Abstract
Colistin is administered as its inactive prodrug colistimethate (CMS). Selection of an individualized CMS dose for each patient is difficult due to its narrow therapeutic window, especially in patients with chronic kidney disease (CKD). Our aim was to analyze CMS use in patients with CKD. Secondary objectives were to assess the safety and efficacy of CMS in this special population. In this prospective observational cohort study of CMS-treated CKD patients, CKD was defined as the presence of a glomerular filtration rate (GFR) < 60 mL/min/m2 for more than 3 months. The administered doses of CMS were compared with those recently published in the literature. Worsened CKD at the end of treatment (EOT) was evaluated with the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) criteria. Colistin plasma concentrations (Css) were measured using high-performance liquid chromatography. Fifty-nine patients were included. Thirty-six (61.2%) were male. The median age was 76 (45–95) years and baseline GFR was 36.6 ± 13.6. The daily mean CMS dosage used was compared with recently recommended doses (3.36 vs. 6.07; p < 0.001). Mean Css was 0.9 (0.2–2.9) mg/L, and Css was <2 mg/L in 50 patients (83.3%). Clinical cure was achieved in 43 (72.9%) patients. Worsened renal function at EOT was present in 20 (33.9%) patients and was reversible in 10 (52.6%). The CMS dosages used in this cohort were almost half those currently recommended. The mean achieved Css were under the recommended target of 2 mg/dL. Despite this, clinical cure rate was high. In this patient cohort, the incidence of nephrotoxicity was similar to those found in other recent studies performed in the general population and was reversible in 52.6%. These results suggest that CMS is safe and effective in patients with CKD and may encourage physicians to adjust dosage regimens to recent recommendations in order to optimize CMS treatments.
1. Introduction
Colistin has emerged as a last resort drug for the treatment of infections caused by multidrug and extensively drug-resistant Gram-negative bacteria such as Pseudomonas aeruginosa and Acinetobacter baumannii [1,2]. Although several clinical studies have evaluated the pharmacokinetics (PK) of colistin and its prodrug colistimethate sodium (CMS) in different types of patients [3,4,5,6,7,8,9,10] in the last decade, there is still little information on both over and underdosing in high-risk clinical settings, such as chronic kidney disease (CKD) and acute kidney injury (AKI) [4,5,11,12].
Colistin is a concentration-dependent bactericidal antibiotic with a narrow therapeutic window, with nephrotoxicity being its major dose limiting adverse effect [13,14,15]. Until recently, the CMS doses used in daily clinical practice were based on product manufacturer information with limited and rudimentary pharmacological information [2,11]. However, population pharmacokinetic (PK) studies performed in the last few years have led to new dosage schedule recommendations for both patients with normal and reduced renal function, including those undergoing renal replacement therapies [8,11,12]. These new guidelines recommend CMS dose selection and adjustment based on baseline estimated glomerular filtration rate (GFR) or creatinine clearance, as well as on the target colistin plasma concentration (Css). This value, based on different data from in vitro and in vivo PK, pharmacodynamic (PD) and toxicodynamic (TD) studies has been defined as 2 mg/L [16]. All this scientific research has led to the publication of the first consensus document [17]. However, a recent publication has suggested that current recommendations on the use of colistin in patients with reduced renal function are likely to be inadequate [11] and have updated the dosing guidelines of both the European and American regulatory agencies [18,19].
The aim of this study was to evaluate colistin use in a cohort of patients with CKD and to assess whether “old recommendations” are suitable from a PK/PD/TD point of view. A secondary objective was to evaluate the potential factors involved in clinical failure and worsening renal function (WRF) at the end of treatment (EOT).
2. Results
During the study period, 59 patients were enrolled, including 36 (61%) men with a median age of 76 years (interquartile range [IQR], 45–95). All of them had infections caused by extensively drug-resistant Pseudomonas aeruginosa: pneumonia in 14 (23.7%), acute bronchitis in 14 (23.7%), urinary tract infection in 16 (27.1%), bacteremia in three (5.1%), skin and soft tissue infections in five (8.6%) and miscellanea in seven (11.9). The mean estimated GFR at baseline was 36.6 ± 13.6 SD and the median CMS daily dose was 3 (IQR, 1 to 9) million international units (IU). A loading dose was administered in six (10.17%) patients, with the median dose being 6 million IU (IQR, 3–6). Among patients receiving a loading dose, two were diagnosed with a UTI, one with pneumonia and three with tracheobronchitis. None of the included patients was undergoing renal replacement therapy during the study period. WRF was present in 20 (33.9%) patients at EOT. Thirty-day all-cause mortality was 28.8%. Patient characteristics and CMS concentrations are shown in Table 1. The median Css was 0.9 mg/L, but individual values varied widely (IQR 0.2–2.9 mg/L) with the physician–selected doses. The distribution of Css in different GFR clusters is shown in Figure 1.

Table 1.
Patients’ clinical, demographic and pharmacokinetic characteristics.
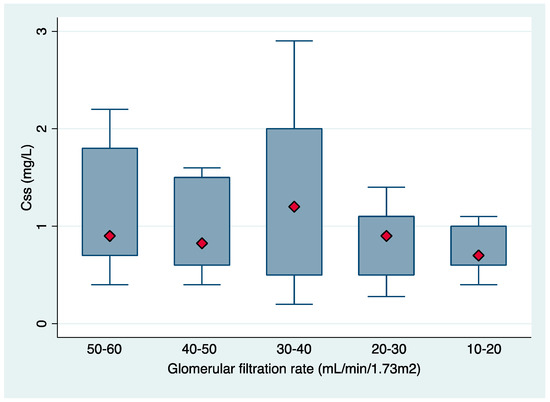
Figure 1.
Boxplot of achieved Css,avg in different intervals of baseline glomerular filtration rate.
The median (IQR) of Css,avg were 0.9 (0.2–2.9), 0.83 (0.4–1.6), 1.2 (0.2–2.9), 0.9 (0.28–1.4) and 0.7 (0.4–1.1) mg/L for the GFR clusters of 50–60, 40–50, 30–40, 20–30 and 10–20 mL/min/1.73m2, respectively. In 50 (83.3%) patients the concentration of formed colistin in plasma was below the suggested therapeutic level of 2 mg/L [12].
Regarding the received CMS doses, 41 (68.3%) patients received daily doses <3 million IU per day, 16 (26.7%) between 3–6 million IU per day and only three (5%) received more than 6 million IU per day. The median (IQR) of Css,avg in these three patient groups was 0.8 (0.2–2.23), 1.25 (0.3–2.9) and 0.68 (0.5–0.86) mg/L, respectively. Table 2 shows the medians of CMS and CBA suggested by Nation et al. [12] for a desired target of Css,avg of 2 mg/L compared with those administered in this cohort of patients.

Table 2.
Table daily doses of colistimethate for a desired target colistin Css of 2 mg/L compared with the mean daily doses used in daily clinical practice for different creatinine clearances.
The mean dose received by patients in our study was significantly lower than those suggested by the study of Nation et al. [12] (3.36 ± 0.23 vs. 6.07 ± 0.11; p < 0.001). Because the recommendations for colistin doses in our center changed in 2014, we analyzed the doses used in the first period (from 2010 to December 2014) and in a second period from 2014 to the present (2014–2018) and there were no changes between these two periods (mean dose 3.15 ± 1.64 vs. 3.57 ± 1.93; p = 0.37).
Clinical cure was achieved in 43 (72.8%) patients. Patients with clinical failure were more severely ill (SOFA 6 vs. SOFA 2; p < 0.001), had achieved higher colistin plasma concentrations (0.8 vs. 1.1, p = 0.03), and had higher rates of 30-day all-cause mortality (81.3% vs. 9.3%); p < 0.001). The characteristics of patients with and without clinical cure are shown in Table 3. On multivariate analysis, the only factor related to clinical failure was SOFA score (OR 0.63; 95% CI, 0.44–0.90; p = 0.012). The overall incidence of WRF at the EOT was 33.9%. The median time from colistin initiation to WRF was 7 (IQR 1 to 16) days. WRF was reversible in 10 (52.6%) patients, but follow up results were lacking in six (31.6%). Patients with nephrotoxicity at EOT had higher median (IQR) SOFA scores (3 (1–9) versus 2 (0–9); p = 0.16), had a longer CMS treatment (15.5 days (6–30) versus 12 days (4–45); p = 0.12), had received more concomitant nephrotoxic drugs (1.6 ± 1 versus 1.2 ± 0.9; p = 0.15) and achieved higher Css,avg (1.2 ( 0.3–2.9) versus 0.83 ( 0.2–2.4); p=0.1) mg/L. Characteristics of patients with and without WRF at EOT are shown in Table 4. In the multivariate analysis, the only factors related to nephrotoxicity at EOT were Css (OR 3.21; 95% CI 1.02–10.1; p = 0.047) and days of CMS treatment (OR 1.11; 95% CI 0.99–12.4; p = 0.069). The results of this analysis are shown in Table 5. Of importance, none of the patients in this cohort developed neurotoxicity.

Table 3.
Univariate analysis of patients with and without clinical cure.

Table 4.
Characteristics of patients with and without acute kidney injury at the end of treatment.

Table 5.
Multivariate analysis of independent risk factors for colistin-associated nephrotoxicity at the end of treatment.
3. Discussion
Colistin use has reemerged in recent years for the treatment of multidrug-resistant Gram-negative infections [1,2,20], which has prompted the performance of a large number of clinical and PK studies of its use in the last decade. However, there is still little pharmacological information in patients at high risk of under- or overdosing, such as those with CKD. Colistin is administered in the inactive form of CMS, which is hydrolyzed spontaneously in vivo to colistin, the active compound. CMS, the pro-drug, is renally eliminated by glomerular filtration and active tubular secretion. Previous experiences have reported that about 60% of the CMS dose is renally excreted in the urine during the first 24 h after dosing [21]. Similarly, a study performed by our group confirmed that there is a rapid urinary excretion of CMS in patients within the first 6 h after intravenous administration [22]. In patients with renal dysfunction, urinary CMS excretion is reduced and, consequently, a larger fraction of this pro-drug can be converted to colistin, leading to drug overexposure in this population [8,21] and consequently to a high risk of kidney injury. Selection of the CMS dose in patients with CKD is therefore a critical issue [13,23,24,25].
Recent toxicodynamic studies performed in the general population indicate that the risk of nephrotoxicity in patients receiving CMS increases with plasma colistin concentration exceeding 2.5 mg/L [13,15]. This point, together with the lack of information on the minimum inhibitory concentrations (MIC) at the beginning of CMS treatment, have led to consideration of an average Css,avg of 2 mg/L as a target when initiating therapy [11]. The median Css,avg in this study was 0.9 mg/L, which is far below the above-mentioned recommended 2 mg/L [11] but is also below those reported in previous studies performed by our group in the general population (1.06 with an IQR of 0.11–5.99) [7,13]. This finding is surprising, since patients with a reduced GFR could have been expected to achieve higher colistin plasma concentrations [8,11,12]. An explanation could be the low CMS doses administered in our patient cohort because of the use of outdated product/hospital CMS dosing recommendations, as well as clinicians’ concern about nephrotoxicity in patients with compromised renal function at baseline. Another important finding of this study is the considerable interpatient variability in colistin plasma concentrations even in patients within the same GFR range (Figure 1). This finding has also been reported in recent pharmacokinetic studies. Nation et al. reported up to ~12-fold inter–patient variability in plasma colistin Css across all four renal function groups (≥80, 50 –<80, 30–<50 and <30 mL/min) [11]. A previous study by our group also reported wide variability in observed plasma colistin concentrations (1.06 with an IQR of 0.11 to 5.99) [13]. In addition, in a pharmacokinetic study, Garonzik et al. found wide interindividual variability with a range of the colistin Css,avg of 0.48–9.38 mg/L (median, 2.36 mg/L) [8].
Until a few years ago, CMS dose selection was guided by the recommendations in the product information sheet, based on outdated pharmacological information [2] and in local protocols. As of 2013, new PK, PD and clinical knowledge led to the new dosing guidelines [17], which has been published by several regulatory agencies such as the European Medicines Agency (EMA) [19] and the US Food and Drug Administration (FDA) [18]. However, their impact and implementation in daily clinical practice are still unknown. Additionally, a recent publication highlights substantial differences between the FDA- and EMA-approved CMS dose recommendations and suggests that these doses are inadequate to achieve the PK target of 2 mg/L in certain clinical settings [11]. In this scenario, based on a population pharmacokinetic study [8], Nation et al. proposed a clinician-friendly dosing algorithm [12]. When we compared the CMS doses proposed in this algorithm with the real doses used in our daily clinical practice, we observed that patients in the present cohort were underdosed (3.36 ± 0.23 vs. 6.07 ± 0.11; p < 0.001)., which could explain the low Css found in the patient cohort in our hospital.
The overall rate of clinical cure in this patient cohort was 72.8%, which is similar to rates reported by other studies [7,26,27,28]. The only studied factor associated with clinical failure was disease severity (SOFA). Of note, colistin plasma levels were not related to clinical cure. This finding was also observed in our previous study [7], and was somewhat in disagreement with recent pharmacokinetic studies suggesting that a Css of 2 mg/L could be a desirable target for the treatment of infections caused by MDR-GNB [8,11,12]. However, the rates of clinical cure differed, depending on the patients’ diagnosis, and this percentage was only 50% in patients diagnosed with pneumonia. This finding confirms the hypothesis of Nation et al. that intravenous CMS is not efficacious against lung infections [16] and therefore a plasma colistin concentration of 2 mg/L may not be adequate for isolates with a colistin MIC >1 mg/L [11]. In this scenario, higher CMS doses to achieve adequate PK/PD targets could lead to the development of nephrotoxicity, since previous studies have identified colistin plasma concentrations >2.5 mg/L as a risk factor for nephrotoxicity [13,14,15].
Although the small sample size of this study does not allow definite conclusions to be drawn, we advocate treatment of lung infections with inhalation or combined therapy, as well as application of therapeutic drug monitoring to optimize PK/PD while minimizing the risk of nephrotoxicity. In contrast, in less severe infections such as UTI, a plasma level lower than the proposed 2 mg/L might be enough. Consequently, we believe that it is difficult to define a single optimal Css level for all types of infections and microorganisms and that it would be better to have a well-defined PK/PD ratio as a target.
The rate of WRF in this cohort of patients with CKD was 33.9% and nephrotoxicity is the main adverse effect of polymyxins. Rates of nephrotoxicity in patients treated with CMS range from 20% to 60% according to recent studies using standardized criteria to evaluate nephrotoxicity [7,13,23,24,25]. Even though WRF was reversible in more than 50% of patients, the diagnosis and management of this complication during CMS treatment is a challenge in daily clinical practice, since nephrotoxicity is potentially related to worse clinical outcomes. In two previous studies by our group, the presence of AKI at EOT was a predictor of 30-day all-cause mortality [7,13]. In another study performed by Falagas et al. in 258 patients with infections caused by multidrug-resistant Gram-negative bacilli (GNB), the development of AKI during treatment was also related to mortality [20]. Several studies have assessed the risk factors for colistin-associated nephrotoxicity with different results [23,24,26,29,30], but recent clinical PK/TD studies have indicated that the risk of nephrotoxicity increased as plasma colistin exposure exceeded approximately 2.5 mg/L [13,14,15]. Indeed, the present study also demonstrates that Css is a predictor of WRF at EOT.
In conclusion, intravenous CMS use in patients with CKD with the currently recommended dosage regimens is just as safe as that in the general population. Our data suggest that the dosage regimens used in daily clinical practice should be updated based on modern PK/PD/TD studies, at least in the treatment of MDR infections. In this scenario, the use of the formula of Nation et al. [12] or possibly the clinician-friendly dosing algorithm proposed by the same group [12] could be a useful clinical tool to guide treatment in this special population. Another important conclusion of this study is that rates of clinical cure in patients with pneumonia are poor with the dosage regimens used in daily clinical practice. Regarding colistin-associated nephrotoxicity, this study confirms the relationship between colistin plasma concentrations and the development of nephrotoxicity during treatment. Finally, all these issues highlight the need for therapeutic drug monitoring in daily clinical practice both to optimize PK/PD and to prevent the development of nephrotoxicity.
4. Materials and Methods
4.1. Study Population
We conducted a prospective observational study in a cohort of patients with CKD and infections caused by extensively drug-resistant P. aeruginosa treated with intravenous CMS for at least 48 h. The study was carried out at Hospital del Mar, a tertiary care university hospital in Barcelona (Spain), from January 2010 to August 2018. The study was approved by the local ethics committee (Comité Ètic d’Investigació Clínica del Parc de Salut Mar. Approval number 2011/4501/I). Exclusion criteria were age <18 years, pregnancy, and breast-feeding during the study period. A pharmacy-generated alarm system was used to identify patients under CMS treatment. The study investigators performed the assessment from the first day of the treatment and informed consent was obtained from all participants or their legal representatives.
4.2. Collected Data
Patient data included demographic information, SOFA score [31], Charlson comorbidity index [32], CMS treatment (indication, daily and total cumulative dose measured in millions of IU and treatment duration). GFR at baseline and at EOT was calculated using the abbreviated Modification of Diet in Renal Disease equation (MDRD-4) [33]. WRF was defined as a decrease in GFR ≥25% and was classified according to the Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) criteria [34]. Patients with WRF were followed up until GFR recovery or until hospital discharge. Information was collected on the concomitant use of other potential nephrotoxic drugs such as aminoglycosides, vancomycin, angiotensin II receptor blockers, angiotensin-converting enzyme (ACE) inhibitors, loop diuretics, intravenous dye, amphotericin B and non-steroidal anti-inflammatory drugs (NSAIDs), as well as the need for vasopressor drugs or discontinuation of CMS due to nephrotoxicity. Combination therapy with other antibiotics with potential activity or synergy with colistin was assessed.
4.3. Definitions
CKD was defined as kidney damage or GFR <60 mL/min/1.73 m2 for 3 months or more, irrespective of cause [35]. Infections were defined according to the Centers for Disease Control and Prevention [36]. Clinical failure was defined as a lack of improvement in patients or in at least one of the initial symptoms, worsening or death. Clinical cure was defined as either the absence of symptoms or as a consistent improvement in the signs and symptoms of the infection. Thirty-day all-cause mortality was considered as death from any cause during the 30 days following EOT. Combined antibiotic treatment consisted of CMS plus meropenem or CMS plus amikacin.
4.4. CMS Administration
The dosage regimen and dose adjustments were determined by the treating physician. Since this study was performed over more than 8 years, colistin daily doses varied during this time. From 2010 to 2014, dose adjustments were made according to the package insert’s recommended dosing as follows: GFR ≥76 mL/min/1.73 m2, 4–6 million IU daily in three doses; GFR 40–75 mL/min/1.73 m2, 2–3 million IU daily in two doses; GFR 25–40 mL/min/1.73 m2, 1.5–2 million IU daily divided in one or two doses and; GFR <25 mL/min/1.73 m2, 0.6–1 million IU daily every 36 h. In 2014, a new protocol based on our previous experience [13] was implemented in our center and CMS was adjusted as follows: GFR ≥90 mL/min/1.73 m2, 9 million IU daily in three doses; GFR 50–89 mL/min/1.73 m2, 3 million IU every 12 h, GFR 10–49 mL/min/1.73 m2, 3 million IU per day; GFR ≤10 mL/min/1.73 m2, 2 million IU/day. However, the final dose was chosen by the treating physician. CMS (colistimethate formulation for intravenous use, GES Genéricos Españoles®, Las Rozas, Spain was diluted in 100 mL of physiological saline before intravenous administration over 30 min. Each vial contained 1 million IU of CMS (equivalent to 80 mg CMS).
4.5. Microbiological Data
Identification and susceptibility testing of P. aeruginosa were first performed by microdilution using the Gram-negative breakpoint panel for non-fermenting GNB of the MicroScan® WalkAway system (Siemens Diagnostic Inc., Los Angeles CA, USA). Colistin MIC was determined by microdilution using cation-adjusted MHB; the isolate was considered susceptible if the MIC was ≤ 2 mg/L according to the Clinical and Laboratory Standards Institute [37].
4.6. Pharmacokinetic Data
Blood samples were obtained just before the next dose on day 3–4 of the treatment. It was assumed that steady state was already achieved considering a half-life of approximately 14 h [10]. Concentrations of CMS and formed colistin in plasma were measured using a validated high-performance liquid chromatography (HPLC) method [13,38]. The limit of quantification of the HPLC methods for colistin and CMS in plasma were 0.20 and 0.50 mg/L, respectively. As the plasma concentration-time profiles of formed colistin are almost flat [8,13], measured colistin concentrations were regarded as Css,avg. The percentage of patients achieving the therapeutic target defined as a Css,avg of 2 mg/L was also analyzed. Finally, the CMS doses used in our cohort of patients in daily clinical practice were compared with those recently recommended by the algorithm of Nation et al. [12]. We calculated the expected Css based on the formula of Nation et al. [12].
4.7. Statistical Analysis
Dichotomous data were compared using a χ2 or Fisher’s exact test. Normally distributed continuous data are expressed as means and standard deviations (SD), and were compared using the t-test. Otherwise, values are presented as means with interquartile range (IQR) and were compared using the Mann-Whitney U-test. The baseline and clinical characteristics of patients receiving different CMS doses were compared using the ANOVA or Kruskal-Wallis test. Multivariate analysis of risk factors for colistin-associated nephrotoxicity was conducted using logistic regression. Univariate analyses were performed separately for each of the risk factor variables to ascertain the odds ratio (OR) and 95% confidence interval (CI). All clinically important covariates and those with p < 0.3 in the univariate analyses were included in the multivariate analysis.
Author Contributions
L.S. conceived and designed the study, data collection, analysis and wrote the manuscript. C.S.; microbiology analysis. N.C. and S.L.; analytical method, management and analysis of clinical specimens. J.S., I.D., X.F. and M.M.: data collection. E.R.: assessment of patients and analysis. S.L., S.G., J.L. and J.P.H.: interpretation of data and drafted the manuscript. All authors read the manuscript and approved.
Funding
This work was supported by Fondo de Investigación Sanitaria (FIS) from Instituto de Salud Carlos III, Spanish Ministry of Health, Grant numberPS09/01634 and from Spanish Ministry of Health and Social Policy, General Pharmacy Subdirection, Grant numbers EC10-165 and EC11-318. J.L. is an Australian National Health and Medical Research Council (NHMRC) Principal Research Fellow and supported by a research grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01 AI132154).
Conflicts of Interest
The authors declare that they have no competing interests. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References
- Li, J.; Nation, R.L.; Milne, R.W.; Turnidge, J.D.; Coulthard, K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- Grégoire, N.; Mimoz, O.; Mégarbane, B.; Comets, E.; Chatelier, D.; Lasocki, S.; Gauzit, R.; Balayn, D.; Gobin, P.; Marchand, S.; et al. New colistin population pharmacokinetic data in critically ill patients suggesting an alternative loading dose rationale. Antimicrob. Agents Chemother. 2014, 58, 7324–7330. [Google Scholar] [CrossRef] [PubMed]
- Jitmuang, A.; Nation, R.L.; Koomanachai, P.; Chen, G.; Lee, H.J.; Wasuwattakul, S.; Sritippayawan, S.; Li, J.; ThamLikitkul, V.; Landersdorfer, C.B. Extracorporeal clearance of colistin methanesulphonate and formed colistin in end-stage renal disease patients receiving intermittent haemodialysis: Implications for dosing. J. Antimicrob. Chemother. 2015, 70, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Koomanachai, P.; Landersdorfer, C.B.; Chen, G.; Lee, H.J.; Jitmuang, A.; Wasuwattakul, S.; Sritippayawan, S.; Li, J.; Nation, R.L.; ThamLikitkul, V. Pharmacokinetics of colistin methanesulfonate and formed colistin in end-Stage renal disease patients receiving continuous ambulatory peritoneal dialysis. Antimicrob. Agents Chemother. 2014, 58, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Rietschel, E.; Kasel, D.; Schwiertz, R.; Starke, K.; Beier, H.; Grasemann, H. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J. Antimicrob. Chemother. 2006, 57, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Sorlí, L.; Luque, S.; Segura, C.; Campillo, N.; Montero, M.; Esteve, E.; Herrera, S.; Benito, N.; Alvarez-Lerma, F.; Grau, S.; et al. Impact of colistin plasma levels on the clinical outcome of patients with infections caused by extremely drug-resistant Pseudomonas aeruginosa. BMC Infect. Dis. 2017, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Garonzik, S.M.; Li, J.; ThamLikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef] [PubMed]
- Luque, S.; Grau, S.; Valle, M.; Sorlí, L.; Horcajada, J.P.; Segura, C.; Álvarez-Lerma, F. Differences in pharmacokinetics and pharmacodynamics of colistimethate sodium (CMS) and colistin between three different CMS dosage regimens in a critically ill patient infected by a multidrug-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents 2013, 42, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Plachouras, D.; Karvanen, M.; Friberg, L.E.; Papadomichelakis, E.; Antoniadou, A.; Tsangaris, I.; Karaiskos, I.; Poulakou, G.; Kontopidou, F.; Armaganidis, A.; et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2009, 53, 3430–3436. [Google Scholar] [CrossRef]
- Nation, R.L.; Garonzik, S.M.; Li, J.; ThamLikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Turnidge, J.D.; Forrest, A.; Silveira, F.P. Updated US and European Dose Recommendations for Intravenous Colistin: How Do They Perform? Clin. Infect. Dis. 2016, 62, 552–558. [Google Scholar] [CrossRef]
- Nation, R.L.; Garonzik, S.M.; ThamLikitkul, V.; Giamarellos-Bourboulis, E.J.; Forrest, A.; Paterson, D.L.; Li, J.; Silveira, F.P. Dosing guidance for intravenous colistin in critically-ill patients. Clin. Infect. Dis. 2016, 64, ciw839. [Google Scholar] [CrossRef] [PubMed]
- Sorlí, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Alvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P.; et al. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Sorlí, L.; Luque, S.; Benito, N.; Segura, C.; Campillo, N.; Montero, M.; Esteve, E.; Mirelis, B.; Pomar, V.; et al. Validation of a colistin plasma concentration breakpoint as a predictor of nephrotoxicity in patients treated with colistin methanesulfonate. Int. J. Antimicrob. Agents 2016, 48, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Forrest, A.; Garonzik, S.M.; ThamLikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Li, J.; Silveira, F.P.; Nation, R.L. Pharmacokinetic/Toxicodynamic Analysis of Colistin-Associated Acute Kidney Injury in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e01367. [Google Scholar] [CrossRef] [PubMed]
- Cheah, S.-E.; Wang, J.; Nguyen, V.T.T.; Turnidge, J.D.; Li, J.; Nation, R.L. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J. Antimicrob. Chemother. 2015, 70, 3291–3297. [Google Scholar] [PubMed]
- Nation, R.L.; Li, J.; Cars, O.; Couet, W.; Dudley, M.N.; Kaye, K.S.; Mouton, J.W.; Paterson, D.L.; Tam, V.H.; Theuretzbacher, U.; et al. Framework for optimisation of the clinical use of colistin and polymyxin B: The Prato polymyxin consensus. Lancet. Infect. Dis. 2014, 15, 225–234. [Google Scholar] [CrossRef]
- Food and Drug Administration. Coly-Mycin M Parenteral (Colistimethate for Injection, USP). Available online: http://www.accessdata.fda.gob/drugsatfda_docs/label/2013/050108s030lbl.pdf (accessed on 31 January 2018).
- European Medicines Agency Completes Review of Polymyxin-Based Medicines|European Medicines Agency. Available online: https://www.ema.europa.eu/news/european-medicines-agency-completes-review-polymyxin-based-medicines (accessed on 23 November 2018).
- Falagas, M.E.; Rafailidis, P.I.; Ioannidou, E.; Alexiou, V.G.; Matthaiou, D.K.; Karageorgopoulos, D.E.; Kapaskelis, A.; Nikita, D.; Michalopoulos, A. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: A retrospective cohort study of 258 patients. Int. J. Antimicrob. Agents 2010, 35, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Couet, W.; Gregoire, N.; Marchand, S.; Mimoz, O. Colistin pharmacokinetics: The fog is lifting. Clin. Microbiol. Infect. 2012, 18, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Luque, S.; Escaño, C.; Sorli, L.; Li, J.; Campillo, N.; Horcajada, J.P.; Salas, E.; Grau, S. Urinary Concentrations of Colistimethate and Formed Colistin after Intravenous Administration in Patients with Multidrug-Resistant Gram-Negative Bacterial Infections. Antimicrob. Agents Chemother. 2017, 61, e02595. [Google Scholar] [CrossRef]
- Deryke, C.A.; Crawford, A.J.; Uddin, N.; Wallace, M.R. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrob. Agents Chemother. 2010, 54, 4503–4505. [Google Scholar] [CrossRef] [PubMed]
- Hartzell, J.D.; Neff, R.; Ake, J.; Howard, R.; Olson, S.; Paolino, K.; Vishnepolsky, M.; Weintrob, A.; Wortmann, G. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 2009, 48, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Lee, J.; Marchaim, D.; Yee, V.; Zhao, J.J.; Chopra, T.; Lephart, P.; Kaye, K.S. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin. Infect. Dis. 2011, 53, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Dalfino, L.; Puntillo, F.; Mosca, A.; Monno, R.; Spada, M.L.; Coppolecchia, S.; Miragliotta, G.; Bruno, F.; Brienza, N. High-dose, extended-interval colistin administration in critically ill patients: Is this the right dosing strategy? A preliminary study. Clin. Infect. Dis. 2012, 54, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Sheng, W.H.; Wang, J.T.; Chen, Y.C.; Chang, S.C. Safety and efficacy of intravenous colistin (colistin methanesulphonate) for severe multidrug-resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents 2010, 35, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Markou, N.; Apostolakos, H.; Koumoudiou, C.; Athanasiou, M.; Koutsoukou, A.; Alamanos, I.; Gregorakos, L. Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients. Crit. Care 2003, 7, R78–R83. [Google Scholar] [CrossRef] [PubMed]
- Rattanaumpawan, P.; Ungprasert, P.; ThamLikitkul, V. Risk factors for colistin-associated nephrotoxicity. J. Infect. 2011, 62, 187–190. [Google Scholar] [CrossRef]
- Falagas, M.E.; Rafailidis, P.I.; Kasiakou, S.K.; Hatzopoulou, P.; Michalopoulos, A. Effectiveness and nephrotoxicity of colistin monotherapy vs. colistin-meropenem combination therapy for multidrug-resistant Gram-negative bacterial infections. Clin. Microbiol. Infect. 2006, 12, 1227–1230. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P.; Workgroup, A.D.Q.I. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]
- Ricci, Z.; Cruz, D.; Ronco, C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008, 73, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; Zeeuw, D.D.E.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- The Clinical & Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Information Supplement; CLSI Document M100-SCLI.; CLSI: Wayne, PA, USA, 2013. [Google Scholar]
- Li, J.; Milne, R.W.; Nation, R.L.; Turnidge, J.D.; Coulthard, K.; Johnson, D.W. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J. Chromatogr. Biomed. Sci. Appl. 2001, 761, 167–175. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).