Design, Synthesis and Biological Evaluation of Nitrate Derivatives of Sauropunol A and B as Potent Vasodilatory Agents
Abstract
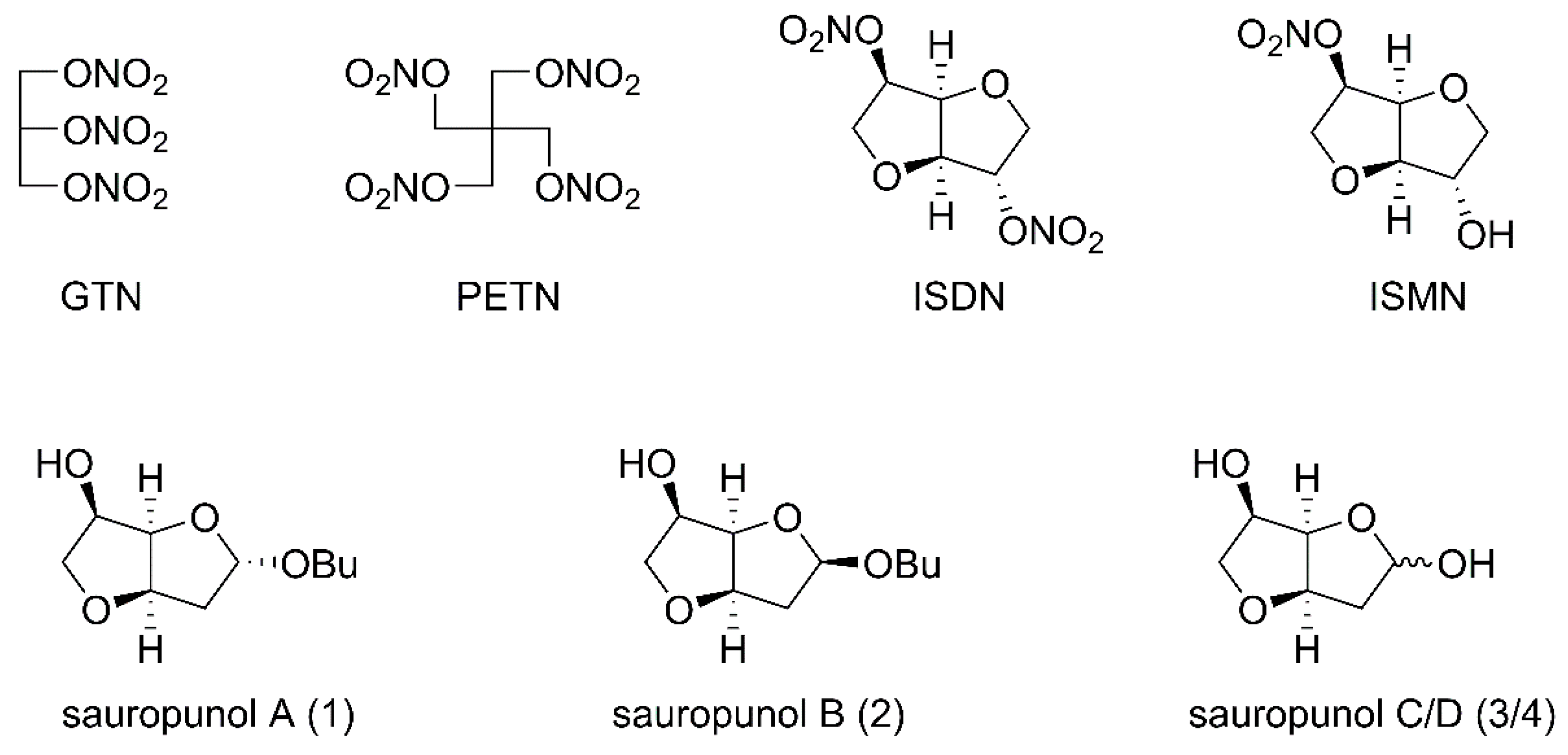
:1. Introduction
2. Results
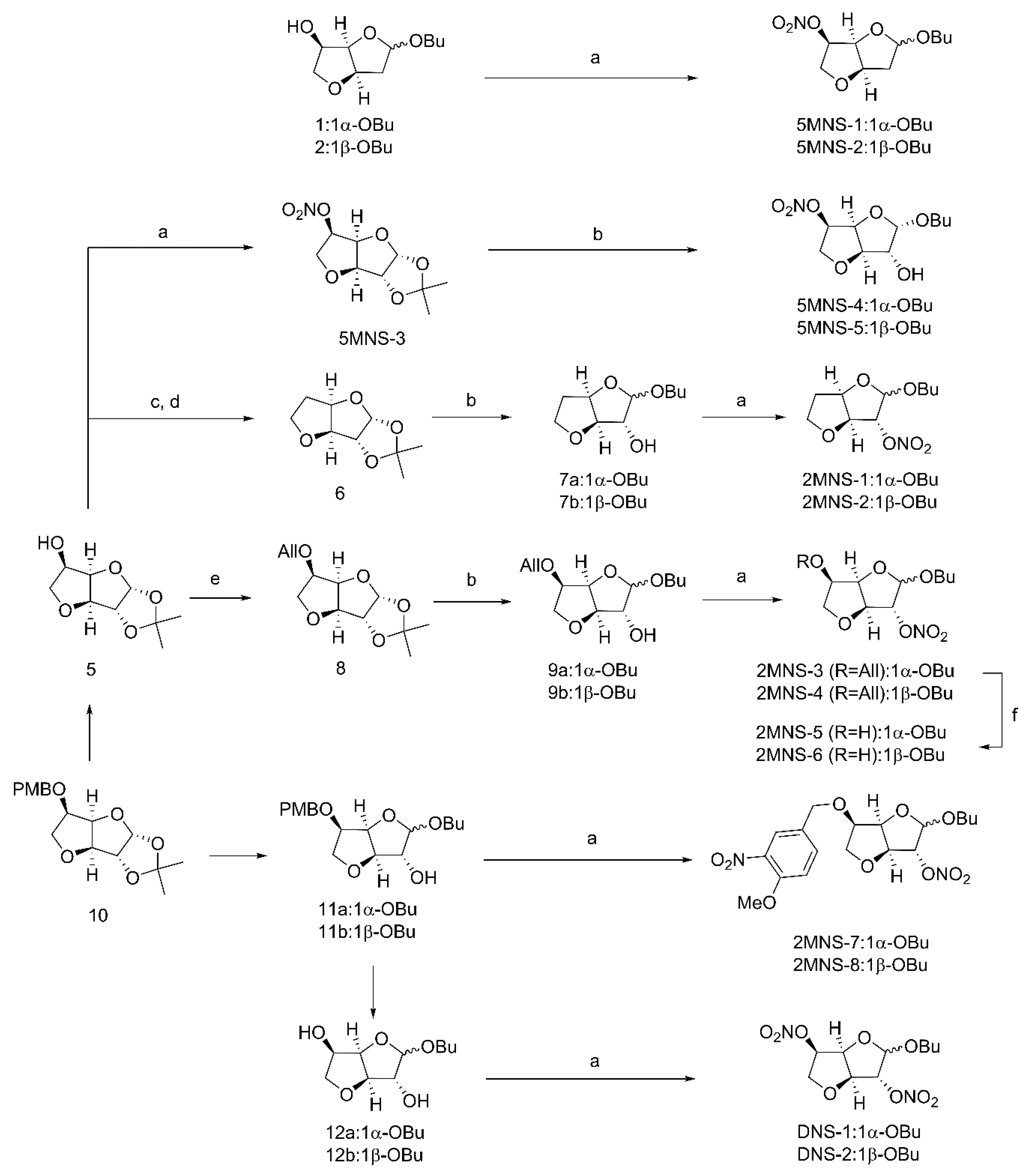
2.1. Chemistry
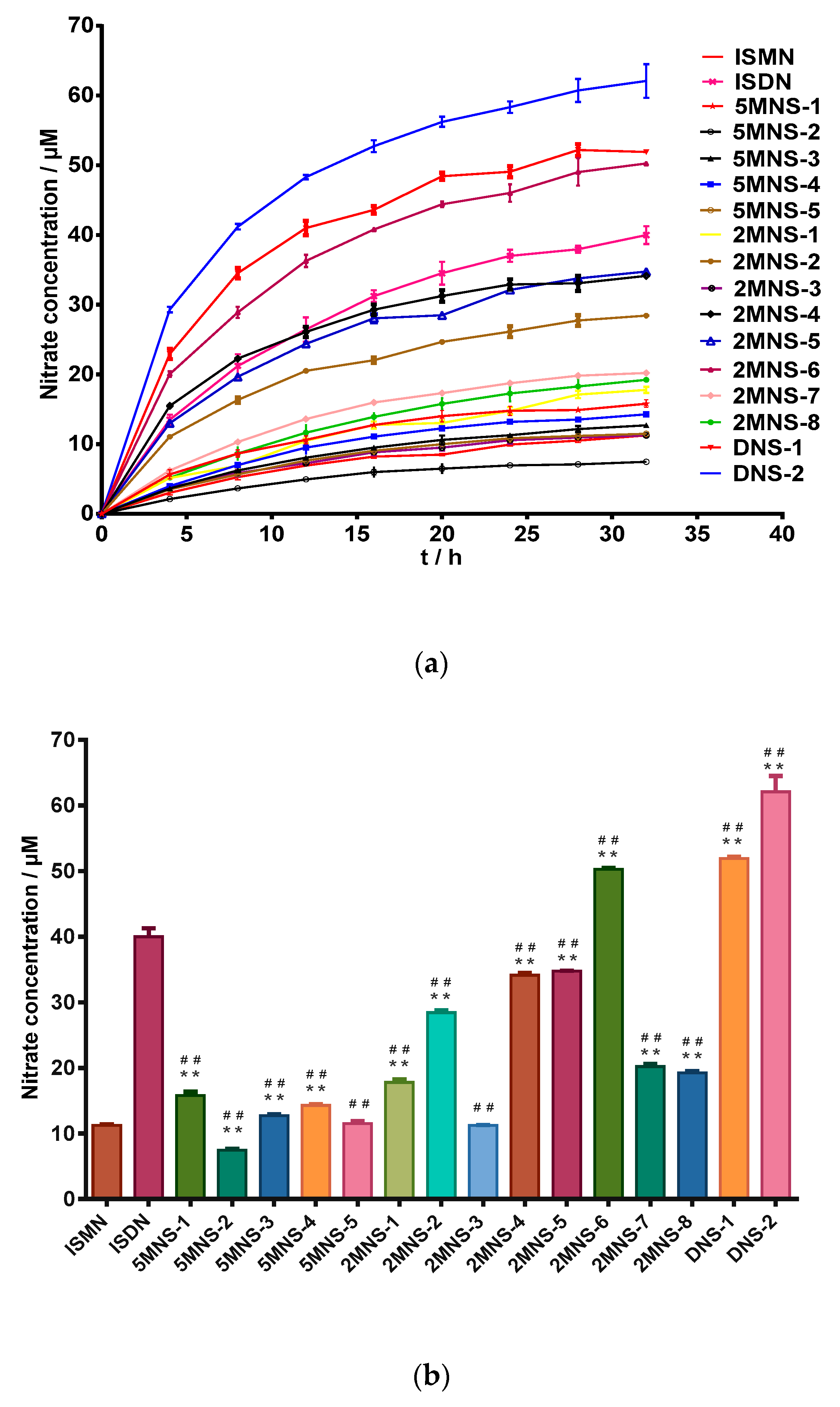
2.2. In Vitro Nitro Oxide Releasing Capacities
2.3. Vasodilatory Effects on Isolated Rat Mesenteric Arterial Rings
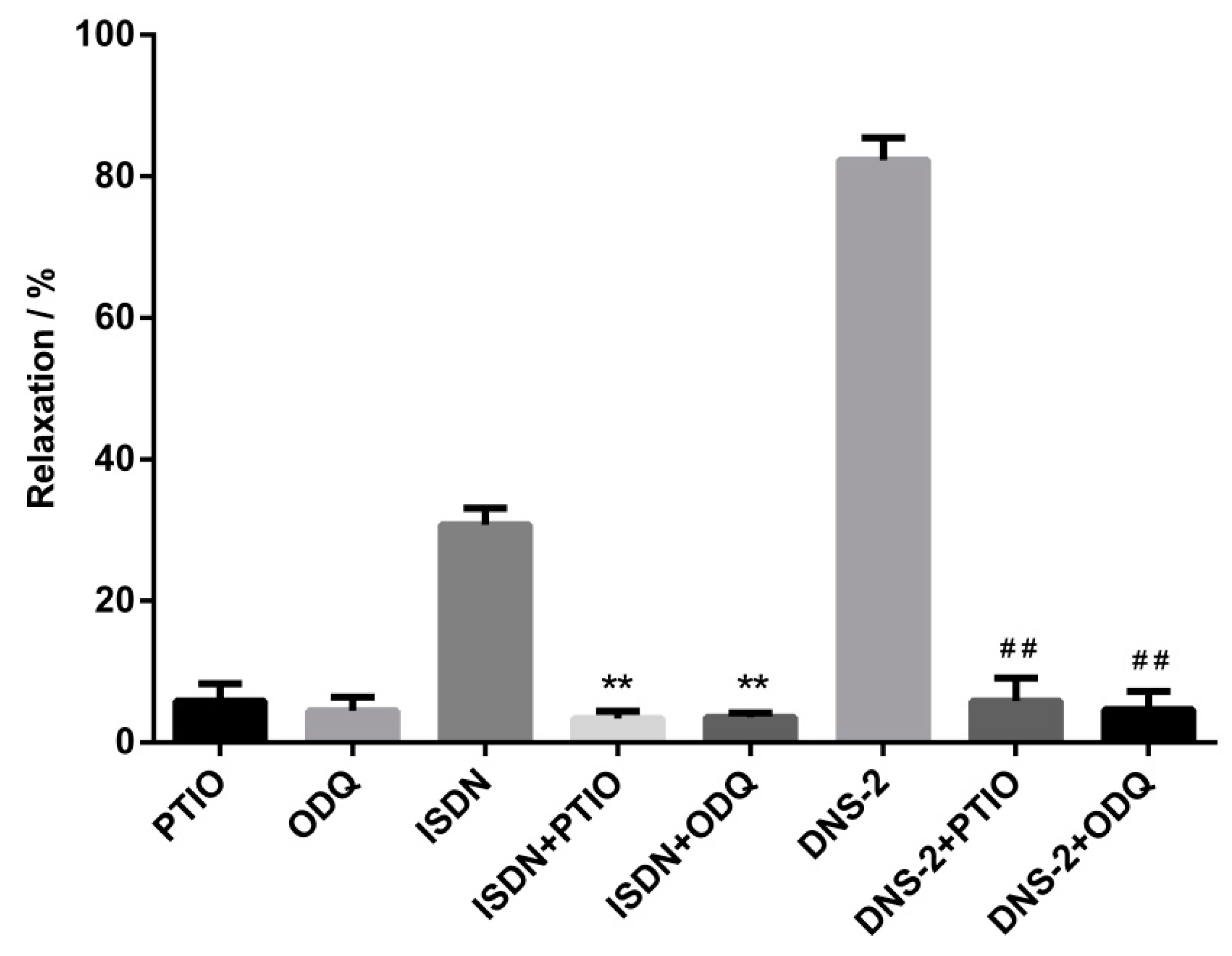
2.4. Effects of ODQ and PITO on Vasodilatory Effects of DNS-2
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Chemistry
4.2.1. Synthesis of Butyl 2-deoxy-3,6-anhydro-5-O-nitro-α-D-arabinohexofuranoside (5MNS-1)
4.2.2. Synthesis of Butyl 2-deoxy-3,6-anhydro-5-O-nitro-β-D-arabinohexofuranoside (5MNS-2)
4.2.3. Synthesis of 1,2-O-Isopropylidene-3,6-anhydro-5-O-nitro-α-d-glucofuranoside (5MNS-3)
4.2.4. Synthesis of Butyl 3,6-anhydro-5-O-nitro-α-d-glucofuranoside (5MNS-4) and Butyl 3,6-anhydro-5-O-nitro-β-d-glucofuranoside (5MNS-5)
4.2.5. Synthesis of Butyl 5-deoxy-3,6-anhydro-α-d-glucofuranoside (7a) and Butyl 5-deoxy-3,6-anhydro-β-d-glucofuranoside (7b)
4.2.6. Synthesis of Butyl 5-deoxy3,6-anhydro-2-O-nitro-α-d-glucofuranoside (2MNS-1) and Butyl 5-deoxy-3,6-anhydro-2-O-nitro-β-d-glucofuranoside (2MNS-2)
4.2.7. Synthesis of 1,2-O-Isopropylidene-3,6-anhydro-5-O-allyl-α-d-glucofuranoside (8)
4.2.8. Synthesis of Butyl 3,6-anhydro-5-O-allyl-α-d-glucofuranoside (9a) and Butyl 3,6-anhydro-5-O-allyl-β-d-glucofuranoside (9b)
4.2.9. Synthesis of Butyl 3,6-anhydro-2-O-nitro-5-O-allyl-α-d-glucofuranoside (2MNS-3) and Butyl 3,6-anhydro-2-O-nitro-5-O-allyl-β-d-glucofuranoside (2MNS-4)
4.2.10. Synthesis of Butyl 3,6-anhydro-2-O-nitro-α-d-glucofuranoside (2MNS-5) and Butyl 3,6-anhydro-2-O-nitro-β-d-glucofuranoside (2MNS-6)
4.2.11. Synthesis of Butyl 3,6-anhydro-5-O-(3-nitro-4-methoxybenzyl)-2-O-nitro-α-d-glucofuranoside (2MNS-7) and Butyl 3,6-anhydro-5-O-(4-methoxybenzyl)-2-O-nitro-β-d-glucofuranoside (2MNS-8)
4.2.12. Synthesis of Butyl 3,6-anhydro-2,5-di-O-nitro-α-d-arabinohexofuranoside (DNS-1) and Butyl 3,6-anhydro -2,5-di-O-nitro-β-d-arabinohexofuranoside (DNS-2)
4.3. Biology Evaluation
4.3.1. Nitric Oxide Releasing Assay
4.3.2. Vasodilatory Potential on Isolated Rat Mesenteric Arterial Rings
4.3.3. Effects of ODQ and PITO on Vasodilatory Effects of DNS-2
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moncada, S. Nitric oxide: Discovery and impact on clinical medicine. J. R. Soc. Med. 1999, 92, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.J.; Palmer, R.M.; Moncada, S. Comparative pharmacology of EDRF and nitric oxide on vascular strips. Eur. J. Pharmacol. 1987, 141, 445–451. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Nat. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef] [PubMed]
- Pörsti, I.; Paakkari, I. Nitric oxide-based possibilities for pharmacotherapy. Ann. Med. 1995, 27, 407–420. [Google Scholar] [CrossRef]
- Schuman, E.M.; Meffert, M.K.; Schulman, H.; Madison, D.V. An ADP-Ribosyltransferase as a Potential Target for Nitric Oxide Action in Hippocampal Long-Term Potentiation. Proc. Nat. Acad. Sci. USA 1994, 91, 11958–11962. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.; Nash, K.; Tackieyarboi, E.; Kostrevski, A.; Novak, A.; Raghavan, A.; Tulsulkar, J.; Alhadidi, Q.; Wamer, N.; Langenderfer, B. Furoxans (Oxadiazole-4N-oxides) with Attenuated Reactivity are Neuroprotective, Cross the Blood Brain Barrier, and Improve Passive Avoidance Memory. J. Med. Chem. 2018, 91, 4593–4607. [Google Scholar] [CrossRef] [PubMed]
- Biava, M.; Battilocchio, C.; Poce, G.; Alfonso, S.; Consalvi, S.; Porretta, G.C.; Schenone, S.; Calderone, V.; Martelli, A.; Testai, L. Improving the solubility of a new class of antiinflammatory pharmacodynamic hybrids, that release nitric oxide and inhibit cycloxygenase-2 isoenzyme. Eur. J. Pharmacol. 2012, 58, 287–298. [Google Scholar] [CrossRef]
- Kang, F.; Ai, Y.; Zhang, Y.; Huang, Z. Design and synthesis of new hybrids from 2-cyano-3,12-dioxooleana- 9-dien-28-oic acid and O2-(2,4-dinitrophenyl) diazeniumdiolate for intervention of drug-resistant lung cancer. Eur. J. Pharmacol. 2018, 149, 269–280. [Google Scholar]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospect. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Shami, P.J.; Saavedra, J.E.; Wang, L.Y.; Bonifant, C.L.; Diwan, B.A.; Singh, S.V.; Gu, Y.; Fox, S.D.; Buzard, G.S.; Citro, M.L. JS-K, a Glutathione/Glutathione S-Transferase-activated Nitric Oxide Donor of the Diazeniumdiolate Class with Potent Antineoplastic Activity1. Mol. Canc. Ther. 2003, 2, 409–417. [Google Scholar]
- Huang, Z.; Wu, J.; Yu, Z.; Yuan, H.; Zhang, Y.; Yue, F.; Bhardwaj, A.; Kaur, J.; Knaus, E.E.; Zhang, Y. Glutathione S-Transferase π-Activatable O2-(Sulfonylethyl Derived) Diazeniumdiolates Potently Suppress Melanoma in Vitro and in Vivo. J. Med. Chem. 2018, 61, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.D.S.; Souza, P.C.D.; Marino, L.B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Fruttero, R.; Chin, C.M.; Pavan, F.R.; Santos, J.L.D. Synthesis and biological activity of furoxan derivatives against Mycobacterium tuberculosis. Eur. J. Pharmacol. 2016, 123, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Jones, R.; Talbot, J.; Mayers, I.; Barrie, J.; Hoskinson, M.; Light, B. Inhaled Nitric Oxide Treatment of Patients with Pulmonary Tuberculosis Evidenced by Positive Sputum Smears. Antimicrob. Agents Chemother. 2005, 49, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Regevshoshani, G.; Ko, M.; Miller, C.; Avgay, Y. Slow Release of Nitric Oxide from Charged Catheters and Its Effect on Biofilm Formation by Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Brynildsen, M.P. A Kinetic Platform to Determine the Fate of Nitric Oxide in Escherichia coli. PLoS Comput. Biol. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.L.; Lanaro, C.; Lima, L.M.; Gambero, S.; Francopenteado, C.F.; Alexandremoreira, M.S.; Wade, M.; Yerigenahally, S.; Kutlar, A.; Meiler, S.E. Design, synthesis, and pharmacological evaluation of novel hybrid compounds to treat sickle cell disease symptoms. J. Med. Chem. 2011, 54, 5811–5819. [Google Scholar] [CrossRef] [PubMed]
- Burgaud, J.L.; Riffaud, J.P.; Del, S.P. Nitric-oxide releasing molecules: D new class of drugs with several major indications. Curr. Pharm. Des. 2002, 8, 201–213. [Google Scholar] [CrossRef]
- De Carvalho, P.S.; Maróstica, M.; Gambero, A.; Pedrazzoli, J., Jr. Synthesis and pharmacological characterization of a novel nitric oxide-releasing diclofenac derivative containing a benzofuroxan moiety. Eur. J. Med. Chem. 2010, 41, 2489–2493. [Google Scholar] [CrossRef]
- Chen, J.; Wang, T.; Xu, S.; Lin, A.; Yao, H.; Xie, W.; Zhu, Z.; Xu, J. Design, synthesis and biological evaluation of novel nitric oxide-donating protoberberine derivatives as antitumor agents. Eur. J. Med. Chem. 2017, 132, 173–183. [Google Scholar] [CrossRef]
- Bhandari, S.V.; Bothara, K.G.; Patil, A.A.; Chitre, T.S.; Sarkate, A.P.; Gore, S.T.; Dangre, S.C.; Khachane, C.V. Design, synthesis and pharmacological screening of novel antihypertensive agents using hybrid approach. Bioorg. Med. Chem. 2009, 17, 390–400. [Google Scholar] [CrossRef]
- Burgaud, J.L.; Ongini, E.; Del, S.P. Nitric oxide-releasing drugs: A novel class of effective and safe therapeutic agents. Ann. N. Y. Acad. Sci. 2002, 962, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Françasilva, M.S.; Balarini, C.M.; Cruz, J.C.; Khan, B.A.; Rampelotto, P.H.; Braga, V.A. Organic nitrates: Past, present and future. Molecules 2014, 19, 15314–15323. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Zhen, H.S.; Wang, Y.; Jiang, J.G.; Yang, Y.Y. Studies on Acute Toxicity and Anti-inflammatory Effect of Root of Sauropus rostratus. Chin. J. Experim. Tradit. Med. Form. 2013, 19, 286–288. (In Chinese) [Google Scholar]
- Huang, Y.; Tan, J.; Ma, W. Studies on antibacterial active of the extracts from Sauropus spatuli folius Beille in vitro. Pop. Sci. Technol. 2014, 2, 68–70. (In Chinese) [Google Scholar]
- Zhen, H.S.; Liu, R.; Qiu, Q.; Jiang, J.G.; Yang, Y.Y. Anti-inflammatory and Analgesic Effect of Sauropus rostratus. Chin. J. Experim. Tradit. Med. Form. 2013, 19, 270–273. (In Chinese) [Google Scholar]
- Subhasree, B.; Baskar, R.; Laxmi, K.R.; Lijina, S.R.; Rajasekaran, P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Liu, H.; Wang, J.; Li, G.; Wang, G.; Li, Y. Five natural carbohydrates from the leaves of Sauropus rostratus. Carbohydr. Res. 2014, 384, 99–101. [Google Scholar] [CrossRef]
- Xie, W.; Lin, A.; Xu, J.; Wu, X. The Construction of Anhydro Monosaccharides. Asian J. Organic Chem. 2017, 6, 6–26. [Google Scholar] [CrossRef]
- D’Alonzo, D.; De, F.M.; Porto, C.; Iacono, R.; Huebecker, M.; Cobucci-Ponzano, B.; Priestman, D.; Platt, F.M.; Parenti, G.; Moracci, M. N-Butyl-l-Deoxynojirimycin (L-NBDNJ): Synthesis of an Allosteric Enhancer of alpha-Glucosidase Activity for the Treatment of Pompe Disease. J. Med. Chem. 2017, 60, 9462–9469. [Google Scholar] [CrossRef]
- Speciale, G.; FarrenDai, M.; Shidmoossavee, F.S.; Williams, S.J.; Bennet, A.J. C2-Oxyanion neighboring group participation: Transition state structure for the hydroxide-promoted hydrolysis of 4-nitrophenyl α-D-mannopyranoside. J. Am. Chem. Soc. 2016, 138, 14012–14019. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.Q.; Liu, D.; He, W.G.; Xu, J.Y.; Lin, A.J.; Yao, H.Q.; Tanabe, G.; Muraoka, O.; Xie, W.J. Construction of 3,6-Anhydrohexosides via Intramolecular Cyclization of Triflates and Its Application to the Synthesis of Natural Product Isolated from Leaves of Sauropus rostratus. Org. Lett. 2014, 16, 5004–5009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Wang, Z.; Tanabe, G.; Muraoka, O.; Lin, A.; Xu, J.; Wu, X.; Wu, L.; Xie, W. Total synthesis, structural elucidation and anti-inflammatory activity evaluation of 2-deoxy-3,6-anhydro hexofuranoside derivatives isolated from Sauropus rostratus. Org. Biomol. Chem. 2016, 14, 10906–10913. [Google Scholar] [CrossRef] [PubMed]
- Stoss, P.; Erhardt, E. Bicycloalkanol nitrates. Arch. Pharm. 1987, 320, 621–624. [Google Scholar] [CrossRef]
- Shi, Z.D.; Yang, B.H.; Wu, Y.L. A stereospecific synthesis of l -deoxyribose, l-ribose and l-ribosides. Tetrahedron 2002, 58, 3287–3296. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Mccombie, S.W. A New Method for the Deoxygenation of Secondary Alcohols. J. Chem. Soc. 1975, 6, 553–559. [Google Scholar] [CrossRef]
- Gurjar, M.K.; Patil, V.J.; Pawar, S.M. Synthesis of (1 R,5 R)-2,6-dioxabicyclo[3.3.0]octan-3-one from d -glucose. Carbohydrate Res. 1987, 165, 313–317. [Google Scholar] [CrossRef]
- Bi, Y.; Yang, X.; Zhang, T.; Liu, Z.; Zhang, X.; Lu, J.; Cheng, K.; Xu, J.; Wang, H.; Lv, G. Design, synthesis, nitric oxide release and antibacterial evaluation of novel nitrated ocotillol-type derivatives. Eur. J. Med. Chem. 2015, 101, 71–80. [Google Scholar] [CrossRef]
- Sorba, G.; Medana, C.; Fruttero, R.; Cena, C.; Stilo, A.D.; Ubaldina Galli, A.; Gasco, A. Water Soluble Furoxan Derivatives as NO Prodrugs. J. Med. Chem. 1997, 40, 463–469. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 851, 51–70. [Google Scholar] [CrossRef]
- Abrams, J. Mechanisms of action of the organic nitrates in the treatment of myocardial ischemia. Am. J. Cardiol. 1992, 70, B30–B42. [Google Scholar] [CrossRef]
- Torfgård, K.E.; Ahlner, J. Mechanisms of action of nitrates. Cardiovasc. Drugs Ther. 1994, 8, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, S.; Hu, C.; Jia, C. Relaxing effect of an flavonoid WX-02 on isolated vascular rings of rabbits. J. Shen. Pharm. Univ. 2013, 9, 709–712. (In Chinese) [Google Scholar]
- Heesen, B.J.; Hotchkiss, R.S.; Karl, I.E. Sepsis decreases phenylephrine- and KCl-induced aortic ring contraction and decreases the frequency of oscillations in active wall tension. Shock 1994, 2, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and carboxy-PTIO with *NO, *NO2, and O2-*. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.J.; Yong, W.J. Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol. 2005, 46, 923–930. [Google Scholar]
- Jian, W.W.; Li, P.Z.; Jian, Y.W.; Ren, X.T. Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and Taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide 2006, 15, 351–358. [Google Scholar]
- Garthwaite, J.; Southam, E.; Boulton, C.L.; Nielsen, E.B.; Schmidt, K.; Mayer, B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995, 48, 184–188. [Google Scholar]
- Schrammel, A.; Behrends, S.; Schmidt, K.; Koesling, D.; Mayer, B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 1996, 50, 1–5. [Google Scholar]
- Xu, W.Q.; Xiong, Z.Z.; Chen, T.T.; Gao, X.Y.; Yu, H.; Zhang, S.Q.; Cao, Y. X, Vasodilation effect of 2-benzyl-5-hydroxy-6-methoxy-3, 4-dihydroisoquinolin-1-one. Arch. Pharmacal. Res. 2012, 35, 1471–1477. [Google Scholar] [CrossRef]
- Lubomirov, L.; Gagov, H.; Petkova-Kirova, P.; Duridanova, D.; Kalentchuk, V.U.; Schubert, R. Urocortin relaxes rat tail arteries by a PKA-mediated reduction of the sensitivity of the contractile apparatus for calcium. British J. Pharmacol. 2010, 134, 1564–1570. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Compounds | Relaxation a (%) | Relaxation b (%) |
|---|---|---|
| ISMN | 8.01 ± 3.59 | 3.05 ± 1.69 |
| ISDN | 30.21 ± 2.55 | 20.13 ± 3.45 |
| 5MNS-1 | 58.13 ± 3.01 **## | 24.21 ± 2.79 ** |
| 5MNS-2 | 30.21 ± 2.90 ** | 15.15 ± 3.01 ** |
| 5MNS-3 | 45.38 ± 3.51 **## | 16.09 ± 2.01 ** |
| 5MNS-4 | 20.11 ± 4.73 *# | 11.86 ± 3.56 *# |
| 5MNS-5 | 4.10 ± 2.01 ## | 2.01 ± 1.00 ## |
| 2MNS-1 | 15.22 ± 3.02 ## | 7.08 ± 2.00 ## |
| 2MNS-2 | 9.89 ± 2.71 ## | 4.96 ± 3.54 ## |
| 2MNS-3 | 31.03 ± 2.66 ** | 5.06 ± 1.79 ## |
| 2MNS-4 | 51.25 ± 5.02 **## | 22.89 ± 2.53 ** |
| 2MNS-5 | 11.00 ± 2.65 ## | 2.02 ± 1.03 ## |
| 2MNS-6 | 8.96 ± 2.06 ## | 52.11 ± 3.66 **## |
| 2MNS-7 | 22.1 ± 4.43 * | 86.27 ± 2.37 **## |
| 2MNS-8 | 67.56 ± 3.86 **## | 85.17 ± 4.54 **## |
| DNS-1 | 29.21 ± 3.66 ** | 80.87 ± 5.31 **## |
| DNS-2 | 81.98 ± 6.10 **## | 85.22 ± 6.01 **## |
| Compounds | IC50 a (μM) | Compounds | IC50 b (μM) |
|---|---|---|---|
| ISMN | 42.30 ± 3.52 | ISMN | 35.34 ± 2.52 |
| ISDN | 13.86 ± 0.56 | ISDN | 16.93 ± 0.98 |
| 5MNS-1 | 24.25 ± 0.50 **## | 2MNS-6 | 32.08 ± 6.40 # |
| 5MNS-3 | 36.16 ± 1.37 *## | 2MNS-7 | 5.94 ± 0.42 **## |
| 2MNS-3 | 12.08 ± 0.65 **# | 2MNS-8 | 5.52 ± 0.47 **## |
| 2MNS-4 | 13.75 ± 1.00 ** | DNS-1 | 11.14 ± 1.29 **## |
| 2MNS-8 | 6.94 ± 0.72 **## | DNS-2 | 10.03 ± 0.72 **## |
| DNS-2 | 6.02 ± 0.40 **## |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Rao, X.; Cong, R.; Zhang, C.; Wang, Z.; Xu, J.; Tanabe, G.; Muraoka, O.; Wu, X.; Xie, W. Design, Synthesis and Biological Evaluation of Nitrate Derivatives of Sauropunol A and B as Potent Vasodilatory Agents. Molecules 2019, 24, 583. https://doi.org/10.3390/molecules24030583
Lu L, Rao X, Cong R, Zhang C, Wang Z, Xu J, Tanabe G, Muraoka O, Wu X, Xie W. Design, Synthesis and Biological Evaluation of Nitrate Derivatives of Sauropunol A and B as Potent Vasodilatory Agents. Molecules. 2019; 24(3):583. https://doi.org/10.3390/molecules24030583
Chicago/Turabian StyleLu, Lu, Xuemin Rao, Rigang Cong, Chenxi Zhang, Zhimei Wang, Jinyi Xu, Genzoh Tanabe, Osamu Muraoka, Xiaoming Wu, and Weijia Xie. 2019. "Design, Synthesis and Biological Evaluation of Nitrate Derivatives of Sauropunol A and B as Potent Vasodilatory Agents" Molecules 24, no. 3: 583. https://doi.org/10.3390/molecules24030583
APA StyleLu, L., Rao, X., Cong, R., Zhang, C., Wang, Z., Xu, J., Tanabe, G., Muraoka, O., Wu, X., & Xie, W. (2019). Design, Synthesis and Biological Evaluation of Nitrate Derivatives of Sauropunol A and B as Potent Vasodilatory Agents. Molecules, 24(3), 583. https://doi.org/10.3390/molecules24030583






