A New 3-D Open-Framework Zinc Borovanadate with Catalytic Potentials in α-Phenethyl Alcohol Oxidation
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthetic Aspects and Characterization
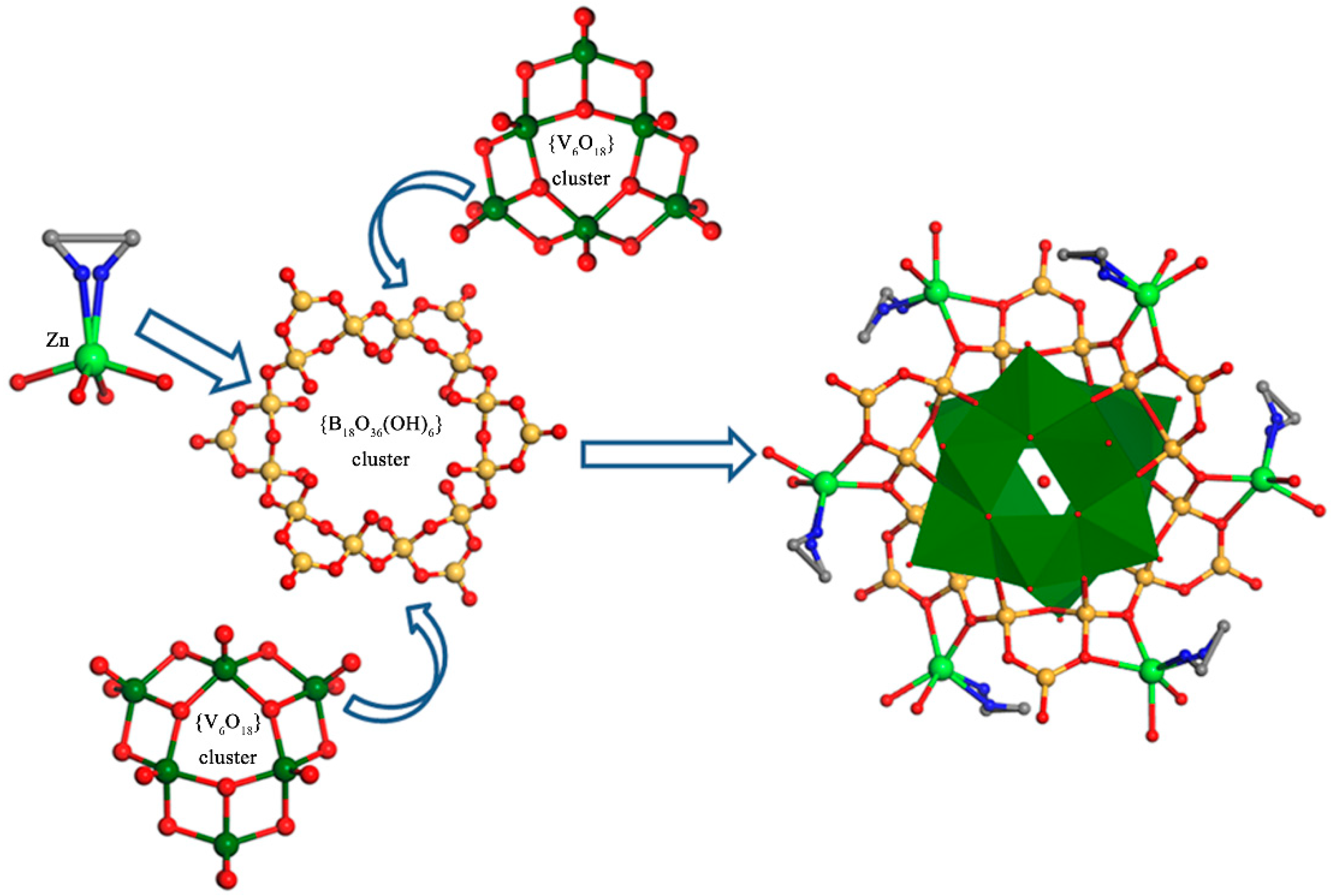
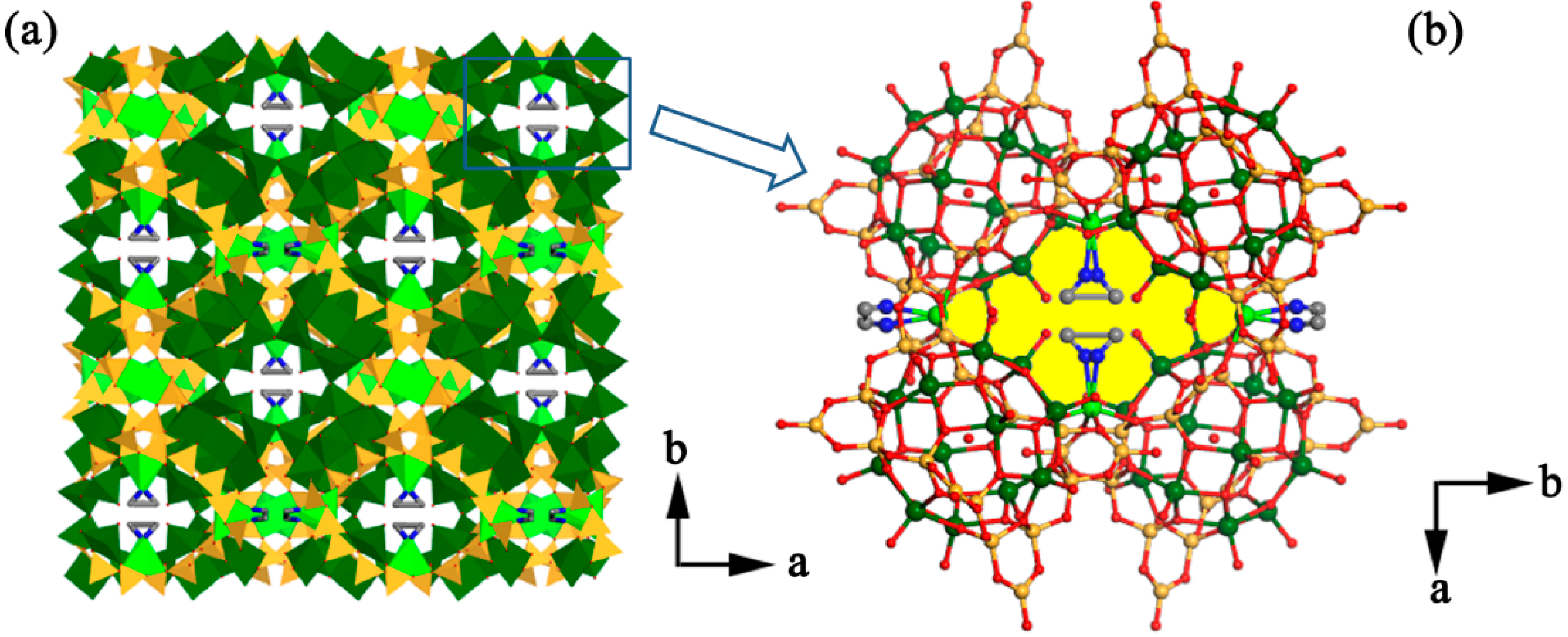
2.2. Structure Description
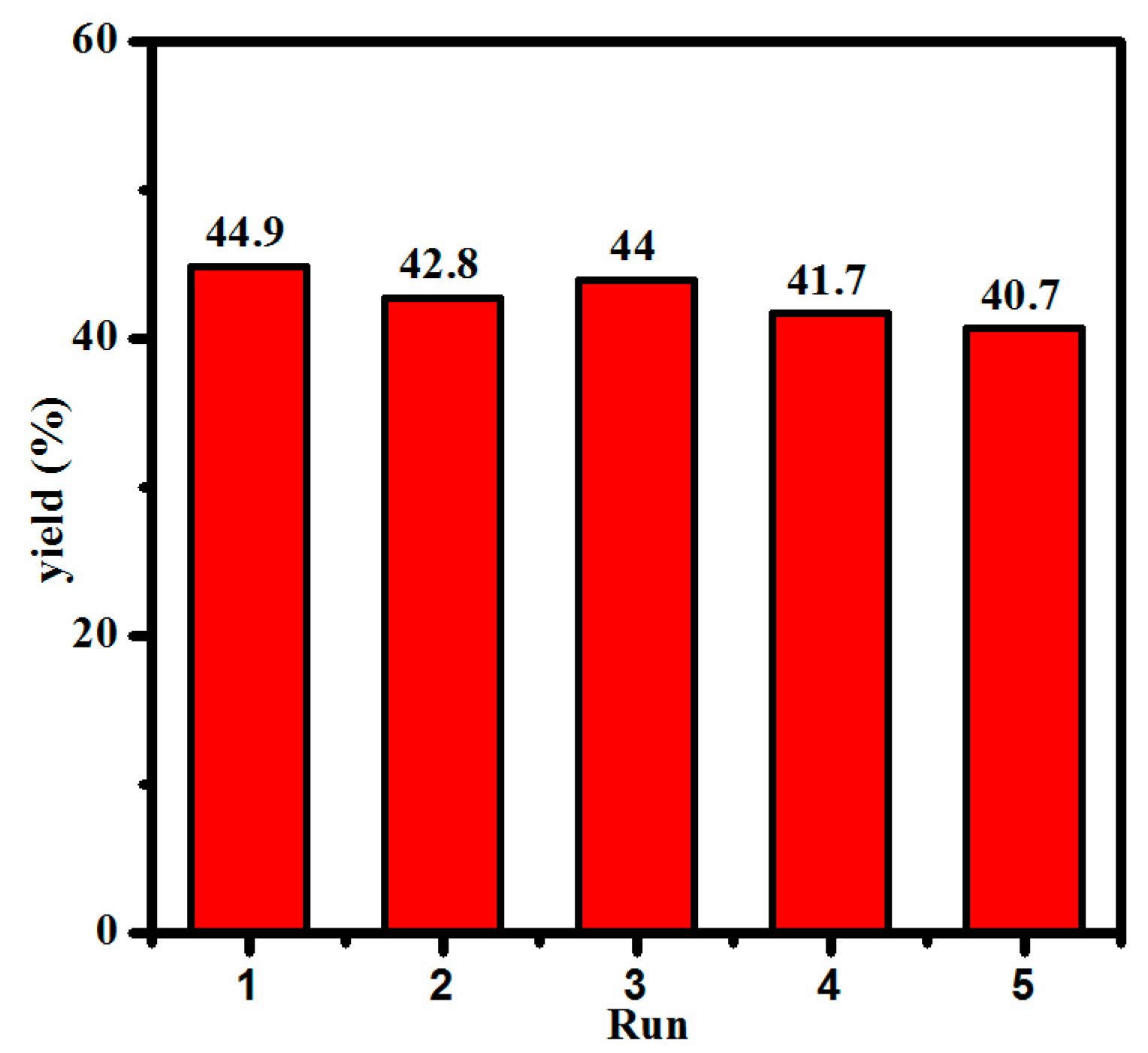
2.3. Catalytic Performance
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maspoch, D.; Ruiz-Molina, D.; Veciana, J. Old Materials with New Tricks: Multifunctional Open-Framework Materials. Chem. Soc. Rev. 2007, 36, 770–818. [Google Scholar] [CrossRef] [PubMed]
- Barrer, R.M. Hydrothermal chemistry of zeolites. Gynaecol. Endosc. 1982, 9, 191–194. [Google Scholar]
- Meng, L.; Cheng, Q.; Kim, C.; Gao, Y.W.; Lukasz, W.; Chen, Y.S.; Michael, J.Z.; Zhang, X.P.; Ma, S.Q. Crystal Engineering of a Microporous, Catalytically Active fcu Topology MOF Using a Custom-Designed Metalloporphyrin Linker. Angew. Chem. Int. Ed. 2012, 51, 10082–10085. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, A. The early history and growth of metal phosphonate chemistry. In Metal Phosphonate Chemistry: From Synthesis to Applications; RSC Publishing: London, UK, 2012. [Google Scholar]
- Yücesan, G.; Zorlu, Y.; Stricker, M.; Beckmann, J. Metal-organic solids derived from arylphosphonic acids. Coord. Chem. Rev. 2018, 369, 105–122. [Google Scholar] [CrossRef]
- Taddei, M.; Costantino, F.; Vivani, R. Robust Metal-Organic Frameworks Based on Tritopic Phosphonoaromatic Ligands. Eur. J. Inorg. Chem. 2016, 4300–4309. [Google Scholar] [CrossRef]
- Chen, H.; Yu, Z.B.; Bacsik, Z.; Zhao, H.; Yao, Q.; Sun, J. Construction of mesoporous frameworks with vanadoborate clusters. Angew. Chem. Int. Ed. 2014, 53, 3608–3611. [Google Scholar] [CrossRef] [PubMed]
- Leithall, R.M.; Shetti, V.N.; Maurelli, S.; Chiesa, M.; Gianotti, E.; Raja, R. Toward understanding the catalytic synergy in the design of bimetallic molecular sieves for selective aerobic oxidations. J. Am. Chem. Soc. 2013, 135, 2915–2918. [Google Scholar] [CrossRef]
- Volodin, A.D.; Korlyukov, A.A.; Zorina-Tikhonova, E.N.; Chistyakov, A.S.; Sidorov, A.A.; Eremenko, I.L.; Vologzhanin, A.V. Diastereoselective solid-state crossed photocycloaddition of olefins in a 3D Zn(II) coordination polymer. Chem. Commun. 2018, 54, 13861–13864. [Google Scholar] [CrossRef]
- Karmakar, A.; Paul, A.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. pH dependent synthesis of Zn(II) and Cd(II) coordination polymers with dicarboxylfunctionalized arylhydrazone of barbituric acid: Photoluminescence properties and catalysts for Knoevenagel condensation. New J. Chem. 2016, 40, 1535–1546. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Sabbatini, A.; Drew, M.G.B.; Martins, L.M.D.R.S.; Pettinari, C.; Pombeiro, A.J.L. Cooperative Metal−Ligand Assisted E/Z Isomerization and Cyano Activation at CuII and CoII Complexes of Arylhydrazones of Active Methylene Nitriles. Inorg. Chem. 2014, 53, 9946–9958. [Google Scholar] [CrossRef]
- Spoon, H.W.W.; Tielens, A.G.G.M.; Armus, L.; Sloar, G.C.; Sargent, B.; Cami, J.; Charmandaris, V.; Houck, J.R.; Soifer, B.T. The Detection of Crystalline Silicates in Ultraluminous Infrared Galaxies. Astrophy. J. 2008, 638, 759–765. [Google Scholar] [CrossRef]
- Murugavel, R.; Choudhury, A.; Walawalkar, M.G.; Pothiraja, R.; Rao, C.N.R. Metal Complexes of Organophosphate Esters and Open-Framework Metal Phosphates: Synthesis, Structure, Transformations, and Applications. Chem. Rev. 2008, 108, 3549–3655. [Google Scholar] [CrossRef] [PubMed]
- Dadachov, M.S.; Sun, K.; Conradsson, T.; Zou, X.D. The First Templated Borogermanate (C2N2H10)2[(BO2.5)2(GeO2)3]: Linkage of Tetrahedra of Significantly Different Sizes. Angew. Chem. Int. Ed. 2000, 39, 3674–3676. [Google Scholar] [CrossRef]
- Zhao, D.; Cheng, W.D.; Zhang, H.; Huang, S.P.; Xie, Z.; Zhang, W.L.; Yang, S.L. KMBP2O8 (M = Sr, Ba): A new kind of noncentrosymmetry borophosphate with the three-dimensional diamond-like framework. Inorg. Chem. 2009, 48, 6623–6629. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, H.; Yu, Z.B.; Wang, L.; Sun, L.; Sun, J. Construct Polyoxometalate Frameworks through Covalent Bonds. Inorg. Chem. 2015, 54, 8699–8704. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, Y.; Yu, Z.; Zhao, H.; Yao, Q.; Zou, X.; Bäckvall, J.-E.; Sun, J. 3D Open-Framework Vanadoborate as a Highly Effective Heterogeneous Pre-catalyst for the Oxidation of Alkylbenzenes. Chem. Mater. 2013, 25, 5031–5036. [Google Scholar] [CrossRef]
- Feng, Y.; Li, M.; Fan, H.; Huang, Q.; Qiu, D.; Shi, H. A novel open-framework copper borophosphate containing 1-D borophosphate anion with 10-MR windows and 12-MR channels. Dalton Trans. 2015, 44, 894–897. [Google Scholar] [CrossRef]
- Warren, C.J.; Rijssenbeek, J.T.; Rose, D.J.; Haushalter, R.C.; Zubieta, J. Hydrothermal synthesis and characterization of an unusual polyoxovanadium borate cluster structure of Rb4[(VO)6{B10O16(OH)6}2]·0.5H2O. Polyhedron 1998, 17, 2599–2605. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; An, L.; Chen, R.; Hu, F.; Tang, Q. Hydrothermal syntheses, crystal structures and characterization of new vanadoborates: The novel decorated cage cluster [V6B22O44(OH)10]. J. Solid State Chem. 2013, 201, 79–84. [Google Scholar] [CrossRef]
- Wu, M.; Law, T.S.; Sung, H.H.; Cai, J.; Williams, I.D. Synthesis of elliptical vanadoborates housing bimetallic centers [Zn4(B2O4H2)(V10B28O74H8)]8− and [Mn4(C2O4)(V10B28O74H8)]10−. Chem. Commun. 2005, 1827–1829. [Google Scholar] [CrossRef]
- Warren, C.J.; Haushalter, R.C.; Rose, D.J.; Zubieta, J. A bimetallic main group oxide cluster of the oxovanadium borate system:(H3NCH2CH2NH3)4[(VO)12O4{B8O17(OH)4}2 {Mn(H2O)2}2]·H2O. lnorg. Chim. Acta 1998, 282, 123–129. [Google Scholar] [CrossRef]
- Warren, C.J.; Rose, D.J.; Haushalter, R.C.; Zubieta, J. A New Transition Metal-Main Group Oxide Cluster in the Oxovanadium-Borate System: Hydrothermal Synthesis and Structure of (H3O)12[(VO)12{B16O32(OH)4}2]·28H2O. Inorg. Chem. 1998, 37, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Rijssenbeek, J.T.; Rose, D.J.; Haushalter, R.C.; Zubieta, J. Novel Clusters of Transition Metals and Main Group Oxides in the Alkylamine/Oxovanadium/Borate System. Angew. Chem. Int. Ed. Engl. 1997, 36, 9. [Google Scholar] [CrossRef]
- Yamase, T.; Suzuki, M.; Ohtaka, K. Structures of photochemically prepared mixed-valence polyoxovanadate clusters: Oblong [V18O44(N3)]14−, superkeggin [V18O42(PO4)]11− and doughnut-shaped [V12B32O84Na4]15− anions. J. Chem. Soc. Dalton Trans. 1997, 14, 2463–2472. [Google Scholar] [CrossRef]
- Hermosilla-Ibáñez, P.; Muñoz-Becerra, K.; Paredes-García, V.; Fur, E.; Spodine, E.; Venegas-Yazigi, D. Structural and Electronic Properties of Polyoxovanadoborates Containing the [V12B18O60] Core in Different Mixed Valence States. Inorganics 2015, 3, 309–331. [Google Scholar] [CrossRef]
- Hermosilla-Ibáñez, P.; Car, P.E.; Vega, A.; Costamagna, J.; Caruso, F.; Pivan, J.-Y.; Fur, E.L.; Spodine, E.; Venegas-Yazigi, D. New structures based on the mixed valence polyoxometalate cluster [V12B18O60H6]n−. Cryst. Eng. Comm. 2012, 14, 5604–5612. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Hu, F.; Zou, H.; Li, X. A new 1-D extended vanadoborate containing triply bridged metal complex units. Inorg. Chem. Commun. 2012, 25, 51–54. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, X.; Pan, S.; Han, J.; Yang, Y.; Zhang, F.; Yu, H. Synthesis, crystal structure, and properties of an interesting elliptical vanadoborate housing crystal: [Ni(en)2]6[(VO)12O6B18O39(OH)3]·5H2O. J. Alloys Compd. 2015, 624, 126–130. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Huang, C.; Yang, Q.; Chen, Y.; Sun, R.; Zhang, F.; Guo, W. Synthesis, crystal structure and two-dimensional infrared correlation spectroscopy of a layer-like transition metal (TM)-oxalate templated polyoxovanadium borate. J. Solid State Chem. 2005, 178, 3563–3570. [Google Scholar] [CrossRef]
- Hermosilla-Ibanez, P.; Canon-Mancisidor, W.; Costamagna, J.; Vega, A.; Paredes-Garcia, V.; Garland, M.T.; Le Fur, E.; Cador, O.; Spodine, E.; Venegas-Yazigi, D. Crystal lattice effect on the quenching of the intracluster magnetic interaction in [V12B18O60H6]10− polyoxometalate. Dalton Trans. 2014, 43, 14132–14141. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Chen, R.; Xiao, H.-P.; Hu, F.; Zou, H.; Zhou, Y.; Liu, C.; Zhu, L. New 3-D polyoxovanadoborate architectures based on [V12B18O60]16− clusters. Cryst. Eng. Comm. 2013, 15, 5057–5063. [Google Scholar] [CrossRef]
- Zhang, L.R.; Shi, Z.; Yang, G.Y.; Chen, X.M.; Feng, S.H. Hydrothermal Synthesis and X-Ray Single Crystal Structure of [Zn(en)2]6[(VO)12O6B18O39(OH)3]·13H2O. J. Solid State Chem. 1999, 148, 450–454. [Google Scholar] [CrossRef]
- Muñoz-Becerra, K.; Hermosilla-Ibáñez, P.; Le Fur, E.; Cador, O.; Paredes-García, V.; Spodine, E.; Venegas-Yazigi, D. First Non-Centrosymmetric Deca-Vanadoborate with Borate Vacancies, Self-Assembled around a 1,3-Propanediammonium Cation. Cryst. Growth Des. 2015, 15, 2561–2564. [Google Scholar] [CrossRef]
- An, L.; Zhou, J.; Xiao, H.-P.; Liu, X.; Zou, H.; Pan, C.-Y.; Liu, M.; Li, J. A series of new 3-D boratopolyoxovanadates containing five types of [KxOy]n building units. Cryst. Eng. Comm. 2014, 16, 4236–4244. [Google Scholar] [CrossRef]
- Feng, Y.; Ding, C.; Fan, H.; Zhong, Z.; Qiu, D.; Shi, H. Employing a new 12-connected topological open-framework copper borovanadate as an effective heterogeneous catalyst for oxidation of benzyl-alkanes. Dalton Trans. 2015, 44, 18731–18736. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla-Ibáñez, P.; Costamagna, J.; Vega, A.; Paredes-Garcia, V.; Le Fur, E.; Spodine, E.; Venegas-Yazigi, D. Coordination interactions in the crystalline lattice of alkaline ions with the polyoxometalate [V12B18O60H6]10− ligand. J. Coord. Chem. 2014, 67, 23–24. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Keene, T.D.; D’Alessandro, D.M.; Kramer, K.W.; Price, J.R.; Price, D.J.; Decurtins, S.; Kepert, C.J. [V16O38(CN)]9−: A Soluble Mixed-Valence Redox-Active Building Block with Strong Antiferromagnetic Coupling. Inorg. Chem. 2012, 51, 9192–9199. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on platinum metal catalysts in aqueous solutions. Catal. Today 1994, 19, 247–283. [Google Scholar] [CrossRef]
- Musawir, M.; Davey, P.N.; Kelly, G.; Kozhevnikov, I.V. Highly efficient liquid-phase oxidation of primary alcohols to aldehydes with oxygen catalysed by Ru–Co oxide. Chem. Commun. 2003, 34, 1414–1415. [Google Scholar] [CrossRef]
- Mizuno, N.; Kamata, K. Catalytic oxidation of hydrocarbons with hydrogen peroxide by vanadium-based polyoxometalates. Coord. Chem. Rev. 2011, 255, 2358–2370. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Ribera, A.; Nunes, A.V.M.; Gahramanova, S.I.; Marchettid, F.; Pombeiro, A.J.L. MnII and CuII complexes with arylhydrazones of active methylene compounds as effective heterogeneous catalysts for solvent- and additive-free microwave-assisted peroxidative oxidation of alcohols. RSC Adv. 2015, 5, 25979–25987. [Google Scholar] [CrossRef]
- Marui, K.; Higashiura, Y.; Kodama, S.; Hashidate, S.; Nomoto, A.; Yano, S.; Ueshima, M.; Ogawa, A. Vanadium-catalyzed green oxidation of benzylic alcohols in water under air atmosphere. Tetrahedron 2014, 70, 2431–2438. [Google Scholar] [CrossRef]
- Feng, Y.; Qiu, D.; Fan, H.; Li, M.; Huang, Q.; Shi, H. A new 3-D open-framework cadmium borovanadate with plane-shaped channels and high catalytic activity for the oxidation of cyclohexanol. Dalton Trans. 2015, 44, 8792–8796. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Nomoto, A.; Yano, S.; Ueshima, M.; Ogawa, A. Novel Heterotetranuclear V2Mo2 or V2W2 Complexes with 4,4′-Di-tert-butyl-2,2′-bipyridine: Syntheses, Crystal structures, and Catalytic Activities. Inorg. Chem. 2011, 50, 9942–9947. [Google Scholar] [CrossRef] [PubMed]
- Maurya, M.R.; Kumar, U.; Manikandan, P. Synthesis and Characterisation of Polymer-Anchored Oxidovanadium(IV) Complexes and Their Use for the Oxidation of Styrene and Cumene. Eur. J. Inorg. Chem. 2007, 2303–2314. [Google Scholar] [CrossRef]
- Bikas, R.; Shahmoradi, E.; Noshiranzadeh, N.; Emami, M.; Reinoso, S. The effects of halogen substituents on the catalytic oxidation of benzylalcohols in the presence of dinuclear oxidovanadium(IV) complex. Inorg. Chim. Acta 2017, 466, 100–109. [Google Scholar] [CrossRef]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalysis by heteropoly compounds—Recent developments. Appl. Catal. A Gen. 2001, 222, 63–77. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97; Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 are available from the authors. |



| [Zn6(en)3] [V12B18O54(OH)6(H2O)]2·14H2O | |
|---|---|
| Empirical formula | C6H68B36Zn6N6O136V24 |
| Formula weight | 4404.60 |
| Temperature/K | 293(2) |
| Crystal system | Cubic |
| Space group | Pn-3 |
| a/Å | 18.850(2) |
| b/Å | 18.850(2) |
| c/Å | 18.850(2) |
| α/° | 90 |
| β/° | 90 |
| γ/° | 90 |
| Volume/Å3 | 6698(2) |
| Z | 2 |
| Density (calc.)/Mg m−3 | 2.184 |
| Absorption coeff./mm−1 | 2.772 |
| F(000) | 4292 |
| Crystal size/mm3 | 0.32 × 0.25 × 0.15 |
| 2θ range for data collection | 6.11 to 54.85° |
| Index ranges | −24 ≤ h ≤ 24 −24 ≤ k ≤ 23 −24 ≤ l ≤ 24 |
| Reflections collected | 63889 |
| Independent reflections | 2569[R(int) = 0.0917] |
| Data/restraints/parameters | 2569/12/160 |
| Goodness-of-fit on F2 | 1.118 |
| Final R indexes [I >= 2σ(I)] | R1 = 0.0473, wR2 = 0.1138 |
| Final R indexes [all data] | R1 = 0.0576, wR2 = 0.1197 |
| Largest diff. peak/hole/e Å−3 | 1.323/−1.263 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Guo, B.; Sun, X.; Zhang, L.; Yuan, H. A New 3-D Open-Framework Zinc Borovanadate with Catalytic Potentials in α-Phenethyl Alcohol Oxidation. Molecules 2019, 24, 531. https://doi.org/10.3390/molecules24030531
Liu X, Guo B, Sun X, Zhang L, Yuan H. A New 3-D Open-Framework Zinc Borovanadate with Catalytic Potentials in α-Phenethyl Alcohol Oxidation. Molecules. 2019; 24(3):531. https://doi.org/10.3390/molecules24030531
Chicago/Turabian StyleLiu, Xinxin, Biao Guo, Xuejiao Sun, Le Zhang, and Hongming Yuan. 2019. "A New 3-D Open-Framework Zinc Borovanadate with Catalytic Potentials in α-Phenethyl Alcohol Oxidation" Molecules 24, no. 3: 531. https://doi.org/10.3390/molecules24030531
APA StyleLiu, X., Guo, B., Sun, X., Zhang, L., & Yuan, H. (2019). A New 3-D Open-Framework Zinc Borovanadate with Catalytic Potentials in α-Phenethyl Alcohol Oxidation. Molecules, 24(3), 531. https://doi.org/10.3390/molecules24030531






