Stainless Steel Wire Mesh Supported Molecularly Imprinted Composite Membranes for Selective Separation of Ebracteolata Compound B from Euphorbia fischeriana
Abstract
1. Introduction
2. Results and Discussion
2.1. Validation of UHPLC Method
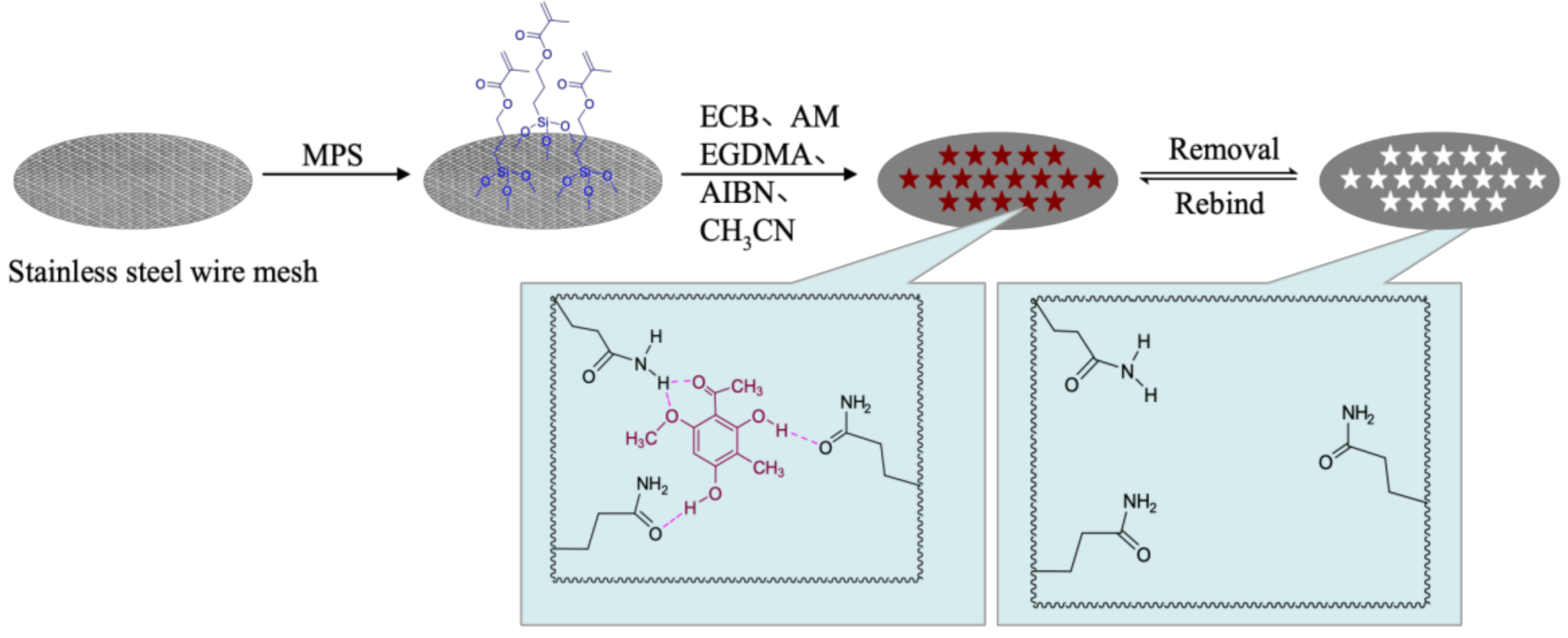
2.2. Preparation of ECB-MICMs
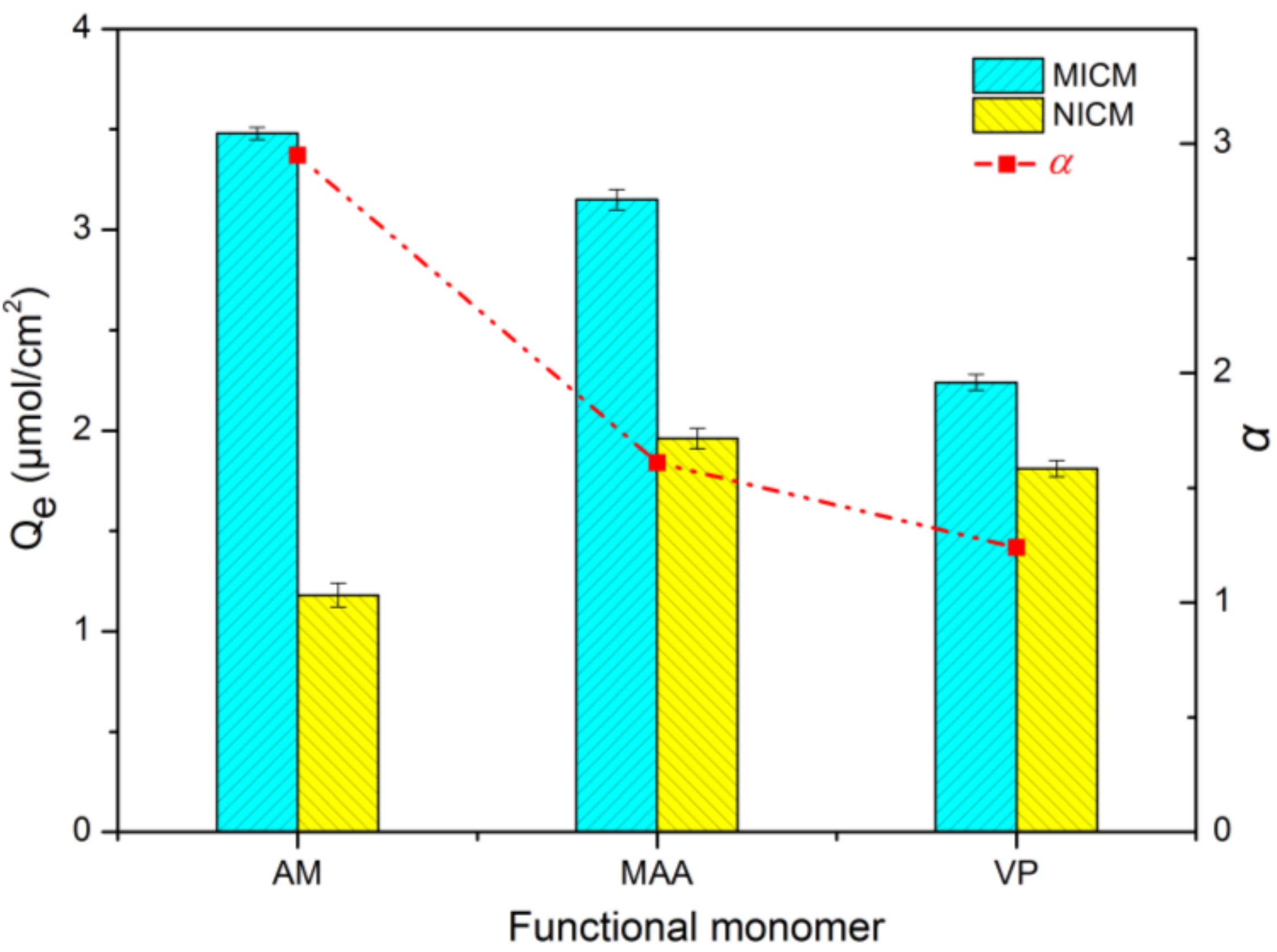
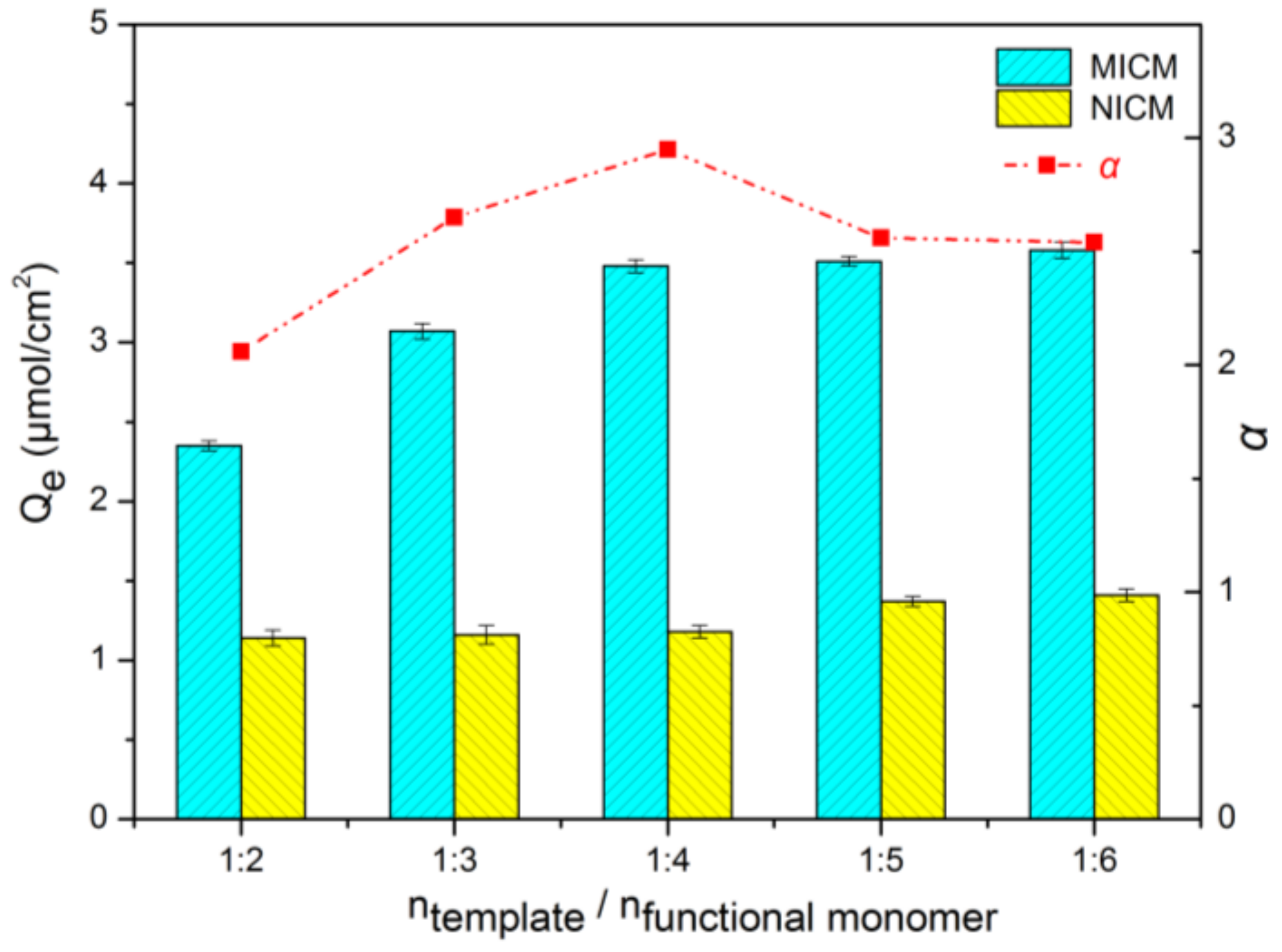
2.3. Screening and Optimization of Functional Monomer
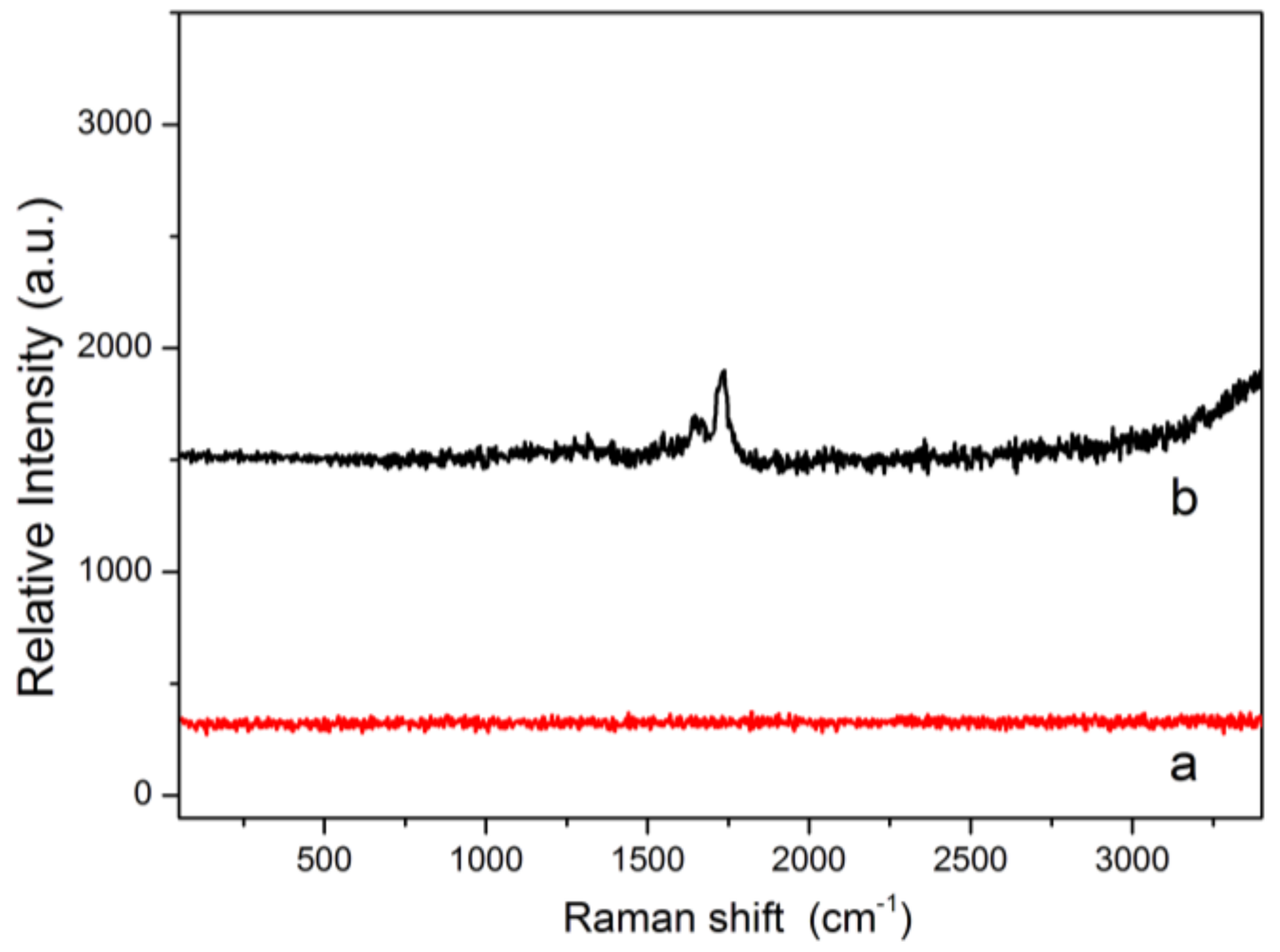
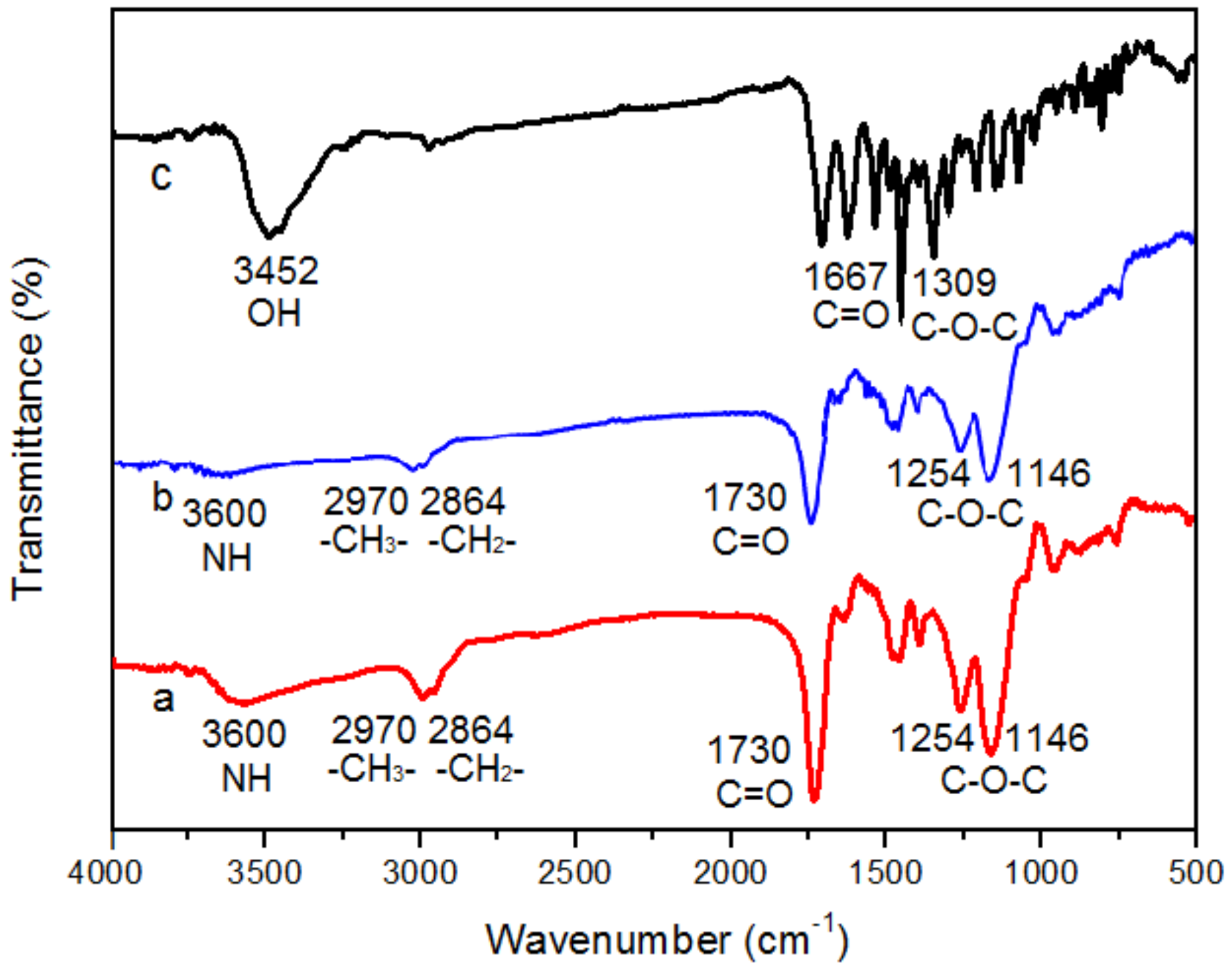
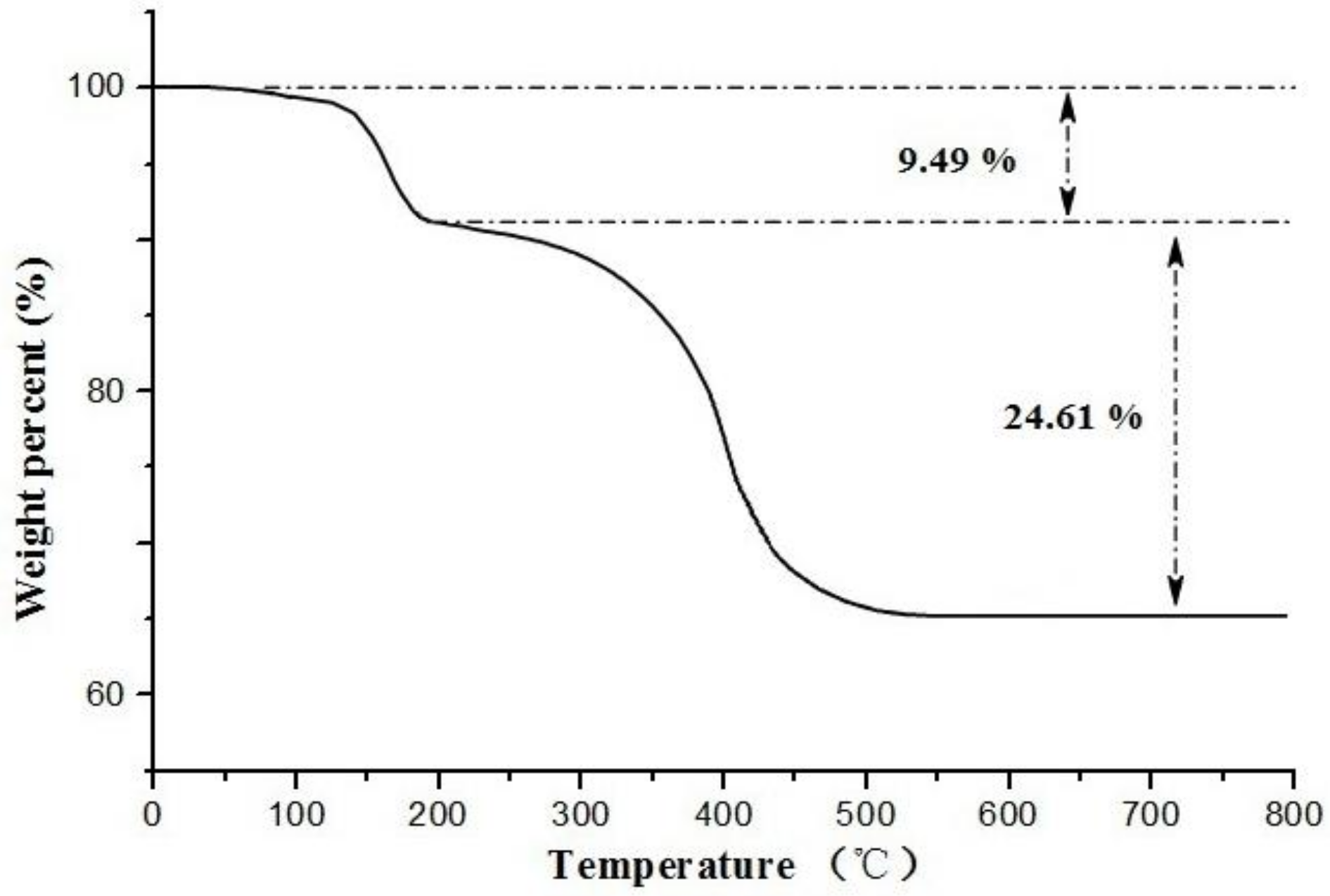
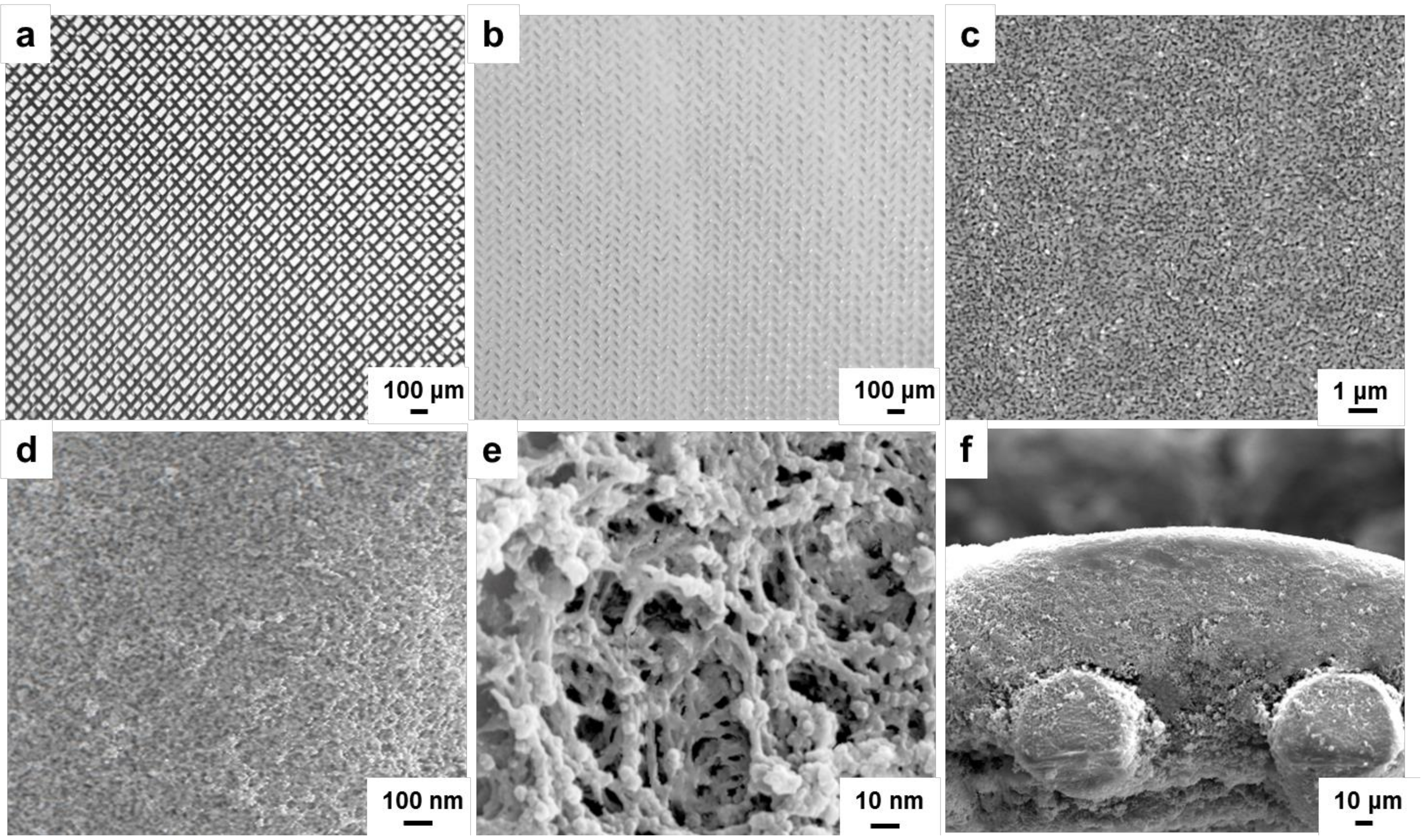
2.4. Characterization of ECB-MICMs
2.5. Adsorption of ECB on ECB-MICMs
2.5.1. Adsorption Kinetics
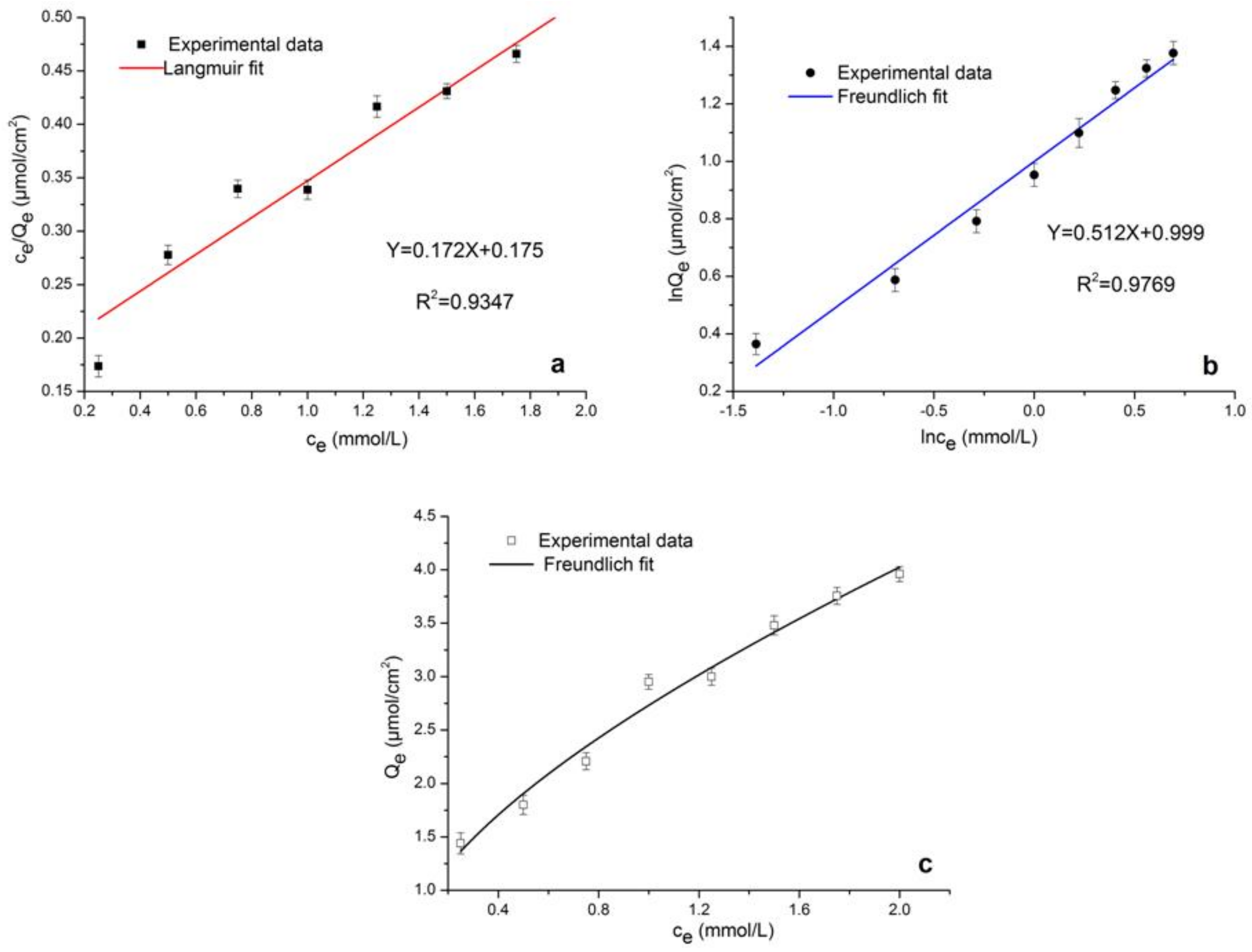
2.5.2. Adsorption Isotherms
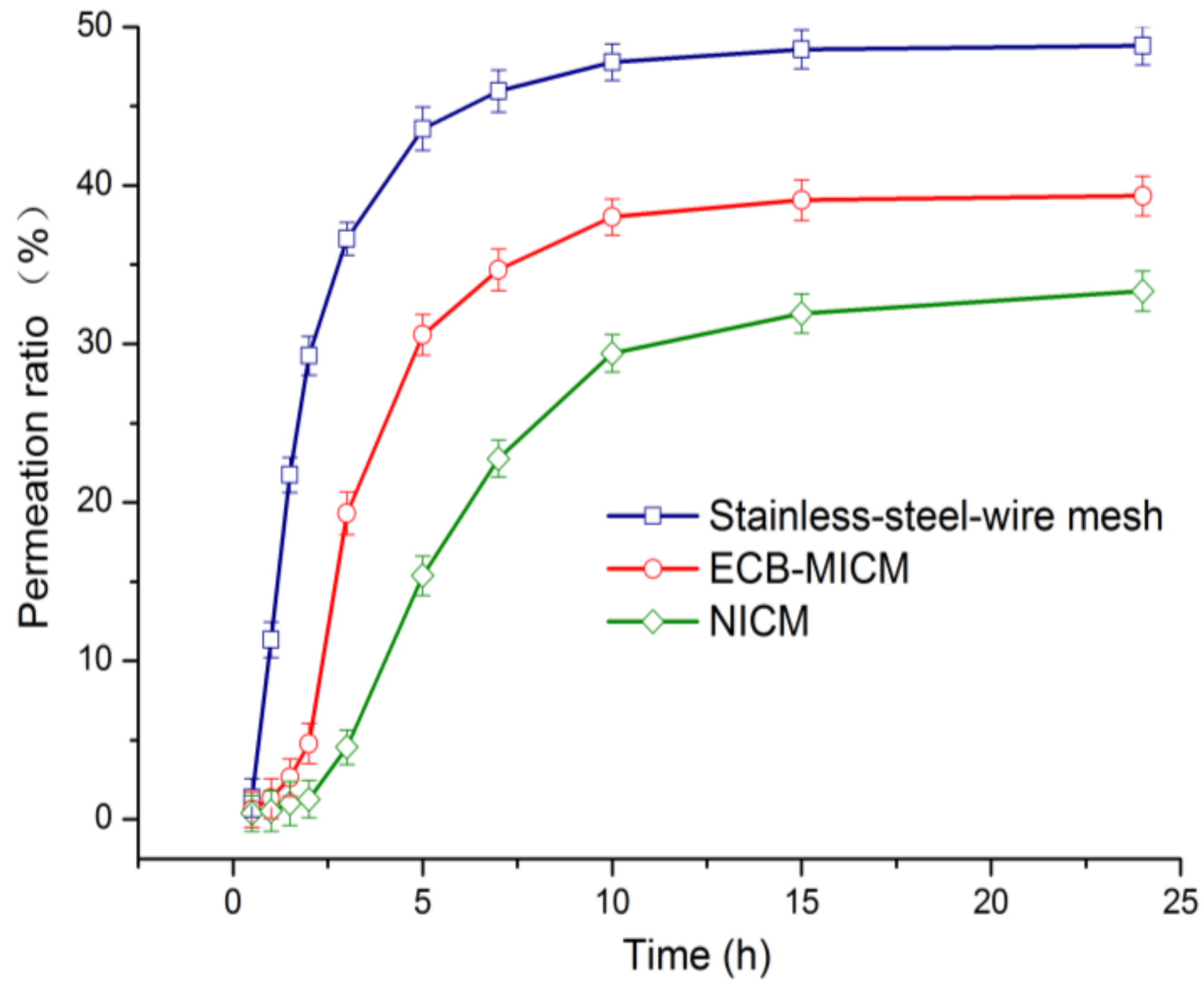
2.6. Permeability of ECB-MICMs
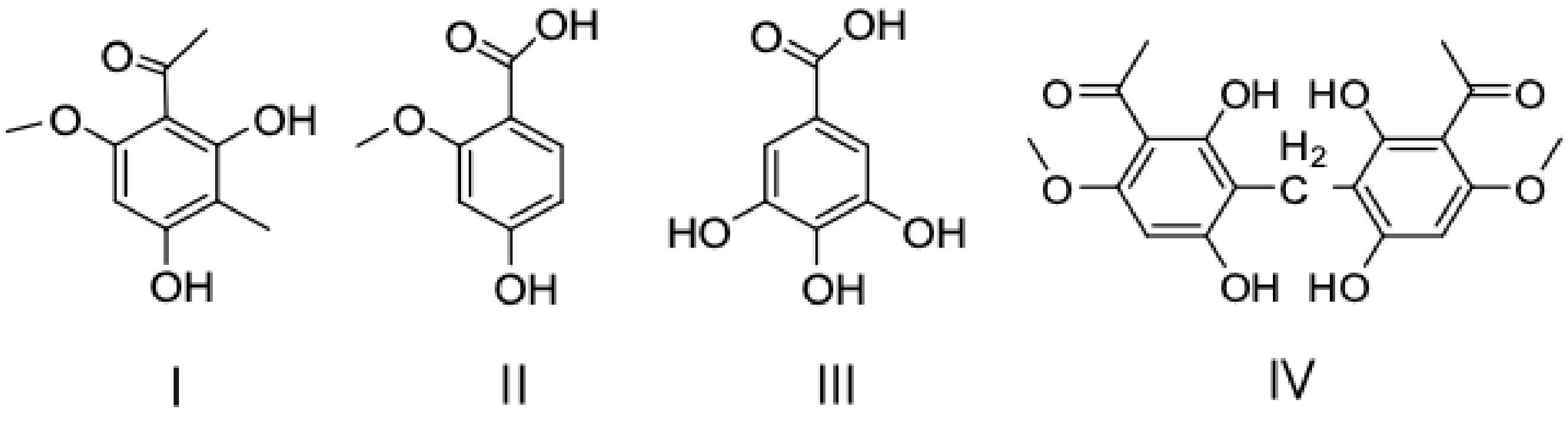
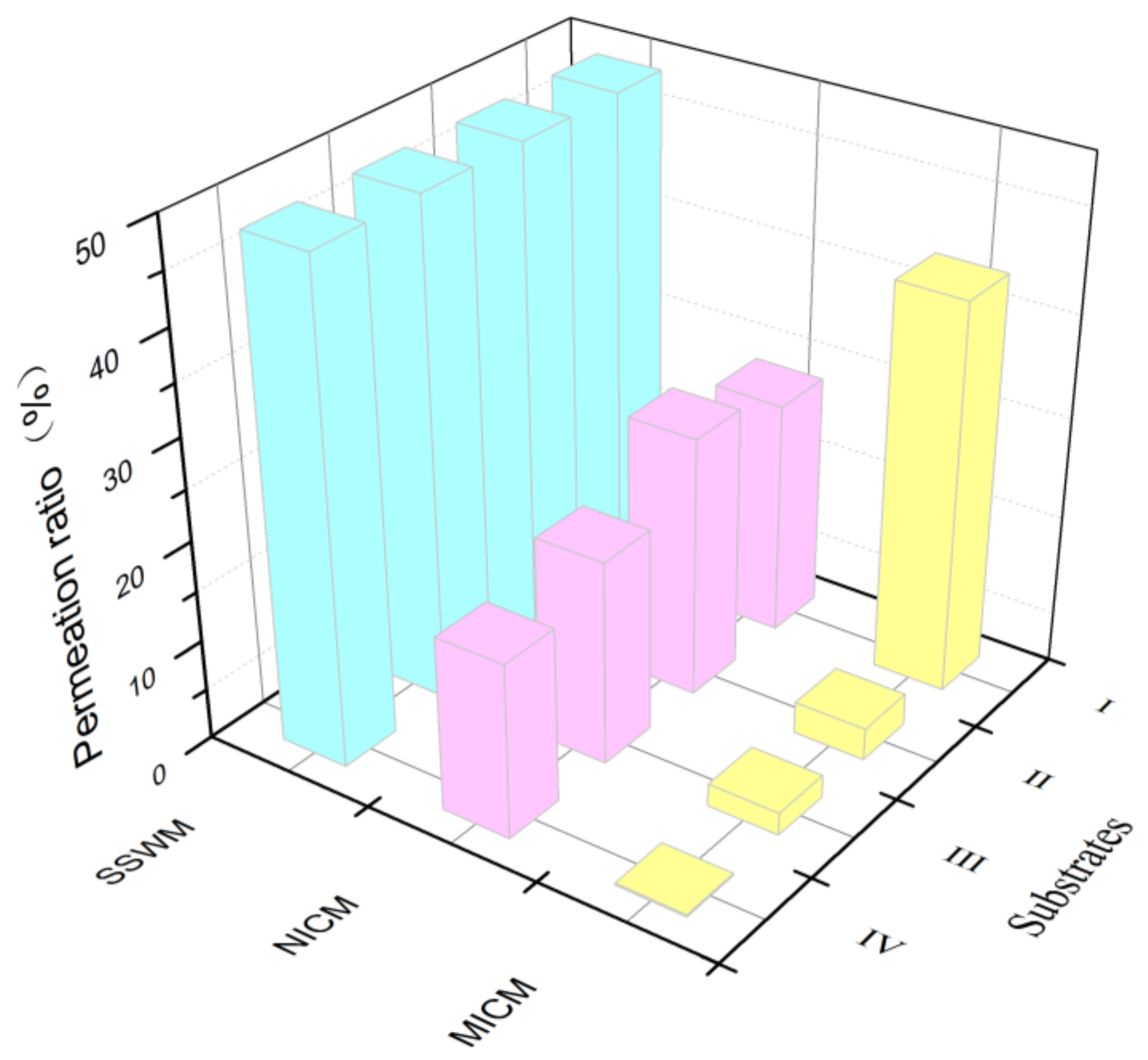
2.7. Permeation Selectivity
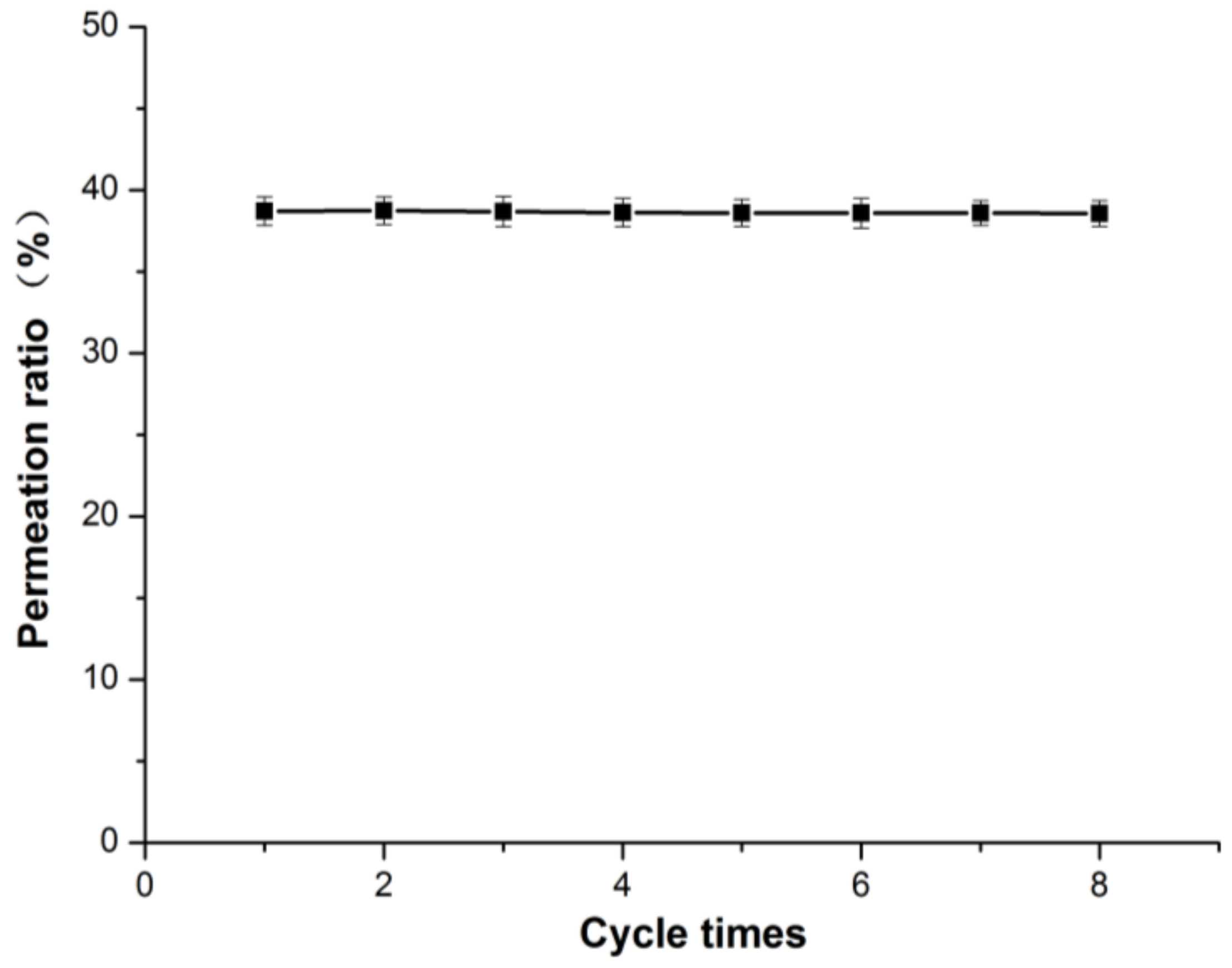
2.8. Reusability of ECB-MICMs
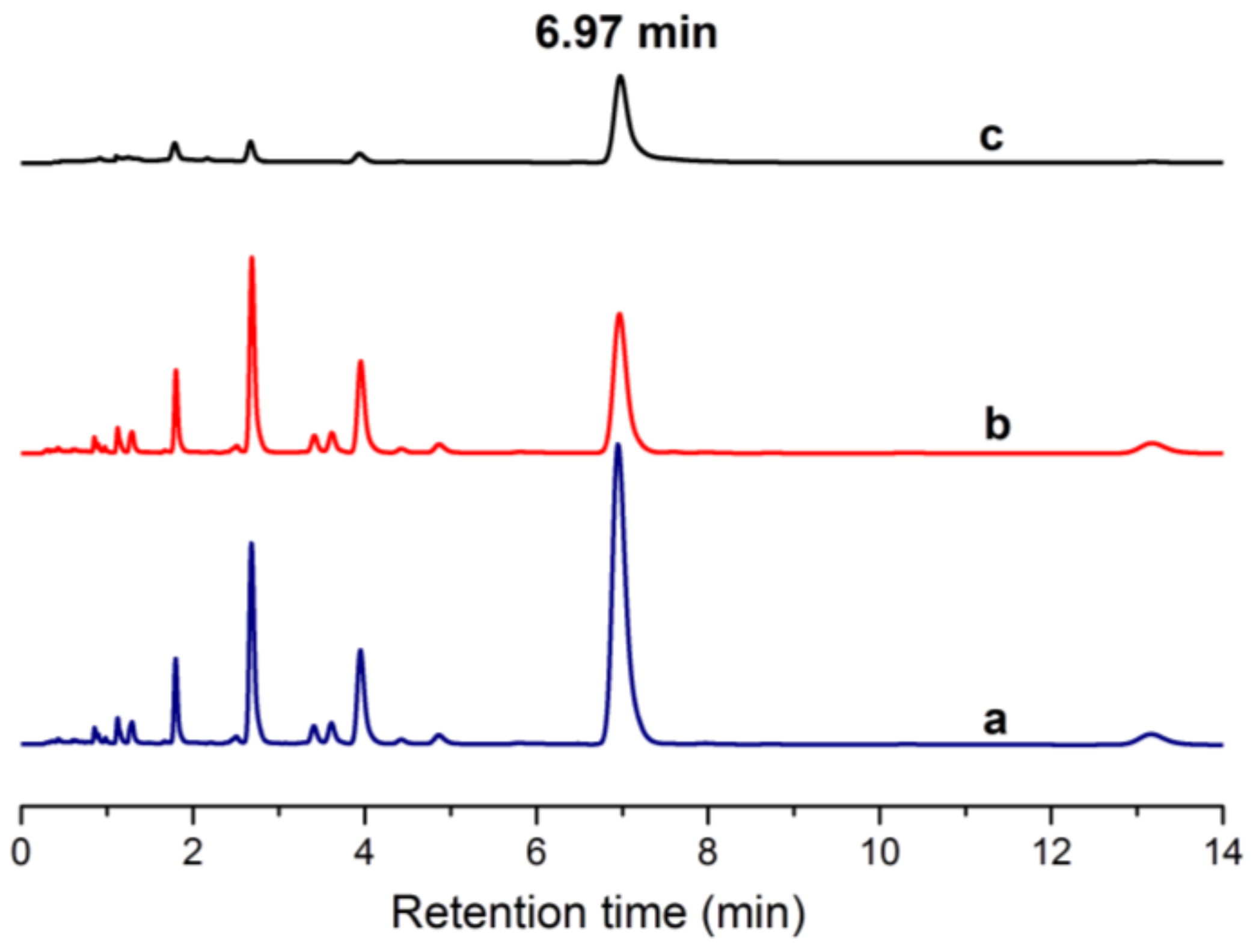
2.9. Separation of ECB in Euphorbia fischeriana Extract
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Experimental
3.3.1. Extraction and Purification of ECB
3.3.2. Purity Measurement
3.3.3. Preparation of ECB-MICMs
3.3.4. Screening of Functional Monomer Experiment
3.3.5. Optimization of Functional Monomer Experiment
3.3.6. Characterization
3.3.7. Adsorption Experiment
3.3.8. Permeation Experiment
3.3.9. Permeation Selectivity Experiment
3.3.10. Reusability Experiment
3.3.11. Separation Performance of ECB-MICMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barrero, R.A.; Chapman, B.; Yang, Y.F.; Moolhuijzen, P.; Keeble-Gagnère, G.; Zhang, N.; Tang, Q.; Bellgard, M.I.; Qiu, D.Y. De novo assembly of Euphorbia fischeriana root transcriptome identifies prostratin pathway related genes. BMC Genomics 2011, 12, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.L.; Fang, P.L.; Zhang, X.J.; Ni, W.; Li, L.; Yang, L.M.; Chen, C.X.; Zheng, Y.T.; Li, C.T.; Hao, X.J.; et al. Tigliane-Type Diterpenoid Glycosides from Euphorbia fischeriana. J. Nat. Prod. 2011, 74, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.Y.; Zhang, H.; Liu, J.C. Structural diversity and biological activities of diterpenoids derived from Euphorbia fischeriana Steud. Molecules 2018, 23, 935. [Google Scholar] [CrossRef]
- Jian, B.Y.; Zhang, H.; Han, C.C.; Liu, J.C. Anti-cancer activities of diterpenoids derived from Euphorbia fischeriana Steud. Molecules 2018, 23, 387. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Shi, Q.W.; Jiang, Y.; Gao, J.L.; Li, M.J.; Ma, H.Y. Microwave-assisted extraction and ionic liquid-based dispersive liquid–liquid microextraction followed by HPLC for the determination of trace components in a Langdu preparation. Anal. Methods 2016, 8, 5218–5227. [Google Scholar] [CrossRef]
- Song, W.G.; Sun, Y.Q.; Liu, L.F.; Wang, L. Research advance in Chinese medicine on anti-mycobacterium tuberculosis. J. Beihua Univ. (Nat. Sci.) 2007, 8, 150–151. [Google Scholar]
- Zhao, K.J.; Yang, J.; Zhang, P.Z. Comparative observation on the inhibitory effects against tuberculous bacillus of 5 extracts of the roots of Euphorbia fischeriana. Lishizhen Med. Mat. Med. Res. 2000, 11, 589–590. [Google Scholar]
- Zhang, H.Q.; Ding, Y.M.; Chen, G.Y.; Dong, Y.F. Studies on active constituents of root of Euphorbia ebracteolata Hayata (Euphorbiaceae). Acta Bot. Sin. 1987, 29, 429–431. [Google Scholar]
- Zhao, K.J.; Liu, S.L.; Li, H.M. Inhibiting effects of different extracts and constituents from Euphorbia fischeriana on tuberculosis bacillus. Chin. Pharm. 2007, 10, 1063–1065. [Google Scholar]
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef]
- Huang, S.S.; Li, P.; Zhang, B.J.; Deng, S.; Zhang, H.L.; Sun, C.P.; Huo, X.K.; Tian, X.G.; Ma, X.C.; Wang, C. Acetophenone glycosides from the roots of Euphorbia fischeriana and their inhibitory effects against Mycobacterium smegmatis. Phytochem. Lett. 2017, 19, 151–155. [Google Scholar] [CrossRef]
- Su, X.L.; Lin, R.C.; Wong, S.K.; Tsui, S.K.; Kwan, S.K. Identification and characterisation of the Chinese herb Langdu by LC-MS/MS analysis. Phytochem. Anal. 2003, 14, 40–47. [Google Scholar] [CrossRef]
- Wierzbicka, C.; Liu, M.Q.; Bauer, D.; Irgum, K.; Sellergren, B. Cationic pTyr/pSer imprinted polymers based on a bisimidazolium host monomer: Phosphopeptide recognition in aqueous buffers demonstrated by µ-liquid chromatography and monolithic columns. J. Mater. Chem. B 2017, 5, 953–960. [Google Scholar] [CrossRef]
- Wu, Y.L.; Liu, X.L.; Cui, J.Y.; Dai, J.D.; Li, C.X.; Yan, Y.S. Bioinspired synthesis of high-performance nanocomposite imprinted membrane by a polydopamine-assisted metal-organic method. J. Hazard. Mater. 2017, 323, 663–673. [Google Scholar] [CrossRef]
- Villar-Navarro, M.; Martín-Valero, M.J.; Fernández-Torres, R.M.; Callejón-Mochón, M.; Bello-López, M.A. Easy, fast and environmental friendly method for the simultaneous extraction of the 16 EPA PAHs using magnetic molecular imprinted polymers (mag-MIPs). J. Chromatogr. B 2017, 1044–1045, 63–69. [Google Scholar] [CrossRef]
- Zhao, K.Y.; Feng, L.Z.; Lin, H.Q.; Fu, Y.F.; Lin, B.B.; Cui, W.K.; Li, S.D.; Wei, J.F. Adsorption and photocatalytic degradation of methyl orange imprinted composite membranes using TiO2/calcium alginate hydrogel as matrix. Catal. Today 2014, 3, 41–48. [Google Scholar] [CrossRef]
- Ansari, S.; Karimi, M. Recent configurations and progressive uses of magnetic molecularly imprinted polymers for drug analysis. Talanta 2017, 167, 470–485. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles in chemical sensing-synthesis, characterisation and application. Sens. Actuators B 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Yu, J.L.; Wang, X.Y.; Kang, Q.; Shen, D.Z.; Chen, L.X. One-pot synthesis of quantum dots based molecular imprinting nanosensor for highly selective and sensitive fluorescent detection of 4-nitrophenol in environmental water. Environ. Sci. Nano 2017, 4, 493–502. [Google Scholar] [CrossRef]
- Ahmad, R.; Griffete, N.; Lamouri, A.; Felidj, N.; Chehimi, M.M.; Mangeney, C. Nanocomposites of gold nanoparticles@molecularly imprinted polymers: Chemistry, processing, and applications in sensors. Chem. Mater. 2015, 27, 5464–5478. [Google Scholar] [CrossRef]
- Ji, J.; Zhou, Z.H.; Zhao, X.L.; Sun, J.D.; Sun, X.L. Electrochemical sensor based on molecularly imprinted film at Au nanoparticles-carbon nanotubes modified electrode for determination of cholesterol. Biosens. Bioelectron. 2015, 66, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.B.; Chen, M.; Ge, H.W.; Lu, Z.W.; Liu, X.; Zou, P.; Wang, X.X.; He, H.; Zeng, X.Y.; Wang, Y.Y. A novel electrochemical sensor based on Au@PANI composites film modified glassy carbon electrode binding molecular imprinting technique for the determination of melamine. Biosens. Bioelectron. 2017, 87, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Suedee, R.; Bodhibukkana, C.; angthong, N.; Amnuaikit, C.; Kaewnopparat, S.; Srichana, T. Development of a reservoir-type transdermal enantioselective-controlled delivery system for racemic propranolol using a molecularly imprinted polymer composite membrane. J. Controlled Release 2008, 129, 170–178. [Google Scholar] [CrossRef]
- Sergeyeva, T.A.; Piletsky, S.A.; Piletska, E.V.; Brovko, O.O.; Karabanova, L.V.; Sergeeva, L.M.; El’skaya, A.V.; Turner, A.P.F. In situ formation of porous molecularly imprinted polymer membranes. Macromolecules 2003, 36, 7352–7357. [Google Scholar] [CrossRef]
- Lehmann, M.; Brunner, H.; Tom, G.E.M. Selective separations and hydrodynamic studies: A new approach using molecularly imprinted nanosphere composite membranes. Desalination 2002, 149, 315–321. [Google Scholar] [CrossRef]
- Yusof, N.A.; Zakaria, N.D.; Maamor, N.A.M.; Abdullah, A.H.; Haron, M.J. Synthesis and Characterization of molecularly imprinted polymer membrane for the removal of 2, 4-Dinitrophenol. Int. J. Mol. Sci. 2013, 14, 3993–4004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kong, K.L.; Qiao, N.; Wang, N.A.; Wang, Q.; Liu, H.; Zhou, Z.Y. Facile preparation of novel layer-by-layer surface ion-imprinted composite membrane for separation of Cu2+ from aqueous solution. Appl. Surface Sci. 2018, 457, 981–990. [Google Scholar] [CrossRef]
- Asman, S.; Yusof, N.A.; Abdullah, A.H.; Haron, M.J. Synthesis and characterization of hybrid molecularly imprinted polymer (MIP) membranes for removal of methylene blue (MB). Molecules 2012, 17, 1916. [Google Scholar] [CrossRef]
- Scorrano, S.; Mergola, L.; Bello, M.P.D.; Lazzoi, M.R.; Vasapollo, G.; Sole, R.D. Molecularly imprinted composite membranes for selective detection of 2-deoxyadenosine in urine samples. Int. J. Mol. Sci. 2015, 16, 13746–13759. [Google Scholar] [CrossRef]
- Mathew-Krotz, J.; Shea, K.J. Imprinted polymer membranes for the selective transport of targeted neutral molecules. J. Am. Chem. Soc. 1996, 118, 8154–8155. [Google Scholar] [CrossRef]
- Shah, N.; Rehan, T.; Park, J.K. Adsorptive molecularly imprinted composite membranes for chiral separation of phenylalanine. Polish J. Chem. Technol. 2016, 18, 22–29. [Google Scholar] [CrossRef]
- Smuleac, V.; Bachas, L.; Bhattacharyya, D. Aqueous-phase synthesis of PAA in PVDF membrane pores for nanoparticle synthesis and dichlorobiphenyl degradation. J. Membr. Sci. 2010, 346, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, R.; Kardani, F. Fabrication of ciprofloxacin molecular imprinted polymer coating on a stainless steel wire as a selective solid-phase microextraction fiber for sensitive determination of fluoroquinolones in biological fluids and tablet formulation using HPLC-UV detection. J. Pharm. Biomed. Anal. 2016, 122, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.J.; Zhu, G.S.; Yang, W.S.; Li, Y.S.; Zhu, G.Q.; Xu, R.; Sun, J.Y.; Qiu, S.L.; Xu, R.R. Stainless-steel net supported Zeolite NaA Membrane with High Permeance and High Permselectivity of Oxygen/Nitrogen in Air Separation. Adv. Mater. 2005, 17, 2006–2010. [Google Scholar] [CrossRef]
- Guo, H.L.; Zhu, G.S.; Li, H.; Zou, X.Q.; Yin, X.J.; Yang, W.S.; Qiu, S.L.; Xu, R.R. Hierarchical growth of large-Scale ordered zeolite silicalite-1 membranes with high permeability and selectivity for recycling CO2. Angew. Chem. Int. Ed. 2007, 45, 7053–7056. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
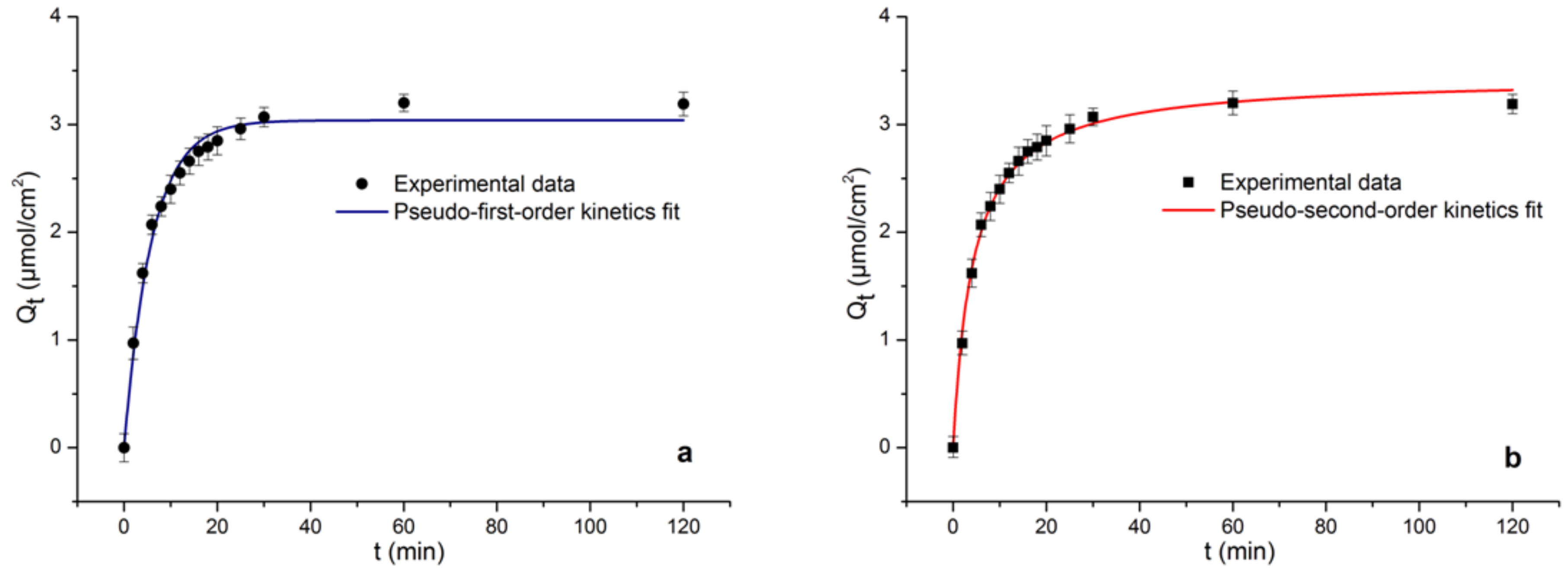
| Kinetics Model | Parameters | ||
|---|---|---|---|
| k | Qt | R2 | |
| Pseudo-first-order | 0.1691 | 3.03901 | 0.9860 |
| Pseudo-second-order | 0.0068 | 3.4379 | 0.9956 |
| Isothermal Asorption Model | Parameters | |
|---|---|---|
| Equation | R2 | |
| Langmuir | Y = 0.172X + 0.175 | 0.9347 |
| Freundlich | Y = 0.512X + 0.999 | 0.9769 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, H.; Guo, M. Stainless Steel Wire Mesh Supported Molecularly Imprinted Composite Membranes for Selective Separation of Ebracteolata Compound B from Euphorbia fischeriana. Molecules 2019, 24, 565. https://doi.org/10.3390/molecules24030565
Ma Y, Wang H, Guo M. Stainless Steel Wire Mesh Supported Molecularly Imprinted Composite Membranes for Selective Separation of Ebracteolata Compound B from Euphorbia fischeriana. Molecules. 2019; 24(3):565. https://doi.org/10.3390/molecules24030565
Chicago/Turabian StyleMa, Yukun, Haijun Wang, and Mengyan Guo. 2019. "Stainless Steel Wire Mesh Supported Molecularly Imprinted Composite Membranes for Selective Separation of Ebracteolata Compound B from Euphorbia fischeriana" Molecules 24, no. 3: 565. https://doi.org/10.3390/molecules24030565
APA StyleMa, Y., Wang, H., & Guo, M. (2019). Stainless Steel Wire Mesh Supported Molecularly Imprinted Composite Membranes for Selective Separation of Ebracteolata Compound B from Euphorbia fischeriana. Molecules, 24(3), 565. https://doi.org/10.3390/molecules24030565




