, 3149–3156. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Entry | SnCl4 (mol%) | MgCl2 (mol%) | Furfural Yield (%) |
|---|---|---|---|
| 1 | 10 | 0 | 71.1 |
| 2 | 8 | 2 | 69.8 |
| 3 | 8 | 0 | 62.0 |
| 4 | 5 | 5 | 68.8 |
| 5 | 5 | 0 | 52.4 |
| 6 | 0 | 10 | 4.5 |
| Entry | Reaction Phase | Extraction Phase | Furfural Yield (%) | ||
|---|---|---|---|---|---|
| Extraction Phase | Reaction Phase | Total | |||
| 1 b | water | / | / | 27.3 | 27.3 |
| 2 c | water | toluene | 6.7 | 25.6 | 32.3 |
| 3 c | water | DMC | 7.0 | 19.1 | 26.1 |
| 4 c | water | MIBK | 5.9 | 39.8 | 45.7 |
| 5 d | EMIMBr | THF | 18.2 | 1.0 | 19.2 |
| 6 d | EMIMBr | toluene | 15.3 | 7.0 | 22.3 |
| 7 d | EMIMBr | DMC | 8.0 | 0.3 | 8.3 |
| 8 d | EMIMBr | EGDE | 7.2 | 4.6 | 11.8 |
| 9 d | EMIMBr | MIBK | 0 | 4.7 | 4.7 |
| Catalyst | Solvent | T/°C | t/h | Loading (wt%) | Con. (%) | Yield (%) | Sel. (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CrCl3 + HCl | water | 145 | 1 | 1 | 39 | [65] | ||
| CrCl3 + HCl | water–toluene | 145 | 2 | 0.5 | 95.8 | 76.3 | 79.6 | [65] |
| BMIMCl–AlCl3 | water–GVL | 140 | 2 | 3 | 99.7 | 79.8 | 80 | [59] |
| FeCl3 | water–2-MTHF | 140 | 2 | 3.2 | 71 | [34] | ||
| terephthalic acid | water–toluene | 190 | 3 | 3.7 | 91.8 | 70.9 | 77.2 | [66] |
| SnCl4 + LiCl | water–DMSO | 130 | 3 | 10 | 63 | [47] | ||
| HCl | water | 140 | 3 | 11.3 | 30 | [68] | ||
| SnCl4 | water | 140 | 5 | 11.3 | 55 | 32 | 58 | [33] |
| SnCl4 | water–n-butanol | 140 | 5 | 4.3 | 90 | 77 | 85 | [33] |
| CO2–H2O | water–THF | 180 | 1 | 1.3 | 82.9 | 69.4 | 83.7 | [67] |
| SnCl4 | EMIMBr | 130 | 1 | 20 | 98.9 | 71.1 | 71.9 | This work |
| SnCl4 | EMIMBr | 130 | 1 | 40 | 54.2 | This work |
| Substrate | Catalyst | Solvent | T/°C | t/h | Loading/wt% | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| corn stalk | CrCl2 + HCl | EMIMCl | 140 | 1 | 22 | [22] | |
| corncob | AlCl3 | BMIMCl | 160 | 0.05 | 2.5 | 19.1 | [24] |
| grass | AlCl3 | BMIMCl | 160 | 2 | 2.5 | 31.4 | [24] |
| pine wood | AlCl3 | BMIMCl | 160 | 2 | 2.5 | 33.6 | [24] |
| corn stalk | BMIMCl–AlCl3 | water–GVL | 140 | 4 | 3 | 48.0 | [59] |
| wheat straw | BMIMHSO4 | BMIMHSO4 | 160 | 2.5 | 10 | 36.2 | [80] |
| wheat straw | CO2–H2O | water–THF/MIBK | 180 | 1 | 44 | [79] | |
| SnCl4 | SnCl4 | EMIMBr | 130 | 3 | 5 | 54.5 | This work |
| SnCl4 | SnCl4 | EMIMBr | 130 | 3 | 10 | 46.4 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Y.; Hou, Q.; Li, W.; Bai, C.; Bai, X.; Ju, M. Efficient Synthesis of Furfural from Biomass Using SnCl4 as Catalyst in Ionic Liquid. Molecules 2019, 24, 594. https://doi.org/10.3390/molecules24030594
Nie Y, Hou Q, Li W, Bai C, Bai X, Ju M. Efficient Synthesis of Furfural from Biomass Using SnCl4 as Catalyst in Ionic Liquid. Molecules. 2019; 24(3):594. https://doi.org/10.3390/molecules24030594
Chicago/Turabian StyleNie, Yifan, Qidong Hou, Weizun Li, Chuanyunlong Bai, Xinyu Bai, and Meiting Ju. 2019. "Efficient Synthesis of Furfural from Biomass Using SnCl4 as Catalyst in Ionic Liquid" Molecules 24, no. 3: 594. https://doi.org/10.3390/molecules24030594
APA StyleNie, Y., Hou, Q., Li, W., Bai, C., Bai, X., & Ju, M. (2019). Efficient Synthesis of Furfural from Biomass Using SnCl4 as Catalyst in Ionic Liquid. Molecules, 24(3), 594. https://doi.org/10.3390/molecules24030594






